Key Points
Olutasidenib induced durable remissions in patients with mIDH1 R/R AML; transfusion independence was achieved across all response groups.
The side effect profile is well-characterized and manageable; olutasidenib represents a therapeutic advance in a poor-prognostic population.
Abstract
Olutasidenib (FT-2102) is a potent, selective, oral, small-molecule inhibitor of mutant isocitrate dehydrogenase 1 (mIDH1). Overall, 153 IDH1 inhibitor–naive patients with mIDH1R132 relapsed/refractory (R/R) acute myeloid leukemia (AML) received olutasidenib monotherapy 150 mg twice daily in the pivotal cohort of this study. The median age of participants was 71 years (range, 32-87 years) and the median number of prior regimens received by patients was 2 (1-7). The rate of complete remission (CR) plus CR with partial hematologic recovery (CRh) was 35%, and the overall response rate was 48%. Response rates were similar in patients who had, and who had not, received prior venetoclax. With 55% of patients censored at the time of data cut-off, the median duration of CR/CRh was 25.9 months. The median duration of overall response was 11.7 months, and the median overall survival was 11.6 months. Of 86 patients who were transfusion dependent at baseline, a 56-day transfusion independence was achieved in 29 (34%), which included patients in all response groups. Grade 3 or 4 treatment-emergent adverse events (≥10%) were febrile neutropenia and anemia (n = 31; 20% each), thrombocytopenia (n = 25; 16%), and neutropenia (n = 20; 13%). Differentiation syndrome adverse events of special interest occurred in 22 (14%) patients, with 14 (9%) grade ≥3 and 1 fatal case reported. Overall, olutasidenib induced durable remissions and transfusion independence with a well-characterized and manageable side effect profile. The observed efficacy represents a therapeutic advance in this molecularly defined, poor-prognostic population of patients with mIDH1 R/R AML. This trial was registered at www.clinicaltrials.gov as #NCT02719574.
Introduction
An isocitrate dehydrogenase 1 gene mutation (mIDH1) is present in between 7% and 14% of patients with acute myeloid leukemia (AML).1-3 IDH1 is a metabolic enzyme that catalyzes oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG). IDH1 gene mutations confer neomorphic enzymatic activity, promoting transformation of α-KG to the oncometabolite, 2-hydroxyglutarate (2-HG). Aberrant accumulation of 2-HG leads to competitive inhibition of α-KG–dependent dioxygenases, which are crucial to DNA and histone demethylation and may block standard differentiation of stem and progenitor cells, thereby promoting neoplastic transformation.4-6 Inhibition of mIDH1 in tumor cells, and the concomitant reduction in 2-HG production, can restore normal cellular differentiation and provide therapeutic benefit in mIDH1-cancers. Inhibitors of mIDH1 have demonstrated efficacy in both newly diagnosed and relapsed/refractory (R/R) AML, and are currently being studied in patients with myelodysplastic syndromes (MDS), cholangiocarcinoma, and glioma.7-12
Olutasidenib (FT-2102) is a potent, selective, oral, small-molecule inhibitor of mIDH1 with the therapeutic potential to restore normal cellular differentiation.12,13 A phase 1/2 trial was conducted to assess the safety, pharmacologic profile, and clinical activity of olutasidenib both as monotherapy and in combination with azacitidine in patients with MDS or AML harboring mIDH1. Here, we report results from a preplanned interim analysis in patients with mIDH1 R/R AML who received olutasidenib monotherapy in the pivotal phase 2 cohort of the trial. Enrollment was stopped early at this second interim analysis when the efficacy stopping criteria were met.
Methods
Study design
Study 2102-HEM-101 (#NCT02719574) is an open-label, multicenter, phase 1/2 trial. During phase 1, the phase 2 dose of 150 mg twice daily was selected.12 Phase 2 includes multiple cohorts of patients with AML and MDS and is ongoing. In this pivotal cohort, patients with mIDH1 R/R AML were enrolled from 57 centers in 9 countries and received 150 mg olutasidenib monotherapy, twice daily, in continuous 28-day cycles.
The study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. The protocol was approved by the institutional review boards or ethics committee at study sites. Written informed consent was provided by all patients.
