Key Points
The gut microbiota in patients with PV is different from that in HCs.
The gut microbiota differs between patients with PV with different treatments.
Abstract
Chronic inflammation is believed to play an important role in the development and disease progression of polycythemia vera (PV). Because an association between gut microbiota, hematopoiesis, and inflammation is well established, we hypothesized that patients with PV have a gut microbiota distinct from healthy control participants (HCs). Recombinant interferon alfa 2 (IFN-α2)-treatment of patients with PV is reportedly disease modifying in terms of normalization of elevated blood cell counts in concert with a reduction in the JAK2V617F allelic burden. Therefore, we hypothesized that patients treated with IFN-α2 might have a composition of the gut microbiota toward normalization. Herein, via amplicon-based next-generation sequencing of the V3 to V4 regions of the 16S ribosomal RNA gene, we report on an abnormal gut microbiota in 102 patients with PV compared with 42 HCs. Patients with PV had a lower alpha diversity and a lower relative abundance of several taxa belonging to Firmicutes (45%) compared with HCs (59%, P <.001). Furthermore, we report the composition of the gut microbiota to differ between the treatment groups (IFN-α2, hydroxyurea, no treatment, and combination therapy with IFN-α2 and ruxolitinib) and the HCs. These observations are highly interesting considering the potential pathogenetic importance of an altered gut microbiota for development of other diseases, including chronic inflammatory diseases. Our observations call for further gut microbiota studies to decipher potential causal associations between treatment and the gut microbiota in PV and related neoplasms.
Introduction
Polycythemia vera (PV) is an acquired clonal stem cell neoplasm within the Philadelphia chromosome negative myeloproliferative neoplasms (MPNs), which also include essential thrombocythemia and primary myelofibrosis.1
Nearly all patients with PV have a Janus kinase-2 (JAK2) driver mutation either in exon 14 (JAK2V617F) (96%) or exon 12 (3%).2-4 Chronic inflammation, partly driven by the malignant clone per se in a self-perpetuating vicious circle consequent to the inflammatory JAK2V617F mutation, is believed to play an important role in the development and progression of MPNs.5-8
Apart from digestion of food, the human gut microbiota assists in the development of the host immune system, protection against pathogens, and has a variety of additional functions.9 Changes in the gut microbiota have been linked to several autoimmune and inflammation-driven diseases such as systemic lupus erythematosus,10,11 obesity, atherosclerosis, and diabetes,9,12 but also response to immunotherapy.13-15
The gut microbiota also influences hematopoiesis. Thus, germ-free mice have lower levels of hematopoietic stem and precursor cells compared with specific pathogen–free mice.16 Other studies have shown that changes in the gut microbiota influence hematopoiesis in both mice and humans.17,18
For decades, patients at low risk (aged < 60 years, no prior thrombosis, and platelet count less than 1500 × 109/L), have been treated with phlebotomies and aspirin only. Patients at high risk have conventionally been treated with hydroxyurea (HU), which normalizes elevated blood cell counts within a few days.19-21 However, HU treatment does not target the malignant stem cell and is not disease modifying. During the last 30 years, evidence has accumulated, being supported most recently by randomized studies, that recombinant interferon alfa 2 (IFN-α2) is a safe and highly efficacious treatment,22,23 which directly targets the malignant clone, and which is also disease modifying as defined by sustained induction of normal blood cell counts and deep molecular remissions.24 Another recently licensed drug is the JAK1-2 inhibitor, ruxolitinib, which is used in patients with PV refractory to or intolerant to HU.25 However, ruxolitinib is highly immunosuppressive and accordingly is associated with an increased risk of infections.26 The effect of ruxolitinib together with IFN-α2 in lower doses (COMBI therapy),27 has been evaluated in a phase 2 study,28 and is currently being evaluated in patients with newly diagnosed PV.
Knowledge about the microbiota in patients with MPNs compared with healthy control participants (HCs) is limited, and no significant differences in alpha diversity have been recorded in 2 smaller studies.29,30 However, 1 study found that patients with MPN had a lower abundance of Phascolarctobacterium compared with HCs.29 Because MPNs are considered inflammation-driven diseases, which are also characterized by immune deregulation, and because the gut microbiota influences the immune system and hematopoiesis, investigations of the microbiota are highly important and relevant.
Therefore, we have investigated the characteristics of the gut microbiota in a larger series of patients with PV (N = 102) compared with HCs (N = 42) and in relation to different treatment regimens, including IFN-α2 monotherapy, combination therapy of IFN-α2 and ruxolitinib (COMBI), and HU monotherapy.
Materials and methods
Ethics approval and dissemination
The project was approved by the National Committee on Health Research Ethics (SJ-698 and SJ-452) and the Danish Data Protection Agency (REG-054-2018, REG-050-2015), and was conducted in accordance with the Declaration of Helsinki.
Patient recruitment
From November 2018 to August 2021, patients from the Department of Hematology, Roskilde, Denmark, aged > 18 years and diagnosed with PV according to the 2016 World Health Organization classification of MPNs31 were invited to participate in the study. Written informed consent was obtained from all patients.
The exclusion criteria were as follows: pregnancy, use of antibiotics within the last 2 months, change in cytoreductive treatment within the last 3 months, or inability to understand the oral and written information. Patients were categorized according to treatment: no cytoreductive treatment, HU, IFN-α2, or COMBI. In total, 10 patients received treatment with other combinations; for example, HU combined with ruxolitinib, and were excluded owing to small group sample sizes. Thus, 102 patients with PV were included for further studies.
HC recruitment
HC (N = 42) were recruited from the Danish General Suburban Population Study (GESUS).32 HCs were defined by the absence of JAK2V617F and CALR mutations, no elevated blood cell counts, and a high-sensitivity C-reactive protein (CRP) ≤ 2 mg/L at the time of examination in GESUS between 2010 and 2013. Eligible HCs were matched 1:4 by age and sex against individuals with JAK2V617F or CALR mutations. HCs were invited by letter, and blood cell count measurements were performed to ensure normal blood cell counts and CRP at the time of stool sample. Written informed consent was obtained from all HCs.
Sample collection
Patients and HCs were given a sampling vial and written instructions on how to collect a stool sample of 2 to 5 mL on the morning of an outpatient visit. The sample was stored at −80°C within 6 hours of sample collection.
