TO THE EDITOR:
With advances in targeted therapy and cellular immunotherapy, older patients who comprise most patients with hematologic malignancies are increasingly being considered for higher intensity, potentially curative treatment such as chimeric antigen receptor T-cell (CAR T) therapy.1,2 However, how older patients (aged ≥ 65 years) respond to CAR T cells’ unique therapeutic mechanism and toxicity profile, remains unknown, and early studies using chronologic age as cutoff found that CAR T cells proliferate and expand in a similar way in older patients.3 Moreover, survival appeared to be similar, despite older patients having increased incidence of immune effector cell–associated neurotoxicity (ICANS), but not cytokine release syndrome (CRS).3-7
Given the effectiveness of comprehensive geriatric assessment (GA) to identify geriatric vulnerabilities such as multimorbidity, functional and cognitive impairments, and polypharmacy to guide treatment intensity; and to manage treatment-related toxicities, the American Society of Clinical Oncology has instituted guidelines recommending that all older patients with cancer receive GA before and during cancer therapy to avoid both over-treatment and under-treatment.8-10 However, whether geriatric frailty and vulnerability contribute to CAR T therapy outcomes have not been examined.
Multidimensional GA and geriatrics consultation was performed before lymphodepletion conditioning at the discretion of treating physicians.6 Comorbidity burden was assessed using the cumulative illness rating scale–geriatric.11 Cognition was assessed using Montreal Cognitive Assessment. Mobility was assessed using timed up and go test. All patients had a diagnosis of relapsed/refractory large B-cell lymphoma after ≥2 lines of therapies based on commercial labeling of axicabtagene ciloleucel (Axi-cel) and tisagenlecleucel (Tisacel) and were treated from January 2018 to May 2021. CAR T therapy eligibility criteria, clinical care, and monitoring, followed standard guidelines. The grading of CRS and ICANS followed the American Society for Transplantation and Cellular Therapy consensus guideline.12 Patient characteristics were summarized using descriptive statistics. Overall survival (OS) was defined from infusion until death or end of follow-up, whereas progression-free survival (PFS) was defined from infusion until progression, death, or end of follow-up in the absence of an event. Survival endpoints were estimated using Kaplan Meier methodology and compared using log-rank tests and a Cox proportional hazards model. Associations between comorbidities and development of ICANS or CRS were estimated using logistic regression. The study was approved by an institutional review board and conducted in accordance with the Declaration of Helsinki.
The cohort comprised 75 consecutive patients with a median age of 71.9 years (range, 65.1-85.9). Overall, 35 patients (47%) received Axi-cel, and 40 patients received Tisacel. In total, 48 patients (64%) had a formal geriatrics consultation with GA before lymphodepletion conditioning (geriatric consult group), whereas the remaining 27 patients did not (usual care group). The baseline characteristics of these 2 groups are tabulated in supplemental Table 1. With a median follow-up of 22 months among survivors, the median OS was 23 months (interquartile range, 12-not reached); and the median PFS was 6 months (interquartile range, 2.9-15) (supplemental Figure 1A). Thirty-six patients died; including 30 from disease relapse/progression and 3 patients each from organ toxicities and infections (2 COVID-19 related), respectively (supplemental Figure 1B). These findings are generally consistent with published reports for older patients.3,4,6,7,13,14
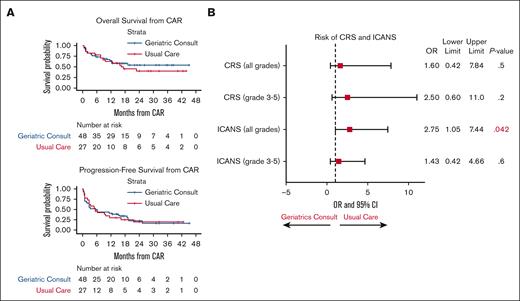
Both groups had similar OS and PFS (Figure 1A). The geriatric consult group had a median length of inpatient stay similar to that of the usual care group; 14 days (range, 5-69), vs 18 days (range, 5-121) to the usual care group (P = .3). The rate of 100-day readmission/emergency room visits was also similar between these 2 groups (P = .5). These results were consistent with a recent randomized, controlled trial of geriatrics consultation among older patients with newly diagnosed hematologic malignancies.15 Interestingly, we found that the odds of CRS and ICANS were lower in the geriatric consult group, reaching statistical significance for all-grade ICANS (odds ratio, 2.75; 95% confidence interval, 1.05-7.44; P = .042; Figure 1B). In a multivariate analysis, we found that tumor burden before lymphodepletion, measured by lactate dehydrogenase level, was strongly associated with survival and ICANS, consistent with prior findings.16-20 We also found that polypharmacy (>5) was significantly associated with the development of ICANS after adjusting for lactate dehydrogenase level and CAR T-cell product (supplemental Figure 2).