Patients
Patients aged ≥18 years, harboring mIDH1R132 with pathologically proven AML, R/R to standard treatment were included. Central confirmation of mutation status was performed using pretreatment peripheral blood and/or bone marrow aspirate samples. Other criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, adequate liver and renal function, and QT interval corrected using Fridericia’s formula (QTcF) ≤450 milliseconds. Patients with symptomatic central nervous system leukemia, uncontrolled infections or metabolic disorders, and/or prior IDH inhibitor therapy were excluded.
Efficacy assessments
The primary efficacy end point was the rate of complete remission (CR) plus CR with partial hematologic recovery (CRh), by investigator assessment, using modified response criteria of the International Working Group in AML.14,15 CRh was defined as bone marrow blasts <5% with an absolute neutrophil count >0.5 × 109/L and platelet count >50 × 109/L.
Overall response rate (ORR) was a secondary end point, defined as the rate of CR, CRh, CR with incomplete blood count recovery (CRi), partial remission (which required recovery of neutrophil and platelet counts consistent with a CR), or morphologic leukemia-free state (MLFS). Other secondary end points included duration of CR + CRh, duration of overall response (DOR), rate of transfusion independence (TI), and overall survival (OS). Patients were classified as transfusion dependent if platelet and/or red blood cell (RBC) transfusion was administered within 8 weeks before baseline and were transfusion independent if platelet and/or RBC transfusion was not administered for at least 56 days during treatment. For the OS, patient data were censored at the last date they were known to be alive. DOR was calculated from the time of first response until death, relapse, or new anticancer therapy; patients without an event were censored at their last response assessment.
Safety assessments
Safety data were summarized descriptively for patients who received at least 1 dose of olutasidenib. Adverse events (AEs) were recorded from the time of first dose until 28 days after the last dose of study drug. AEs were coded using MedDRA version 19.1 and graded via the NCI-CTCAE version 4.03.
Translational analyses
Peripheral blood samples were collected for pharmacodynamic assessments, including for 2-HG, a biomarker of the on-target activity of olutasidenib. Bone marrow and peripheral blood samples were collected for mutational analyses, including for IDH1 variant allele frequency (VAF) and comutations. Methods for translational analyses are described in the supplemental Appendix.
Statistical analysis
A group sequential design using O’Brien Fleming boundaries was used with 2 planned interim analyses and a final test. A sample size of 173 patients would provide 90% power to test the null hypothesis that the true rate of CR + CRh is ≤15% with a 2.5% significance level, using a one-sided exact test for a binomial proportion, assuming the true response rate was 25%. At the first interim analysis, if ≤6 responses in 58 evaluable participants were observed, the trial would be stopped early for futility. At the second interim analysis (at 67% information or an efficacy-evaluable population of 115), the trial may be stopped for either futility or further enrollment may be stopped for efficacy. An early stopping criterion for efficacy was met when ≤28 responses were observed in 115 evaluable participants, and enrollment was stopped.
Time to OS and time to response were measured from the first dose to the event; time to OS and DOR were measured using Kaplan-Meier methods. The survival probability and 95% confidence interval (CI) were estimated at 3, 6, 12, and 24 months based on the Kaplan-Meier product-limit method and Greenwood formula.
Results
Patient disposition and baseline characteristics
Between April 2018 and June 2020, 153 patients with mIDH1 R/R AML were enrolled and received at least 1 dose of olutasidenib (150 mg, twice daily). Olutasidenib was administered in the outpatient setting throughout the trial, except for 2 days during which pharmacokinetic sampling was performed. Compliance with medication was tracked using patient diaries. Mean (±standard deviation) treatment compliance was 99.4% (±2.1%).
At data cut-off on 18 June 2021, 21 (14%) patients remained on treatment and 132 (86%) had discontinued. The most common reasons for discontinuation were disease progression (n = 62; 41%), AE (n = 26; 17%), hematopoietic stem cell transplantation (HSCT) (n = 15; 10%), and death (n = 14; 9%). Median treatment duration was 142 days (range, 3-1045 days).
The efficacy-evaluable population comprised 147 patients who received the first dose of olutasidenib at least 6 months before data cut-off. Patients had received a median of 2 prior regimens (range, 1-7). Most patients had received prior induction therapy (n = 143; 97%) and prior cytarabine (n = 105; 71%). Data on specific induction regimens were not collected. In total, 12 patients had received prior treatment with venetoclax. No patient had received prior IDH1 inhibitor therapy. Patient and disease characteristics are presented in Table 1.