Clinical and biochemical data
Clinical and biochemical data were collected for each patient retrospectively from the day of stool sampling using electronic medical records.
Clinical data included time (years) since diagnosis, previous hematologic treatment, current hematologic treatment, body mass index (kg/m2), smoking status, hypertension, comorbidities, and use of statins or metformin.
Comorbidities were defined using Charlson Comorbidity Index (CCI)33 and were categorized as “no comorbidities” (CCI score = 0), “low burden” (CCI score = 1-2), and “moderate to high burden” (CCI score > 2). Hypertension was defined based on the use of either antihypertensive treatment or ICD-10 (International Classification of Diseases, 10th revision) codes (DI10 and DI109).
Biochemical data included hemoglobin concentration, hematocrit, leukocytes (×109/L), thrombocytes (×109/L), creatinine (μmol/L), and CRP, and estimated glomerular filtration rate (eGFR) was calculated with the eGFR EPI-CKD formula by Levey (mL/minute per 1.73m2).34
16S ribosomal RNA (rRNA) gene sequencing and taxonomic classification
To characterize the gut microbiota, fecal samples were subjected to amplicon-based next-generation sequencing of the V3-V4 regions of the 16S rRNA gene, using a MiSeq sequencer (Illumina Inc). Trimmed reads were queried against DADA2 (version 1.12.1) for quality control, inference of high resolution amplicon sequence variants (ASVs), and taxonomic classification,35 based on the Silva database.36
Removal of contamination was carried out using the Decontam R package.37 For further details, refer to the supplemental Methods.
Statistical analysis
Graphical visualizations and statistical analyses were performed in R (version 4.0.3)38 The R code documenting our statistical analyses is available at https://doi.org/10.6084/m9.figshare.21252783.
Sequence data and clinical and paraclinical data were merged to a phyloseq object using the phyloseq package39 and plots were generated using ggplot2.40
For comparison of normally distributed continuous data, analysis of variance was used, and when data were not normally distributed, pairwise Wilcoxon rank sum tests were used. When comparing categorical data, Fisher exact test was used. When applicable, tests were adjusted for multiple testing using Benjamini-Hochberg correction, and P <.05 were considered to indicate statistical significance.
Alpha diversity (within sample diversity based on evenness and richness in the sample; here, Inverse Simpson Index) and observed richness (number of ASV per sample) were calculated based on untransformed counts. A pairwise Wilcoxon rank sum test was used to compare HCs and patients with PV, as well as to compare PV treatment groups. Differences in bacterial composition between groups were visualized using principal coordinate analyses (PCoAs) based on Bray-Curtis dissimilarities of Hellinger-transformed counts. Analysis of similarities (ANOSIM, package vegan)41 was used to compare differences in composition between the groups. The ANOSIM statistic ‘R’ indicates the difference between groups. The higher the R value (ie, the closer R is to 1), the greater the difference in composition between the groups.
Differences in relative abundance across taxonomic levels (phylum to genus) between the groups were visualized in taxonomic heat trees (package metacoder).42 Linear discriminant analysis effect size (LefSe) was used to identify significant differences in relative abundance at several taxonomical levels (here, phylum to genus) between groups. LefSe was run with total sum scaling normalization and a linear discriminant analysis cutoff of 0.005 (package microbiomeMarker).43
Results
Cohort characteristics and demographic data
This study included 102 patients with PV with a median age of 70 years (range, 31-85) and 42 HCs with a median age of 71 years (range, 66-74) (Table 1). The 2 groups did not differ in terms of sex, age, body mass index, smoking status, hypertension, comorbidities, leukocyte count, CRP, and eGFR. The thrombocyte count was lower in HCs (median, 227 × 109/L; range, 125-355 × 109/L) than in patients with PV (median, 309 × 109/L; range, 113-884, P < .0001). The hematocrit was higher in HCs (mean, 43%; standard deviation [SD], 4%) than in patients with PV (mean, 42%; SD, 3%; P = .037) (Table 1).
Baseline characteristics of patients with PV vs HCs
| Characteristics . | Group . | P value . | |
|---|---|---|---|
| PV . | HCs . | ||
| Patients, N | 102 | 42 | |
| Sex | |||
| Female, n (%) | 45 (44.1) | 17 (40.5) | .72 |
| Age, y | |||
| Median (range) | 70 (31-85) | 71 (66-74) | .083∗ |
| Body mass index, kg/m2 | |||
| Mean (SD) | 26.0 (3.9) | 24.9 (2.4) | .34† |
| Smoking | |||
| Current smoker, n (%) | 8 (7.8) | 1 (2.4) | .28 |
| Former smoker, n (%) | 43 (42.2) | 13 (31) | .35 |
| Never smoker, n (%) | 50 (49) | 26 (61.9) | .28 |
| Unknown, n (%) | 1 (1) | 2 (4.8) | |
| Hypertension | |||
| Yes, n (%) | 55 (53.9) | 16 (38.1) | .1 |
| No, n (%) | 47 (46.1) | 26 (61.9) | .36 |
| Unknown, n (%) | 0 (0) | 0 (0) | |
| Comorbidity index‡ | |||
| Median (range) | 1 (0-9) | 0 (0-4) | .058∗ |
| n (%) | |||
| No comorbidities (CCI = 0) | 36 (35.3) | 22 (52.4) | .064 |
| Low burden (CCI = 1-2) | 42 (41.2) | 16 (38.1) | .85 |
| Moderate to high (CCI > 2) | 24 (23.5) | 4 (9.5) | .065 |
| Blood test | |||
| Leukocyte, ×109/L | |||
| Median (range) | 5.7 (2.5-21.8) | 6.2 (4.5-9.4) | .15∗ |
| Hematocrit, % | |||
| Mean (SD) | 42 (4) | 43 (3) | .037† |
| Thrombocyte, ×109/L | |||
| Median (range) | 309 (113-884) | 227 (125-355) | <.001∗ |
| eGFR | |||
| Mean (SD) | 78.2 (16.5) | 73.1 (13.2) | .14† |
| Characteristics . | Group . | P value . | |
|---|---|---|---|
| PV . | HCs . | ||
| Patients, N | 102 | 42 | |
| Sex | |||
| Female, n (%) | 45 (44.1) | 17 (40.5) | .72 |
| Age, y | |||
| Median (range) | 70 (31-85) | 71 (66-74) | .083∗ |
| Body mass index, kg/m2 | |||
| Mean (SD) | 26.0 (3.9) | 24.9 (2.4) | .34† |
| Smoking | |||
| Current smoker, n (%) | 8 (7.8) | 1 (2.4) | .28 |
| Former smoker, n (%) | 43 (42.2) | 13 (31) | .35 |
| Never smoker, n (%) | 50 (49) | 26 (61.9) | .28 |
| Unknown, n (%) | 1 (1) | 2 (4.8) | |
| Hypertension | |||
| Yes, n (%) | 55 (53.9) | 16 (38.1) | .1 |
| No, n (%) | 47 (46.1) | 26 (61.9) | .36 |
| Unknown, n (%) | 0 (0) | 0 (0) | |
| Comorbidity index‡ | |||
| Median (range) | 1 (0-9) | 0 (0-4) | .058∗ |
| n (%) | |||
| No comorbidities (CCI = 0) | 36 (35.3) | 22 (52.4) | .064 |
| Low burden (CCI = 1-2) | 42 (41.2) | 16 (38.1) | .85 |
| Moderate to high (CCI > 2) | 24 (23.5) | 4 (9.5) | .065 |
| Blood test | |||
| Leukocyte, ×109/L | |||
| Median (range) | 5.7 (2.5-21.8) | 6.2 (4.5-9.4) | .15∗ |
| Hematocrit, % | |||
| Mean (SD) | 42 (4) | 43 (3) | .037† |
| Thrombocyte, ×109/L | |||
| Median (range) | 309 (113-884) | 227 (125-355) | <.001∗ |
| eGFR | |||
| Mean (SD) | 78.2 (16.5) | 73.1 (13.2) | .14† |
CCI, Charlson Comorbidity Index; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Pairwise Wilcoxon rank sum test.