Impact of geriatric consultation on CAR T-cell therapy outcomes. (A) Kaplan-Meyer analysis of OS and PFS of the cohort, stratified by either usual care group (red line) or the geriatric consult group (blue line). The life table is depicted below the graph. (B) Forest plot of the risk of CRS and ICANS according to either the geriatric consult or the usual care group. The OR, 95% confidence interval, and P values were tabulated on the side of the plot. Statistically significant P value is depicted in red.
Impact of geriatric consultation on CAR T-cell therapy outcomes. (A) Kaplan-Meyer analysis of OS and PFS of the cohort, stratified by either usual care group (red line) or the geriatric consult group (blue line). The life table is depicted below the graph. (B) Forest plot of the risk of CRS and ICANS according to either the geriatric consult or the usual care group. The OR, 95% confidence interval, and P values were tabulated on the side of the plot. Statistically significant P value is depicted in red.
The mechanism leading to the reduction of CRS and ICANS is unclear, but it could be related to the clinical team’s increased awareness of, and efforts to, remedy identified GA deficits, many of which were associated with the development of toxicities including CRS and ICANS (refer to further discussion). It should be noted, however, that the uptake of the recommendations from geriatrics consultation was not tracked, and follow-up consultation was not consistently performed. Because GA and GA-guided management can help improve older patients’ functional and mobility impairment, manage polypharmacy and potentially inappropriate medication use, and increase communication and awareness among patients and providers,9,10 we postulate that similar mechanisms may help older recipients of CAR T therapy during the intensive treatment. Finally, our findings support that geriatric polypharmacy, which is likely modifiable in the short term, could significantly worsen outcomes of older patients undergoing intensive, hospital-based, high-risk treatments such as CAR T therapy or transplantation.21
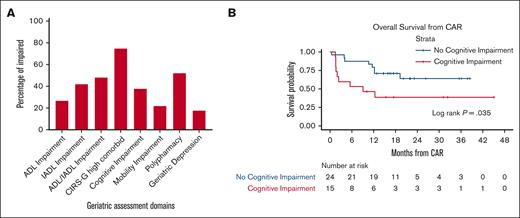
Among patients in the geriatric consult group, impairments in ≥1 GA domains were common: 75% with high Cumulative Illness Rating Scale–Geriatric; 50% with functional impairment, and 38% with cognitive impairment (Figure 2A). These highly prevalent baseline deficits depicted a generally more vulnerable and frailer cohort of older patients with lymphoma than patients who are newly diagnosed because of multiple relapsed, refractory, and heavily pretreated disease.22,23 The median number of geriatrics visits until 1 year after CAR T therapy was 1 (range, 1-7 visits). Most importantly, we examined the association of various GA deficits with CAR T therapy outcomes and found that cognitive impairment (defined by a Montreal Cognitive Assessment score of <26) was significantly associated with worse OS (P value = .035 by log-rank test) (Figure 2B). We also found that cognitive impairment was associated with the development of CRS, whereas mobility impairment was associated with the development of ICANS (supplemental Table 2). In contrast, neither functional impairment nor psychologic impairment were associated with either CRS or ICANS.
Prevalence and impact of geriatric impairment on CAR T-cell therapy outcomes. (A) Prevalence of geriatric deficits as depicted in red bar graph. (B) Kaplan-Meyer analysis of OS stratified pre–CAR T therapy cognitive impairment (no, blue line; or yes, red line). The life table is depicted below the graph. ADL, activities of daily living; CIRS-G, cumulative illness rating scale–geriatric; IADL, instrumental activities of daily living.
Prevalence and impact of geriatric impairment on CAR T-cell therapy outcomes. (A) Prevalence of geriatric deficits as depicted in red bar graph. (B) Kaplan-Meyer analysis of OS stratified pre–CAR T therapy cognitive impairment (no, blue line; or yes, red line). The life table is depicted below the graph. ADL, activities of daily living; CIRS-G, cumulative illness rating scale–geriatric; IADL, instrumental activities of daily living.