Patient demographic and baseline disease characteristics in the efficacy-evaluable population
| Parameter . | Efficacy-evaluable population (n = 147) . |
|---|---|
| Sex, n (%) | |
| Male/female | 74 (50)/73 (50) |
| Age (y) | |
| Median (range) | 71 (32-87) |
| ECOG PS, n (%) | |
| 0 | 45 (31) |
| 1 | 76 (52) |
| 2 | 23 (16) |
| AML type, n (%) | |
| Primary de novo | 97 (66) |
| Secondary: | 50 (34) |
| MDS | 39 (78) |
| Therapy related | 4 (8) |
| Other∗ | 7 (14) |
| AML cytogenetic risk category (per either NCCN or ELN guidelines),†n (%) | |
| Favorable | 6 (4) |
| Intermediate | 107 (73) |
| Poor | 25 (17) |
| Unknown | 9 (6) |
| Prior AML therapy outcome, n (%) | |
| Refractory | 51 (35) |
| Relapsed | 96 (65) |
| Remission duration ≤12 months | 67 (70) |
| Remission duration >12 months | 29 (30) |
| Number of priorregimens | |
| Median (range) | 2 (1-7) |
| 1 regimen, n (%) | 48 (33) |
| 2 regimens, n (%) | 45 (31) |
| ≥3 regimens, n (%) | 54 (37) |
| Prior treatments received,‡n (%) | |
| Induction therapy§ | 143 (97) |
| Cytarabine | 105 (71) |
| Idarubicin | 64 (44) |
| Daunorubicin | 31 (21) |
| Fludarabine | 25 (17) |
| Hypomethylating agent | 58 (39) |
| As a single agent | 21 (14) |
| In combination with venetoclax | 8 (5) |
| Gemtuzumab based combinations | 11 (7) |
| Venetoclax | 12 (8) |
| Allogeneic HSCT | 17 (12) |
| Hematologic laboratory parameters, median (range) | |
| Percentage of bone marrow blasts | 42 (4-98) |
| Percentage of peripheral blood blasts | 22.5 (1-96) |
| White blood cells ×109/L | 2 (0.1-75) |
| Absolute neutrophil count ×109/L | 0.35 (0-5.3) |
| Renal function (creatinine clearance), n (%) | |
| Normal (≥90 mL/min) | 60 (41) |
| Mildly impaired (60-89 mL/min) | 66 (45) |
| Moderately impaired (30-59 mL/min) | 21 (14) |
| Severely impaired (15-29 mL/min) | 0 |
| IDH1mutation type (as determined by investigator), n (%) | |
| R132C | 90 (61) |
| R132H | 34 (23) |
| R132G | 10 (7) |
| R132S | 10 (7) |
| R132L | 3 (2) |
| Number of co-occurring mutations, n (%)‖ | |
| 1 to 3 | 91 (62) |
| 4 to 7 | 19 (13) |
| None | 4 (3) |
| Not done/unknown | 33 (22) |
| Co-occurring mutations in >5% patients, n (%)‖ | |
| Epigenetic | 80 (54) |
| DNMT3A | 67 (46) |
| TET2 | 22 (15) |
| Differentiation | 62 (42) |
| NPM1 | 31 (21) |
| RUNX1 | 20 (14) |
| PHF6 | 8 (5) |
| RTK pathways | 58 (40) |
| FLT3 | 15 (10) |
| NRAS | 14 (10) |
| JAK2 | 12 (8) |
| Chromatin | 50 (34) |
| ASXL1 | 23 (16) |
| STAG2 | 12 (8) |
| BCOR | 12 (8) |
| Splicing | 43 (29) |
| SRSF2 | 25 (17) |
| U2AF1 | 10 (7) |
| Other | 15 (10) |
| TP53 | 9 (6) |
| Parameter . | Efficacy-evaluable population (n = 147) . |
|---|---|
| Sex, n (%) | |
| Male/female | 74 (50)/73 (50) |
| Age (y) | |
| Median (range) | 71 (32-87) |
| ECOG PS, n (%) | |
| 0 | 45 (31) |
| 1 | 76 (52) |
| 2 | 23 (16) |
| AML type, n (%) | |
| Primary de novo | 97 (66) |
| Secondary: | 50 (34) |
| MDS | 39 (78) |
| Therapy related | 4 (8) |
| Other∗ | 7 (14) |