Analysis of variance with post hoc Tukey honest significant difference test.
Comorbidity scores were calculated using CCI.
Each of the 102 patients with PV belonged to 1 of 4 treatment groups; 19 received no treatment (NT) besides low-dose aspirin and phlebotomy, 37 received HU, 23 received IFN-α2, and 23 were treated with COMBI.
Patients treated with HU had a median age of 74 years (range, 53-85) and were older than the patients in the other treatment groups but not older than the HCs. The HCs were older than patients receiving NT, who had a median age of 62 years (range, 38-79; P = .002), and patients treated with IFN-α2, who had a median age 65 years (range, 31-81; P = .001). No significant difference was seen in CRP between the treatment groups.
A significant difference was observed between the treatment groups and HCs, and within the treatment groups, in terms of leukocyte count, hematocrit, thrombocyte count, treatment length, and eGFR; for P values, refer to Table 2.
Baseline characteristics by treatment group
| Characteristics . | Group . | P value . | ||||
|---|---|---|---|---|---|---|
| NT (A) . | HU (B) . | COMBI (C) . | IFN-α2 (D) . | HCs (E) . | ||
| Patients, n | 19 | 37 | 23 | 23 | 42 | |
| Sex | ||||||
| Female, n (%) | 9 (47.4) | 17 (45.9) | 11 (47.8) | 8 (34.8) | 17 (40.5) | NS |
| Age, y | E vs A, P = .002∗ | |||||
| Median (range) | 62 (38-79) | 74 (53-85) | 68 (47-79) | 65 (31-81) | 71(66-74) | E vs D, P = .001; B vs A + D, P = .001; B vs C, P = .01 |
| Disease length, wk† | ||||||
| Median (range) | 207 (0-1214) | 290 (13-2124) | 76 (16-1170) | 452 (44-1183) | NA | NS |
| Treatment length, wk | ||||||
| Median (range) | NA | 210 (13-1028) | 47 (13-369) | 186 (35-587) | NA | C vs B + D, P < .01∗ |
| Body mass index, kg/m2 | ||||||
| Mean (SD) | 25.4 (4.6) | 26.3 (4.1) | 26.3 (4.1) | 26.0 (3.9) | 24.9 (2.4) | NS |
| Smoking, n (%) | ||||||
| Current smoker | 3 (15.8) | 2 (5.4) | 3 (13.0) | 0 (0) | 1(2.4) | NS |
| Former smoker | 7 (36.8) | 17 (45.9) | 9 (39.1) | 10 (34.5) | 13 (31) | |
| Never smoker | 9 (47.4) | 18 (48.6) | 11 (47.8) | 12 (52.2) | 26(61.9) | |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (4.3) | 2 (4.8) | |
| Hypertension, n (%) | ||||||
| Yes | 10 (52.6) | 22 (59.5) | 14 (60.9) | 9 (39.1) | 16 (38.1) | |
| No | 9 (47.4) | 15 (40.5) | 9 (39.1) | 14 (60.9) | 26 (61.9) | NS |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | |
| Comorbidity index‡ | ||||||
| Median (range) | 0 (0-9) | 1 (0-5) | 2 (0-8) | 1 (0-6) | 0 (0-4) | |
| n (%) | NS | |||||
| No comorbidities (CCI = 0) | 10 (52.6) | 11 (29.7) | 5 (21.7) | 10 (43.5) | 22 (52.4) | |
| Low burden (CCI = 1-2) | 7 (36.8) | 14 (37.8) | 13 (56.5) | 8 (34.8) | 16 (38.1) | |
| Moderate to high (CCI > 2) | 2 (10.5) | 12 (32.4) | 5 (21.7) | 5 (21.7) | 4 (9.5) | |
| Blood test | ||||||
| Leukocyte, ×109/L | ||||||
| Median (range) | 8.5 (5.2-20.0) | 6.7 (2.9-21.8) | 4.6 (2.9-7.3) | 4.1 (2.5-13.2) | 6.2 (4.5-9.4) | A vs C + D + E, P < .001∗ |
| A vs B, P = .03 | ||||||
| B vs C + D, P < .001 | ||||||
| E vs C + D, P < .001 | ||||||
| Hematocrit, % | ||||||
| Mean (SD) | 44 (4) | 42 (3) | 38 (5) | 42 (3) | 43 (3) | A vs C, P < .001§ |
| B vs C, P = .002 | ||||||
| C vs D, P = .002; C vs E, P < .001 | ||||||
| Thrombocyte, ×109/L | ||||||
| Median (range) | 415 (147-884) | 316 (120-635) | 288 (118-494) | 206 (113-820) | 227 (125-355) | A vs C, P = .03; A vs D, P < .01; A vs E, P < .001∗ |
| B vs D, P = .01; B vs E, P < .001 | ||||||
| eGFR | ||||||
| Mean (SD) | 83.8 (18.4) | 72.8 (12.2) | 75.2(15.3) | 85.1(12.2) | 73.1 (13.2) | B vs D, P = .02§ |
| JAK2 V617F allele burden‖, median (range) | 16 (0.11-49) | 8.4 (5.7-96) | 22 (0.42-59) | 6.65 (0.21-49) | 0 (0-0) | C vs D, P = .012∗ |