Our results suggest that, with adequate institutional support and appropriate coordination, it is feasible to perform GA and potentially act on identified deficits before highly time-sensitive, intensive treatment such as CAR T therapy. Moreover, selected geriatric impairments such as functional decline, polypharmacy and potentially inappropriate medication use, and comorbidity management could be remedied and/or optimized in the short term.9,10 These potential benefits of GA/consultation may have led to the apparent reduced incidence of toxicities we observed. Finally, it is also very possible that GA can be used to guide appropriate patient selection for intensive treatments such as CAR T therapy. However, we did not have patients who were not undergoing CAR T therapy for comparison and prospective studies are needed to address this question. Mechanistically, GA deficits could contribute to CAR T toxicities and survival in several ways. First, GA deficits may be exacerbated by higher disease burden, which in turn was associated with lower CAR T efficacy and increased toxicities.3,16,19,20 Second, geriatric frailty, exemplified by various impairments, could create a proinflammatory milieu detrimental to CAR T therapy.24 This notion will require comparisons of T-cell fitness, CAR T-cell expansion and persistence, and proinflammatory cascade after treatment among a larger cohort of patients who are fit vs those who are frail. Finally, GA deficits increased risk of side effects and may lead to functional and cognitive decline and accumulation aging-related deficits, which may reduce the ability of these patients to receive/tolerate subsequent therapy.25
Our study is limited by the single institution design, the lack of randomization thus creating potential biases between 2 groups, and the lack of details on geriatric management. However, this is 1 of the largest, oldest cohorts of older patients with lymphoma receiving CAR T therapy published to date, with many of these patients having significant GA deficits and suboptimal performance status who would be excluded from clinical trials. Our results suggest that older patients with lymphoma could achieve substantial benefits from curative-intent CAR T therapy with acceptable side effects; and that GA and geriatric consultation could benefit these older patients because of reductions of toxicities and managements of non-oncologic, geriatric issues before, during, and after the intensive treatment period. Moreover, we show here, to the best of our knowledge, for the first time, that certain GA deficits including cognitive and mobility impairment, polypharmacy, and multimorbidity, are associated with survival and toxicities in a nonchemotherapy based, cellular immunotherapy setting. These findings continue to validate the importance and the added value of GA/GA-guided management in the care of older patients with cancer and call for its integration into standard CAR T management pathways and algorithms.
Acknowledgments: The authors thank the patients and their families, and all the investigators and research staff involved in data collection and analyses. The authors acknowledge National Institutes of Health/National Cancer Institute grant P01 CA23766 and National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748 (M.S.K.). R.J.L. is supported by American Society of Hematology and Elsa U. Pardee Foundation for Cancer Research.
Contribution: R.J.L., P.A.H., and S.A.G. conceived and designed the study; S.J.K. and B.K.-G. performed geriatric assessment and interpretation; T.A.E., J.D.R., D.M.H., A.A.T., R.S., N.L., E.A.P., and D.E.E. collected and assembled the data; S.B. and S.M.D. performed statistical analysis; and all authors provided study material, patients, and administrative support, participated in manuscript writing, and provided final approval for the article.
Conflict-of-interest disclosure: S.A.G. serves on an advisory board for Amgen, Actinuum, Celgene, Johnson & Johnson, Jazz Pharmaceutical, Takeda, Novartis, Kite, and Spectrum Pharma, and receives research funding from Amgen, Actinuum, Celgene, Johnson & Johnson, Miltenyi, and Takeda. P.A.H. receives research support and consulting fees from Portola Pharmaceuticals, Inc. G.A.S. reports consultancy with AbbVie Inc., Allogene Therapeutics, Autolus Therapeutics, BeiGene Ltd., Bristol Myers Squibb Company, Celgene Corporation, Debiopharm Group, Genmab, Kite (a Gilead Company), Incyte Corporation, Janssen Biotech Inc., Miltenyi Biotec, MorphoSys, Novartis, and Roche. J.H.P. reports consultancy with Amgen, Novartis, Kite Pharma, Incyte, Allogene, Autolus, Intellia, Artiva, AstraZeneca, Umoja, Kura Oncology, Innate Pharma, Takeda, and Servier. M.A.P. received honoraria from AbbVie, Bellicum, Bristol Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda; serves on data safety monitoring boards for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune; and has received research support for clinical trials from Incyte, Kite (Gilead), and Miltenyi Biotec. C.S.S. reports consultancy with Juno Therapeutics, Sanofi Genzyme, Spectrum Pharmaceuticals, Novartis, Precision Biosciences, Kite, a Gilead Company, and GSK. M.L.P. serves on an advisory board for Celgene and consults for Merck and Pharmacyclics. G.L.S. reports research support from Janssen, Amgen, Beyond Spring, Bristol Myers Squibb, and Arcellx. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience Inc., and Omeros Corporation; received research funding from Angiocrine Bioscience Inc., Omeros Corporation, and Amgen Inc.; served on ad hoc advisory boards for Kite (a Gilead Company); and received honoraria from i3Health and Medscape for continuing medical education-related activity. R.S. reports serving on the advisory board with Medexus. P.B.D. reports consultancy with Kite (a Gilead Company). R.J.L. reports consultancy with Kite (a Gilead Company). The remaining authors declare no competing financial interests.
Correspondence: Richard J. Lin, Adult BMT and Cellular Therapy Services, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: linr@mskcc.org.
References
Author notes
The current affiliation for C.S.S. is Cleveland Clinic Foundation, Cleveland, OH.
The current affiliation for C.L.B. is Genentech, San Francisco, CA.
The current affiliation for A.A.T. is Hospital Universitario Gregorio Marañón, Madrid, Spain.
Data are available on request from the corresponding author, Richard J. Lin (linr@mskcc.org).
The full-text version of this article contains a data supplement.