| AML cytogenetic risk category (per either NCCN or ELN guidelines),†n (%) | |
| Favorable | 6 (4) |
| Intermediate | 107 (73) |
| Poor | 25 (17) |
| Unknown | 9 (6) |
| Prior AML therapy outcome, n (%) | |
| Refractory | 51 (35) |
| Relapsed | 96 (65) |
| Remission duration ≤12 months | 67 (70) |
| Remission duration >12 months | 29 (30) |
| Number of priorregimens | |
| Median (range) | 2 (1-7) |
| 1 regimen, n (%) | 48 (33) |
| 2 regimens, n (%) | 45 (31) |
| ≥3 regimens, n (%) | 54 (37) |
| Prior treatments received,‡n (%) | |
| Induction therapy§ | 143 (97) |
| Cytarabine | 105 (71) |
| Idarubicin | 64 (44) |
| Daunorubicin | 31 (21) |
| Fludarabine | 25 (17) |
| Hypomethylating agent | 58 (39) |
| As a single agent | 21 (14) |
| In combination with venetoclax | 8 (5) |
| Gemtuzumab based combinations | 11 (7) |
| Venetoclax | 12 (8) |
| Allogeneic HSCT | 17 (12) |
| Hematologic laboratory parameters, median (range) | |
| Percentage of bone marrow blasts | 42 (4-98) |
| Percentage of peripheral blood blasts | 22.5 (1-96) |
| White blood cells ×109/L | 2 (0.1-75) |
| Absolute neutrophil count ×109/L | 0.35 (0-5.3) |
| Renal function (creatinine clearance), n (%) | |
| Normal (≥90 mL/min) | 60 (41) |
| Mildly impaired (60-89 mL/min) | 66 (45) |
| Moderately impaired (30-59 mL/min) | 21 (14) |
| Severely impaired (15-29 mL/min) | 0 |
| IDH1mutation type (as determined by investigator), n (%) | |
| R132C | 90 (61) |
| R132H | 34 (23) |
| R132G | 10 (7) |
| R132S | 10 (7) |
| R132L | 3 (2) |
| Number of co-occurring mutations, n (%)‖ | |
| 1 to 3 | 91 (62) |
| 4 to 7 | 19 (13) |
| None | 4 (3) |
| Not done/unknown | 33 (22) |
| Co-occurring mutations in >5% patients, n (%)‖ | |
| Epigenetic | 80 (54) |
| DNMT3A | 67 (46) |
| TET2 | 22 (15) |
| Differentiation | 62 (42) |
| NPM1 | 31 (21) |
| RUNX1 | 20 (14) |
| PHF6 | 8 (5) |
| RTK pathways | 58 (40) |
| FLT3 | 15 (10) |
| NRAS | 14 (10) |
| JAK2 | 12 (8) |
| Chromatin | 50 (34) |
| ASXL1 | 23 (16) |
| STAG2 | 12 (8) |
| BCOR | 12 (8) |
| Splicing | 43 (29) |
| SRSF2 | 25 (17) |
| U2AF1 | 10 (7) |
| Other | 15 (10) |
| TP53 | 9 (6) |
ECOG, Eastern Cooperative Oncology Group; ELN, European Leukemia Net; NCCN, National Comprehensive Cancer Network; PS, performance status; RTK, receptor tyrosine kinase.
Other secondary AML types were essential thrombocytopenia and myelofibrosis (each 2 patients; 1%) and chronic myelomonocytic leukemia myeloproliferative neoplasm and polycythemia vera (each 1 patient; 1%).
Data on the type of classification system of cytogenetic risk was used (either NCCN or ELN guidelines) were not collected.
Other prior antineoplastic agents received were lomustine (11 patients; 7%); mitoxantrone (9 patients; 6%); etoposide (5 patients; 3%); amsacrine (3 patients; 2%); cladribine, doxorubicin, durvalumab, melphalan, and midostaurin (each 2 patients; 1%); and alemtuzumab, cyclophosphamide, dinaciclib, enasidenib, glasdegib, hydroxycarbamide, idasanutlin, mercaptopurine, and methotrexate (each 1 patient; 1%).