| E vs A + B + C + D, P < .001 | ||||||
| Characteristics . | Group . | P value . | ||||
|---|---|---|---|---|---|---|
| NT (A) . | HU (B) . | COMBI (C) . | IFN-α2 (D) . | HCs (E) . | ||
| Patients, n | 19 | 37 | 23 | 23 | 42 | |
| Sex | ||||||
| Female, n (%) | 9 (47.4) | 17 (45.9) | 11 (47.8) | 8 (34.8) | 17 (40.5) | NS |
| Age, y | E vs A, P = .002∗ | |||||
| Median (range) | 62 (38-79) | 74 (53-85) | 68 (47-79) | 65 (31-81) | 71(66-74) | E vs D, P = .001; B vs A + D, P = .001; B vs C, P = .01 |
| Disease length, wk† | ||||||
| Median (range) | 207 (0-1214) | 290 (13-2124) | 76 (16-1170) | 452 (44-1183) | NA | NS |
| Treatment length, wk | ||||||
| Median (range) | NA | 210 (13-1028) | 47 (13-369) | 186 (35-587) | NA | C vs B + D, P < .01∗ |
| Body mass index, kg/m2 | ||||||
| Mean (SD) | 25.4 (4.6) | 26.3 (4.1) | 26.3 (4.1) | 26.0 (3.9) | 24.9 (2.4) | NS |
| Smoking, n (%) | ||||||
| Current smoker | 3 (15.8) | 2 (5.4) | 3 (13.0) | 0 (0) | 1(2.4) | NS |
| Former smoker | 7 (36.8) | 17 (45.9) | 9 (39.1) | 10 (34.5) | 13 (31) | |
| Never smoker | 9 (47.4) | 18 (48.6) | 11 (47.8) | 12 (52.2) | 26(61.9) | |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 1 (4.3) | 2 (4.8) | |
| Hypertension, n (%) | ||||||
| Yes | 10 (52.6) | 22 (59.5) | 14 (60.9) | 9 (39.1) | 16 (38.1) | |
| No | 9 (47.4) | 15 (40.5) | 9 (39.1) | 14 (60.9) | 26 (61.9) | NS |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | |
| Comorbidity index‡ | ||||||
| Median (range) | 0 (0-9) | 1 (0-5) | 2 (0-8) | 1 (0-6) | 0 (0-4) | |
| n (%) | NS | |||||
| No comorbidities (CCI = 0) | 10 (52.6) | 11 (29.7) | 5 (21.7) | 10 (43.5) | 22 (52.4) | |
| Low burden (CCI = 1-2) | 7 (36.8) | 14 (37.8) | 13 (56.5) | 8 (34.8) | 16 (38.1) | |
| Moderate to high (CCI > 2) | 2 (10.5) | 12 (32.4) | 5 (21.7) | 5 (21.7) | 4 (9.5) | |
| Blood test | ||||||
| Leukocyte, ×109/L | ||||||
| Median (range) | 8.5 (5.2-20.0) | 6.7 (2.9-21.8) | 4.6 (2.9-7.3) | 4.1 (2.5-13.2) | 6.2 (4.5-9.4) | A vs C + D + E, P < .001∗ |
| A vs B, P = .03 | ||||||
| B vs C + D, P < .001 | ||||||
| E vs C + D, P < .001 | ||||||
| Hematocrit, % | ||||||
| Mean (SD) | 44 (4) | 42 (3) | 38 (5) | 42 (3) | 43 (3) | A vs C, P < .001§ |
| B vs C, P = .002 | ||||||
| C vs D, P = .002; C vs E, P < .001 | ||||||
| Thrombocyte, ×109/L | ||||||
| Median (range) | 415 (147-884) | 316 (120-635) | 288 (118-494) | 206 (113-820) | 227 (125-355) | A vs C, P = .03; A vs D, P < .01; A vs E, P < .001∗ |
| B vs D, P = .01; B vs E, P < .001 | ||||||
| eGFR | ||||||
| Mean (SD) | 83.8 (18.4) | 72.8 (12.2) | 75.2(15.3) | 85.1(12.2) | 73.1 (13.2) | B vs D, P = .02§ |
| JAK2 V617F allele burden‖, median (range) | 16 (0.11-49) | 8.4 (5.7-96) | 22 (0.42-59) | 6.65 (0.21-49) | 0 (0-0) | C vs D, P = .012∗ |
| E vs A + B + C + D, P < .001 | ||||||
CCI, Charlson Comorbidity Index; eGFR, estimated glomerular filtration rate; NA, not applicable; NS, not significant; SD, standard deviation.
Pairwise Wilcoxon rank sum test.
Weeks since diagnosis of PV.
Comorbidity scores were calculated using CCI.
Analysis of variance with post hoc Tukey honest significant difference test.
JAK2 V617F allele burden at sample time.
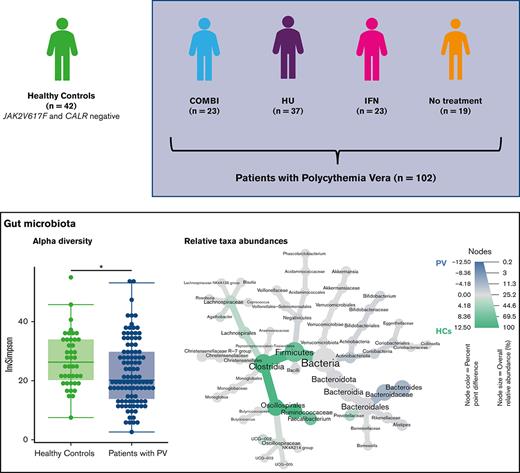
Lower alpha diversity in patients with PV than in HCs
Gut microbiota alpha diversity (within sample diversity based on evenness and richness in the sample) was significantly lower in patients with PV (median Inverse Simpson Index, 20.2) than in HCs (median Inverse Simpson Index, 26.3; P = .016) (Figure 1A). However, patients with PV had a higher observed richness (number of ASV per sample; median observed ASVs, 209.5) compared with HCs (median observed ASVs, 190; P = .034) (supplemental Figure 1A).
Gut microbiota alpha diversity of patients with PV, of the different PV treatment groups, and of HCs. (A) Gut microbiota alpha diversity (median Inverse Simpson Index [InvSimpson]) of HCs and patients with PV. Alpha diversity differed significantly between patients with PV (median InvSimpson, 20.2) and HCs (median InvSimpson, 26.3) (P = .016). (B) Alpha diversity (InvSimpson) of HCs, COMBI, HU, IFN-α2, and NT group. After adjusting for multiple testing, no significant difference was found.
Gut microbiota alpha diversity of patients with PV, of the different PV treatment groups, and of HCs. (A) Gut microbiota alpha diversity (median Inverse Simpson Index [InvSimpson]) of HCs and patients with PV. Alpha diversity differed significantly between patients with PV (median InvSimpson, 20.2) and HCs (median InvSimpson, 26.3) (P = .016). (B) Alpha diversity (InvSimpson) of HCs, COMBI, HU, IFN-α2, and NT group. After adjusting for multiple testing, no significant difference was found.
When comparing the different treatment groups with each other and with the HCs, we observed lower alpha diversity in patients treated with COMBI (median Inverse Simpson Index, 18.8) and patients receiving NT (median Inverse Simpson Index, 19.4) than in HCs (median Inverse Simpson Index, 26.3). These differences were not significant after adjusting for multiple testing (Figure 1B). No difference in observed richness was found when the different treatment groups were compared with each other (supplemental Figure 1B).
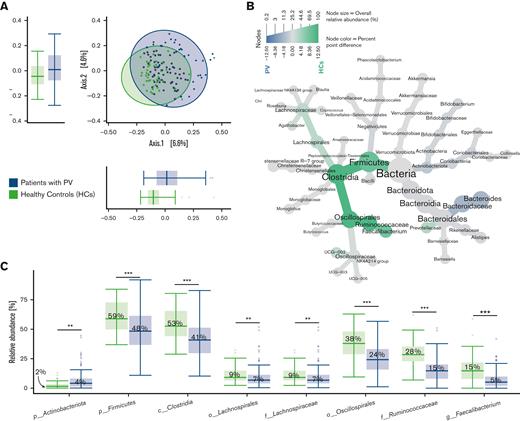
Lower abundance of taxa within Firmicutes in patients with PV compared with HCs
The overall bacterial composition as assessed by PCoA did not differ between patients with PV and HCs (Figure 2A). However, a heat tree depicting relative abundances across taxonomic levels revealed lower abundances of taxa affiliated with the phylum Firmicutes (specifically Clostridia and Ruminococcaceae), as well as Prevotellaceae (phylum Bacteroidota, formerly known as Bacteroidetes) in patients with PV compared with HCs (Figure 2B). In contrast, Bacteroides (phylum Bacteroidota) was more abundant in patients with PV when compared with HCs (Figure 2B).
Fecal bacterial composition and differential abundance analysis of patients with PV and HCs. (A) Fecal bacterial composition did not differ between patients with PV and HCs, illustrated by PCoA based on Bray-Curtis dissimilarities. Boxplots display the distribution of samples for each group on the first 2 principal coordinates. (B) The taxonomy of the bacterial community in patients with PV and HCs is illustrated in a heat tree, where taxonomic levels are represented by junctions and the connection by branches. Green nodes and branches indicate the taxa with a higher relative abundance in HCs, and blue nodes and branches indicate the taxa with a higher relative abundance in patients with PV. Only taxa with at least 0.1% point difference in relative abundance between groups are displayed. (C) Differential abundance analysis of the gut microbiota in patients with PV compared with HCs identified by LefSe. Here, we show taxa with a linear discriminant analysis score > 0.005 and an overall median proportion > 1%. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. c, class; f, family; g, genus; p, phylum; o, order.
Fecal bacterial composition and differential abundance analysis of patients with PV and HCs. (A) Fecal bacterial composition did not differ between patients with PV and HCs, illustrated by PCoA based on Bray-Curtis dissimilarities. Boxplots display the distribution of samples for each group on the first 2 principal coordinates. (B) The taxonomy of the bacterial community in patients with PV and HCs is illustrated in a heat tree, where taxonomic levels are represented by junctions and the connection by branches. Green nodes and branches indicate the taxa with a higher relative abundance in HCs, and blue nodes and branches indicate the taxa with a higher relative abundance in patients with PV. Only taxa with at least 0.1% point difference in relative abundance between groups are displayed. (C) Differential abundance analysis of the gut microbiota in patients with PV compared with HCs identified by LefSe. Here, we show taxa with a linear discriminant analysis score > 0.005 and an overall median proportion > 1%. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. c, class; f, family; g, genus; p, phylum; o, order.
Differential abundance analysis conducted through LefSe identified 13 taxa that differed significantly in abundance between patients with PV and HCs (supplemental Figure 2).
The taxa with a median relative abundance > 1% that differed significantly between the 2 groups are shown in Figure 2C.
Compared with HCs, patients with PV had a significantly lower relative abundance of taxa belonging to Firmicutes (48% in patients with PV vs 59% in HC, P < .001). The same tendency was observed at class level for Clostridia (41% vs 53%, P < .001), at order level for Oscillospirales (24% vs 38%, P < .001) and Lachnospirales (7% vs 9%, P < .01), at family level for Lachnospiraceae (7% vs 9%, P < .01) and Ruminococcaceae (15% vs 28%, P < .001), and at genus level for Faecalibacterium (5% vs 15%, P < .001) (Figure 2C).
Overall, patients with PV had a significantly lower abundance of certain butyrate-producing taxa, such as Faecalibacterium, from the Firmicutes phylum compared with HCs.