Data on the specific induction regimen used were not collected.
Mutations apart from IDH1.
Response
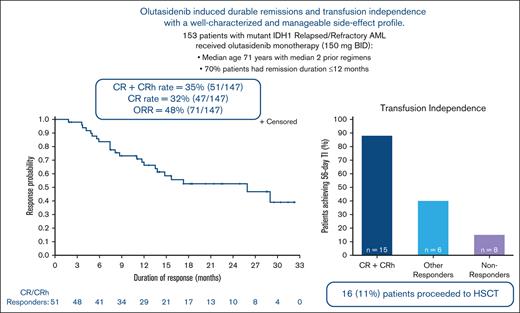
In the primary efficacy evaluation, 51 of 147 patients achieved CR or CRh, yielding a CR + CRh rate of 35% (95% CI, 27.0-43.0). Most patients who achieved CR/CRh responded early, with a median time to response of 1.9 months (range, 0.9-5.6 months). The CR rate was 32% (n = 47; 95% CI, 24.5-40.2). The ORR was 48% (n = 71; 95% CI, 40.0-56.7) (Table 2).
Response rates in the efficacy-evaluable population
| Response rates . | Efficacy-evaluable population (n = 147) . |
|---|---|
| CR∗or CRh | |
| n (%) [95% CI] | 51 (35) [27.0-43.0] |
| Median time to CR/CRh, mo (range) | 1.9 (0.9-5.6) |
| CR∗ | |
| n (%) [95% CI] | 47 (32) [24.5-40.2] |
| Median time to CR, months (range) | 2.8 (0.9-7.4) |
| Overall response | |
| N (%) [95% CI] | 71 (48) [40.0-56.7] |
| Median time to first overall response, mo (range) | 1.9 (0.9-10.2) |
| Best overall response, n (%) | |
| CR∗ | 47 (32) |
| CRh | 4 (3) |
| CRi | 15 (10) |
| PR | 3 (2) |
| MLFS | 2 (1) |
| SD† | 42 (29) |
| Progressive disease | 10 (7) |
| Not evaluable/not done | 6 (4) / 18 (12) |
| Response rates . | Efficacy-evaluable population (n = 147) . |
|---|---|
| CR∗or CRh | |
| n (%) [95% CI] | 51 (35) [27.0-43.0] |
| Median time to CR/CRh, mo (range) | 1.9 (0.9-5.6) |
| CR∗ | |
| n (%) [95% CI] | 47 (32) [24.5-40.2] |
| Median time to CR, months (range) | 2.8 (0.9-7.4) |
| Overall response | |
| N (%) [95% CI] | 71 (48) [40.0-56.7] |
| Median time to first overall response, mo (range) | 1.9 (0.9-10.2) |
| Best overall response, n (%) | |
| CR∗ | 47 (32) |
| CRh | 4 (3) |
| CRi | 15 (10) |
| PR | 3 (2) |
| MLFS | 2 (1) |
| SD† | 42 (29) |
| Progressive disease | 10 (7) |
| Not evaluable/not done | 6 (4) / 18 (12) |
CRi, CR with incomplete blood count recovery; PR, partial remission; SD, stable disease (failure to achieve at least a PR but not meeting criteria for progressive disease. SD for a period of ≥8 weeks indicates clinical benefit).
Includes patients with CR, complete cytogenetic remission, and complete molecular remission.
Includes 3 patients considered to have clinical benefit by the treating physician and 2 patients considered to have resistant but stable disease.
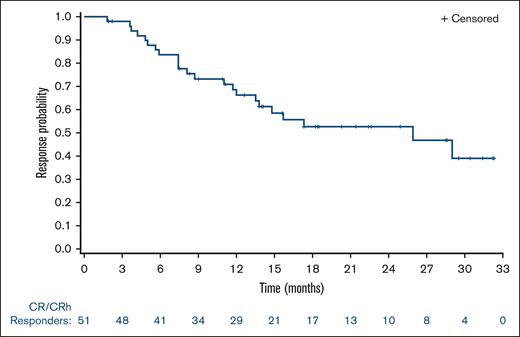
Median duration of CR/CRh was 25.9 months (95% CI, 13.5-NE [not evaluable]) with 55% of patients censored; all 28 censored patients were still being followed-up for duration of response at data cut-off. The estimated percentages of patients who maintained CR/CRh at 6 and 12 months were 78% and 63%, respectively. (Figure 1). Median duration of CR was 28.1 months (95% CI, 13.8-NE). Median DOR was 11.7 months (95% CI, 6.9-25.9).