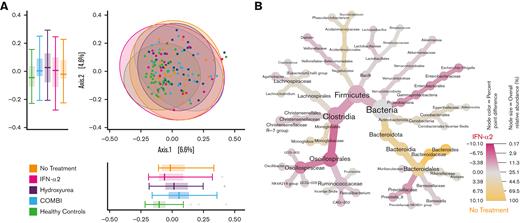
Composition and relative abundance of the gut microbiota in the different treatment groups
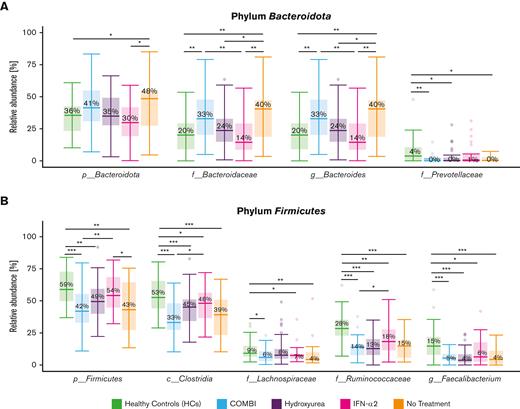
When assessing the overall gut bacterial composition by PCoA according to PV treatments (NT, HU, IFN-α2, and COMBI), no significant differences were observed (Figure 3A). Differential abundance analysis using LefSe revealed a higher relative abundance of Firmicutes (especially Oscillospirales and Clostridia) in patients treated with IFN-α2 (54%) compared with patients receiving NT (43%, P = .039) (Figures 3B and 4B; supplemental Figure 3A). Bacteroides (phylum Bacteroidota) were more abundant in the NT group (40%) compared with the IFN-α2 group (14%, P < .01) (Figures 4A and 3B; supplemental Figure 3A) and the HU group (24%, P = .033) (Figure 4A). Figure 4 displays the comparison of those taxa found to be differentially abundant across most of the groups, whereas supplemental Figure 3 shows additional differentially abundant taxa specific for selected groups.
Fecal bacterial composition of the different treatment groups and HCs. (A) PCoA based on Bray-Curtis dissimilarities. Boxplots display the distribution of samples for each group on the first 2 principal coordinates. All treatment groups differed significantly from the HCs but not from each other; NT vs HCs (ANOSIM, P = .001, R = 0.31), HU vs HCs (ANOSIM, P = .001, R = 0.17), IFN-α2 vs HCs (ANOSIM, P = .002, R = 0.2), and COMBI vs HCs (ANOSIM, P = .001, R = 0.17). (B) The taxonomy of the bacterial community in the IFN-α2 group and the NT group are illustrated in a heat tree, where taxonomic levels are represented by junctions and the connection by branches. Pink nodes and branches indicate the taxa with a higher relative abundance in the IFN-α2 treatment group, and yellow nodes and branches indicate the taxa with a higher relative abundance in the NT group. Only taxa with at least 0.1% difference in relative abundance between groups are displayed.
Fecal bacterial composition of the different treatment groups and HCs. (A) PCoA based on Bray-Curtis dissimilarities. Boxplots display the distribution of samples for each group on the first 2 principal coordinates. All treatment groups differed significantly from the HCs but not from each other; NT vs HCs (ANOSIM, P = .001, R = 0.31), HU vs HCs (ANOSIM, P = .001, R = 0.17), IFN-α2 vs HCs (ANOSIM, P = .002, R = 0.2), and COMBI vs HCs (ANOSIM, P = .001, R = 0.17). (B) The taxonomy of the bacterial community in the IFN-α2 group and the NT group are illustrated in a heat tree, where taxonomic levels are represented by junctions and the connection by branches. Pink nodes and branches indicate the taxa with a higher relative abundance in the IFN-α2 treatment group, and yellow nodes and branches indicate the taxa with a higher relative abundance in the NT group. Only taxa with at least 0.1% difference in relative abundance between groups are displayed.
Differential abundance analysis of the different treatment groups and HCs. LefSe analysis of the 2 dominating phyla, Bacteroidota (A) and Firmicutes (B), across HCs and the 4 different treatment groups. Taxa that differed significantly in abundance between the groups are indicated by a line and asterisks. HCs had a gut microbiota dominated by butyrate-producing taxa of the Firmicutes phyla, such as Faecalibacterium of the family Ruminococcaceae. Here, we show taxa with a linear discriminant analysis score > 0.005 and an overall median proportion > 1%. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Differential abundance analysis of the different treatment groups and HCs. LefSe analysis of the 2 dominating phyla, Bacteroidota (A) and Firmicutes (B), across HCs and the 4 different treatment groups. Taxa that differed significantly in abundance between the groups are indicated by a line and asterisks. HCs had a gut microbiota dominated by butyrate-producing taxa of the Firmicutes phyla, such as Faecalibacterium of the family Ruminococcaceae. Here, we show taxa with a linear discriminant analysis score > 0.005 and an overall median proportion > 1%. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Bacteroides (phylum Bacteroidota) was more abundant in the COMBI group (33%) than in the IFN-α2 group (14%, P < .01) (Figure 4A; supplemental Figure 3B). Patients treated with COMBI had a lower relative abundance of Firmicutes compared with the IFN-α2 group (42% vs 54%, P < .01), especially the taxa Clostridia (33% vs 48%, P = .034) and Ruminococcaceae (14% vs 18%, P = .049) (Figure 4B; supplemental Figure 3B).
No taxa differed significantly in abundance between the COMBI and HU groups or between the IFN-α2 and HU groups. All taxa with a relative abundance above 1% that differed significantly are shown in supplemental Figure 3.
Composition and relative abundance of the gut microbiota in the different PV treatment groups compared with HCs
Even though the overall community structure did not differ between the PV treatment groups, the composition of all treatment groups (NT, HU, IFN-α2, and COMBI) differed significantly from that of HCs; NT vs HCs (ANOSIM; P = .001; R = 0.31), HU vs HCs (ANOSIM; P = .001; R = 0.17), IFN-α2 vs HCs (ANOSIM; P = .002; R = 0.2), COMBI vs HCs (ANOSIM; P = .001; R = 0.28) (Figure 3A).
Differential abundance analysis using LefSe showed that the taxa affiliated with Firmicutes, which differed between patients with PV and HCs, also differed when comparing PV treatment groups with HCs. All significant taxa with a relative abundance above 1% are shown in supplemental Figure 4.