For 12 patients previously treated with venetoclax, the CR + CRh rate was 33% (n = 4; 95% CI, 9.9-65.1) and the CR rate was 25% (n = 3; 95% CI, 5.5-57.2); moreover, 2 patients achieved CRi and 2 achieved stable disease, providing an ORR of 50% (n = 6; 95% CI, 21.1-78.9). Two of 3 patients who achieved CR and 1 who achieved CRh in the postvenetoclax population remained in remission and were receiving study treatment at the time of data cut-off; 1 patient who achieved CR discontinued olutasidenib because of an AE.
OS
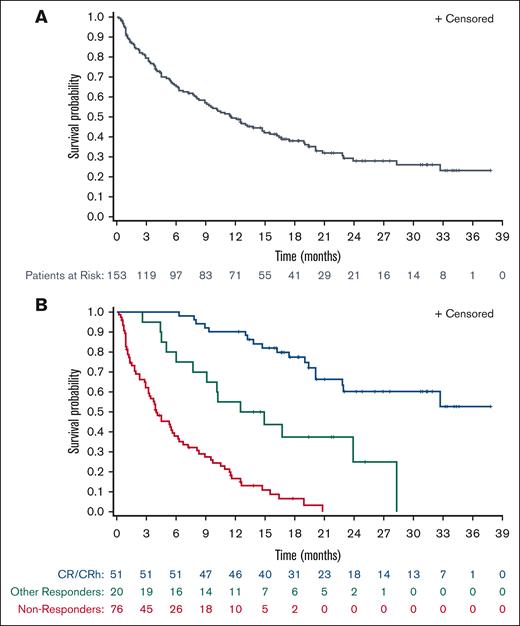
The median OS was 11.6 months (95% CI, 8.9-15.5) in the overall population of 153 patients. (Figure 2A). Better response was strongly associated with longer survival. In patients who achieved CR/CRh, the median OS was not reached (95% CI, 22.8-NE), and the estimated 18-month survival was 78%. For patients who responded but did not achieve CR/CRh, the median OS was 13.7 months (95% CI, 6.0-NE). In nonresponders, the median OS was 4.0 months (95% CI, 3.2-5.8) (Figure 2B).
OS. (A) OS for patients in the full analysis population (N = 153). (B) OS for patients in the efficacy-evaluable population with CR/CRh (n = 51), other responders (n = 20), and nonresponders (n = 76). Other responders are patients with CRi, PR, or MLFS. Nonresponders are patients in response assessment categories other than CR/CRh, CRi, PR, and MLFS.
OS. (A) OS for patients in the full analysis population (N = 153). (B) OS for patients in the efficacy-evaluable population with CR/CRh (n = 51), other responders (n = 20), and nonresponders (n = 76). Other responders are patients with CRi, PR, or MLFS. Nonresponders are patients in response assessment categories other than CR/CRh, CRi, PR, and MLFS.
Transplantation
In total, 16 patients underwent transplantation, including 15 who discontinued olutasidenib to receive HSCT and 1 who discontinued because of a grade 3 AE of bone marrow hypoplasia but subsequently proceeded to transplantation. Patients who received HSCT were followed-up for DOR and OS because HSCT was not an event in time-to-event analysis. To address the possibility that use of HSCT after olutasidenib treatment could favorably influence DOR and OS, sensitivity analyses were performed in which patients had censored observation at the time of transplantation (supplemental Figures 2 and 3). Similar outcomes were observed irrespective of censoring for transplantation.
Hematologic analyses
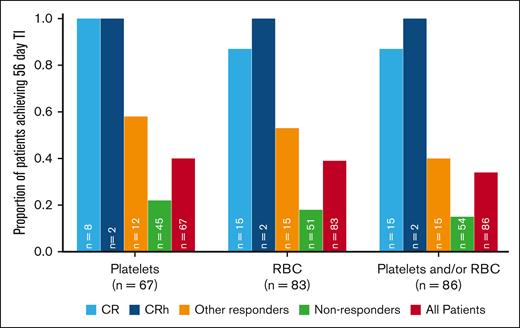
TI was observed in patients in all response groups. Of 86 patients who were RBC and/or platelet transfusion dependent at baseline, 29 (34%) achieved TI during any 56-day postbaseline period. High rates of both platelet and RBC TI (100% and 88%, respectively) were observed among patients who were transfusion dependent at baseline and achieved CR/CRh. In contrast, responders who did not achieve CR/CRh had platelet and RBC TI rates of 58% and 53%, respectively (Figure 3).
Patients who were transfusion dependent at baseline and achieved TI for ≥56 days. Other responders are patients with CRi, PR, or MLFS. Nonresponders are patients in response assessment categories other than CR/CRh, CRi, PR, and MLFS.
Patients who were transfusion dependent at baseline and achieved TI for ≥56 days. Other responders are patients with CRi, PR, or MLFS. Nonresponders are patients in response assessment categories other than CR/CRh, CRi, PR, and MLFS.
Translational analyses
IDH1 mutation subtype and comutations at baseline are presented in Table 1. Patients who achieved CR/CRh had fewer comutations compared with other responders (supplemental Table 3). Although the ORR was not significantly reduced, the rate of CR/CRh in patients with receptor tyrosine kinase pathway mutations was lower than that in patients without receptor tyrosine kinase pathway mutations. Response rates tended to be lower in patients with concurrent FLT3 mutations. Patients in CR/CRh were more likely to have VAF clearance (28%; 11 of 39) than other responders (8%, 1 of 12) or nonresponders (7%, 2 of 30). After 2 cycles of treatment, the median 2-HG concentration was reduced by 82% (83% in CR/CRh responders, and 80% in other responders). The observed reductions in 2-HG were sustained throughout subsequent treatment cycles.
Safety
At least 1 treatment-emergent AE (TEAE) was reported in all 153 patients. The most frequent TEAEs (≥20%), irrespective of relationship to olutasidenib, were nausea (n = 58; 38%), constipation and anemia (n = 40; 26% each), leukocytosis (n = 38; 25%), pyrexia (n = 36; 24%), fatigue (n = 35, 23%), febrile neutropenia (n = 33; 22%), dyspnea and diarrhea (n = 31; 20% each), and thrombocytopenia and hypokalemia (n = 30, 20% each).
Grade 3 and 4 TEAEs, irrespective of relationship to olutasidenib, were reported in 84% of patients, of which the most frequent (≥10%) were febrile neutropenia and anemia (n = 31; 20% each), thrombocytopenia (n = 25; 16%), and neutropenia (n = 20; 13%). The most frequent TEAEs of any grade (≥5%) and grades 3 and 4 (>1%) assessed as treatment-related are summarized in Table 3.
All TEAEs of all grades (greater than or equal to 20%) and of grade 3 or 4 severity (greater than or equal to 10%); and TEAEs assessed as related to treatment with olutasidenib of all grades (greater than or equal to 5%) and of grade 3 or 4 severity (greater than 1%)
| AE . | All TEAEs . | Treatment-related TEAEs . | ||
|---|---|---|---|---|
| All grades (≥20%) n (%) . | Grade 3 or 4 (≥10%) n (%) . | All grades (≥5%) n (%) . | Grade 3 or 4 (>1%) n (%) . | |
| Patients with any AE | 153 (100) | 129 (84) | 111 (73) | 59 (39) |
| Nausea | 58 (38) | 0 (0) | 35 (23) | 0 (0) |
| Anemia | 40 (26) | 31 (20) | 9 (6) | 7 (5) |
| Constipation | 40 (26) | 0 (0) | 12 (8) | 0 (0) |
| Leukocytosis | 38 (25) | 14 (9) | 20 (13) | 7 (5) |
| Pyrexia | 36 (24) | 1 (1) | 2 (1) | 0 (0) |
| Fatigue | 35 (23) | 2 (1) | 11 (7) | 1 (1) |
| Febrile neutropenia | 33 (22) | 31 (20) | 2 (1) | 2 (1) |
| Dyspnea | 31 (20) | 5 (3) | 5 (3) | 1 (1) |