HCs had a significantly higher relative abundance of Firmicutes (59%) compared with patients with PV receiving NT (43%, P < .01), COMBI (42%, P < .001), or HU (49%, P < .01), but not patients treated with IFN-α2 (54%) (Figure 4B; supplemental Figures 4 and 5A-B).
Clostridia, Ruminococcaceae, and Faecalibacterium were more abundant in HCs (53%, 28%, and 15%, respectively) compared with all of the different treatment groups; 39%, 15%, and 4%, respectively, in NT (P < .001); 33%, 14%, and 5%, respectively, in COMBI (P < .001); 45%, 13%, and 4%, respectively, in HU (P < .001); and 48% (P = .02), 18% (P < .01), and 6% (P = .025), respectively, in the IFN-α2 group. Lachnospiraceae were more abundant in HCs (9%) compared with patients receiving NT (4%, P < .01) and patients treated with COMBI (6%, P =.02) or IFN-α2 (7%, P = .04) (Figure 4B; supplemental Figure 5A-B).
Several taxa within Bacteroidota (eg, Bacteroides) were more abundant in patients receiving NT (40%, P < .01) and in patients treated with COMBI (33%, P < .01) compared with the HCs (20%) (Figure 4A; supplemental Figure 4). However, Prevotellaceae were more abundant in the HCs (Figure 4A; supplemental Figure 4).
Overall, the HCs had a more Firmicutes-dominated microbiota with the greatest differences when compared with patients receiving NT and COMBI, and the smallest differences when compared with patients treated with IFN-α2. Patients treated with IFN-α2 appeared to have a gut microbiota more similar to HCs. This is also illustrated in Figure 3A, showing least overall compositional difference between IFN-α2 and HC, especially on principal coordinate 1.
Discussion
Few studies on the gut microbiota29,30 have been conducted in patients with MPNs. Herein, we report, to the best of our knowledge, the largest gut microbiota study in patients with PV, showing that the microbiota in PV indeed differs substantially from that of HCs. A recent study investigating the phenotype and microbial DNA cargo of circulating extracellular vesicles, and the gut microbiota of 38 patients with PV and 30 HCs, found no difference in the microbiota profile between HCs and patients with PV.30 However, patients with PV had an increased proportion of lipopolysaccharide-associated extracellular vesicles, suggesting an increased intestinal permeability, and potentially a state of inflammation in PV.30 Another study investigating the microbiota in patients with MPNs and HCs found no difference in alpha diversity and richness between the HCs and patients with MPN, but discovered that patients with MPN had a lower abundance of Phascolarctobacterium compared with HCs.29 However, both studies were small and did not separate the patients according to treatment, which we have shown here to be associated with differences in the gut microbiota. It must be borne in mind that outcomes of microbiota taxonomic profiling studies are sensitive to different types of bias. Among these are specimen handling, extraction protocol, applying amplicon or shotgun methodology, choice of 16S rRNA gene variable–region primers, contamination, and choice of bioinformatics pipeline including the database(s) used.44
The 2 aforementioned studies used other extraction methods, primer sets, and bioinformatic pipelines, all of which might have influenced the results. Furthermore, the studies were set in different countries with different dietary preferences, all influencing the microbiota. In this study, we compared the composition of the gut microbiota in 102 patients with PV as well as that of the different PV treatment groups with that of 42 HCs. Importantly, in contrast to previously published studies, we found that patients with PV exhibited lower gut microbiota alpha diversity (within sample diversity based on evenness and richness as detected by Inverse Simpson Index analysis) compared with HCs. The higher Inverse Simpson Diversity Index in HCs indicates that the higher alpha diversity observed in this group is mostly attributable to a high evenness of taxa weighted highest by this index, rather than richness of taxa.
Highly interesting, we found that patients treated with IFN-α2 appeared to have a gut microbiota more similar to that of HCs with a significantly lower abundance of, for example, Bacteroides. It is intriguing to consider that IFN-α2 treatment implies conservation of a more healthy microbiota and accordingly a dampening of the microbial inflammatory signals that have recently been proposed to elicit MPNs.45,46 This consideration is even more relevant taking into account that INF-1, as antiinflammatory immunomodulators, have a protective role in the gut by ensuring a proper intestinal barrier function.47 These important aspects will be addressed in our future studies, encompassing microbiota studies at the very earliest stage of MPN development, clonal hematopoiesis of indeterminate potential, and in newly diagnosed patients with MPNs before and during treatment with IFN-α2.
The different treatment groups all differed in composition (beta diversity) from the HCs, but the patients with PV as a group did not differ from HCs. This could possibly be explained by the high interindividual variation in patients with PV, that is, bacterial compositions differed substantially between patients with PV, whereas the gut microbiota profiles of HCs clustered closer together. In this study, the HCs had a gut microbiota dominated by butyrate-producing taxa of the Firmicutes phyla, such as Faecalibacterium of the family Ruminococcaceae,48 with a significantly higher relative abundance compared with patients with PV. Moreover, the same taxa (Clostridia, Lachnospiraceae, Oscillospirales, Ruminococcaceae, and Faecalibacterium) differed when the HCs were compared with the different treatment groups, with the largest differences in the NT and COMBI groups and the smallest when compared with patients treated with IFN-α2. Patients with NT and patients treated with COMBI had a higher abundance of specific taxa capable of producing acetate and propionate, for example, Bacteroides, compared with patients treated with IFN-α2, who, similarly to the HCs, exhibited higher abundances of certain butyrate-producing taxa (phylum Firmicutes). Several members of the phylum Firmicutes are known as butyrate producers, whereas some members of the phylum Bacteroidota primarily produce other types of short-chain fatty acids (SCFAs), such as propionate and acetate.49 These differences might be important, since SCFAs are known modulators of inflammation.50 On the one hand, an initial shift in SCFA-producing bacterial abundances might promote inflammation and thereby the progression of PV. In contrast, PV-associated inflammation might contribute to impaired growth conditions for certain SCFA producers in the gut. Therefore, cause and effect of the associations we observed concerning microbial signatures in PV cannot be clearly deciphered yet.
It is well recognized that inflammation plays an important role in the development and progression of MPNs,51 which is in line with our findings that suggest an important role of the gut microbiota in patients with PV and MPNs in general. A shift in the relative abundance of Firmicutes and Bacteroidetes has been described for other inflammation-driven diseases such as systemic lupus erythematosus.52 Interestingly, in this study, all treatment groups, except IFN-α2, exhibited a higher Bacteroides abundance than did the HCs; however, this finding was not significant in the HU group. A shift in the abundance of Bacteroides has previously been reported in older individuals with some studies reporting the abundance to increase with age, whereas others have reported a decrease.53 Except for the HU group, HCs in this study were older (median age, 71 years) compared with the different treatment groups, COMBI (median age, 68 years), NT group (median age, 62 years), and IFN-α2 (median age, 65 years). It could thus be argued that the differences observed could be owing to higher age of the HCs. However, the same differences in Bacteroides abundance were observed between the 2 groups with the youngest patients (IFN-α2 and NT), as were seen between the youngest group (NT) vs HCs, indicating a higher impact of the disease and treatment rather than age.
It is also worth to consider that HU per se is cytotoxic to certain prokaryotes, for example, Escherichia coli, but also parasites that could result in changes owing to the drug’s effect directly on the microbiota.54,55
Interestingly, patients treated with COMBI did not resemble the patients treated with IFN-α2. In this study, patients treated with IFN-α2 had received the treatment for several years (median,186 weeks), whereas the COMBI treated patients had received treatment for a significantly shorter period (median, 46 weeks). Furthermore, a higher JAK2V617F allele burden in the COMBI group was observed. Both these factors might affect the gut microbiota. Owing to small numbers, we were unable to investigate whether ruxolitinib in itself had an effect. The differences in the gut microbiota in the IFN-α2 group might also reflect patients with treatment response, as described for trastuzumab in a recent study of women with breast cancer56; di Modica et al reported that responders had a Clostridiales-enriched microbiota, especially from the family Lachnnospiraceae, whereas nonresponders had a higher abundance of Bacteroidota.56 The same patterns were found in patients with melanoma treated with anti–PD-1 therapy.57
An association between the gut microbiota and hematopoiesis is increasingly being recognized.17,58 Furthermore, dysbiosis has been linked to hematologic abnormalities in humans18,59 owing to dysregulation of gut microbiota products, which promote production of cytokines that support normal hematopoiesis. A model has been proposed, where microbe-associated molecular patterns activate a Toll-like receptor pathway leading to IFN-1 production, thereby activating the STAT1 pathway and promoting hematopoiesis.18 Furthermore, the effect of the microbiota is reportedly important in other hematological malignancies, regarding both the prediction of side effects after hematopoietic stem cell transplantation60 and graft-versus-host disease.61
This study has several limitations. We were not able to perform in-depth functional annotation, which would have been helpful in further exploring differences in the distribution of SCFA producers. Furthermore, the treatment groups were small, making the results less robust for each treatment group. Finally, stool samples were not taken at the same time point from the start of treatment, that is, some patients had been on treatment for several years and some patients for only 3 months, which might skew the findings.
In conclusion, we have demonstrated that patients with PV have a distinct gut microbiota with a lower abundance of Firmicutes compared with HCs. In addition, the microbiota differs among patients with PV receiving different types of treatment, with Bacteroides being more prevalent in patients receiving NT and COMBI compared with patients treated with IFN-α2. We observed that when compared with the other treatment groups, patients treated with IFN-α2, although still different, seemed to have a gut microbiota more similar to that of HCs. However, whether the difference is owing to treatment with IFN-α2 per se, or owing to the effect of IFN-α2 treatment on PV, or whether certain microbiota signatures facilitate response to IFN-α2 would need to be clarified.
Acknowledgments
The authors thank all participants of the study. The authors convey their gratitude to Mette Grymer Jensen (Medical and Research secretary) for organizing the settings and the collection of stools samples. Furthermore, the authors thank all the doctors at the Department of Hematology, Zealand University Hospital, Region Zealand, Denmark for their help with recruiting patients to the study. The authors also thank the laboratory technicians at the Department of Bacteria, Parasites & Fungi, Statens Serum Institut, Copenhagen, Denmark and Regional Department of Clinical Microbiology, University Hospital of Region Zealand, Region Zealand, Denmark for their great help with dividing and analyzing the samples. Furthermore, the authors are grateful to Marilynn Eickhardt-Sørensen and David Griffith for the linguistic review.
This work was founded by the Region Zealand Foundation for Health Research, and Department of Bacteria, Parasites & Fungi, Statens Serum Institut, Copenhagen, Denmark. The authors are also grateful for the funding received from the Region Zealand and the University of Copenhagen’s research promotion pool.
Authorship
Contribution: J.J.E.C., X.C.N., H.C.H., V.S., L.K., K.F., H.V.N., and C.S.E.-D. designed the study; J.J.E.C., X.C.N., T.A.K., V.S., L.K., K.F., H.V.N., and H.C.H. raised the funding; H.C.H. and C.S.E.-D. recruited the patients; M.K.L., C.E., and C.S.E.-D. recruited the healthy control participants; A.C.I. and L.O.A. performed the bioinformatics; A.C.I. and C.S.E.-D. performed the statistical analyses and produced the figures and tables; A.C.I., C.S.E.-D., K.F., C.R.S., H.V.N., L.K., V.S., H.C.H., X.C.N., and J.J.E.C. interpreted the results; C.S.E.-D. and A.C.I. wrote the initial draft; and all authors contributed substantially to the revision of the manuscript and approved the final version.
Conflict-of-interest disclosure: H.C.H. is on the data monitoring board for AOP Orphan, and has received research funding from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Christina Schjellerup Eickhardt-Dalbøge, The Regional Department of Clinical Microbiology, Zealand University Hospital, Ingemannsvej 18, Slagelse, 4200 Region Zealand, Denmark; e-mail: chsd@regionsjaelland.dk.
References
Author notes
∗H.C.H., X.C.N., and J.J.E.C. contributed equally to this study.
Sequence data, anonymous on the patients with polycythemia vera, will be made available upon publication at the European Nucleotide Archive under project number PRJEB54576 (https://www.ebi.ac.uk/ena/browser/view/PRJEB54576). Owing to the European General Data Protection Regulations, lack of consent from participants to share data, and lack of approval from institutional review board to share data from participants, the data set on healthy control participants cannot be shared publicly.
Data are available on request from authors, Christina Ellervik (cel@regionsjaelland.dk) and Christina Schjellerup Eickhardt-Dalbøge (chsd@regionsjaelland.dk).
The full-text version of this article contains a data supplement.