| Diarrhea | 31 (20) | 2 (1) | 8 (5) | 1 (1) |
| Thrombocytopenia | 30 (20) | 25 (16) | 8 (5) | 6 (4) |
| Hypokalemia | 30 (20) | 9 (6) | 3 (2) | 0 (0) |
| Neutropenia | 20 (13) | 20 (13) | 8 (5) | 8 (5) |
| Decreased appetite | 25 (16) | 3 (2) | 8 (5) | 0 (0) |
| DS | 22 (14) | 13 (8) | 22 (14) | 13 (8) |
| Alanine aminotransferase increased | 18 (12) | 7 (5) | 13 (8) | 4 (3) |
| Aspartate aminotransferase increased | 14 (9) | 4 (3) | 9 (6) | 3 (2) |
| Gamma-glutamyltransferase increased | 10 (7) | 8 (5) | 8 (5) | 7 (5) |
| Hepatic enzyme increased | 7 (5) | 5 (3) | 6 (4) | 5 (3) |
| Tumor lysis syndrome | 4 (3) | 4 (3) | 3 (2) | 3 (2) |
| Deviating liver function test | 3 (2) | 3 (2) | 3 (2) | 3 (2) |
| AE . | All TEAEs . | Treatment-related TEAEs . | ||
|---|---|---|---|---|
| All grades (≥20%) n (%) . | Grade 3 or 4 (≥10%) n (%) . | All grades (≥5%) n (%) . | Grade 3 or 4 (>1%) n (%) . | |
| Patients with any AE | 153 (100) | 129 (84) | 111 (73) | 59 (39) |
| Nausea | 58 (38) | 0 (0) | 35 (23) | 0 (0) |
| Anemia | 40 (26) | 31 (20) | 9 (6) | 7 (5) |
| Constipation | 40 (26) | 0 (0) | 12 (8) | 0 (0) |
| Leukocytosis | 38 (25) | 14 (9) | 20 (13) | 7 (5) |
| Pyrexia | 36 (24) | 1 (1) | 2 (1) | 0 (0) |
| Fatigue | 35 (23) | 2 (1) | 11 (7) | 1 (1) |
| Febrile neutropenia | 33 (22) | 31 (20) | 2 (1) | 2 (1) |
| Dyspnea | 31 (20) | 5 (3) | 5 (3) | 1 (1) |
| Diarrhea | 31 (20) | 2 (1) | 8 (5) | 1 (1) |
| Thrombocytopenia | 30 (20) | 25 (16) | 8 (5) | 6 (4) |
| Hypokalemia | 30 (20) | 9 (6) | 3 (2) | 0 (0) |
| Neutropenia | 20 (13) | 20 (13) | 8 (5) | 8 (5) |
| Decreased appetite | 25 (16) | 3 (2) | 8 (5) | 0 (0) |
| DS | 22 (14) | 13 (8) | 22 (14) | 13 (8) |
| Alanine aminotransferase increased | 18 (12) | 7 (5) | 13 (8) | 4 (3) |
| Aspartate aminotransferase increased | 14 (9) | 4 (3) | 9 (6) | 3 (2) |
| Gamma-glutamyltransferase increased | 10 (7) | 8 (5) | 8 (5) | 7 (5) |
| Hepatic enzyme increased | 7 (5) | 5 (3) | 6 (4) | 5 (3) |
| Tumor lysis syndrome | 4 (3) | 4 (3) | 3 (2) | 3 (2) |
| Deviating liver function test | 3 (2) | 3 (2) | 3 (2) | 3 (2) |
Serious TEAEs, irrespective of relationship to olutasidenib, were reported in 111 (73%) patients, of which the most frequent (≥5%) were disease progression (n = 25; 16%), febrile neutropenia (n = 23; 15%), and pneumonia and differentiation syndrome (DS) (n = 14; 9% each).
In total, 80 (52%) patients experienced TEAEs resulting in dose interruption, including DS (n = 11; 7%), increased alanine aminotransferase (n = 8; 5%), and febrile neutropenia (n = 7; 5%). Twenty-four (16%) patients had TEAEs resulting in dose reduction, including increased alanine aminotransferase (n = 5; 3%), unspecified abnormal liver function test (n = 3; 2%), and increased aspartate aminotransferase, increased gamma-glutamyltransferase, and DS (n = 2; 1% each).
Deaths on treatment or within 28 days of the final dose were reported in 48 (31%) patients; the majority were related to progression of AML or its complications. Events leading to death in >1 patient were disease progression (n = 22; 14%), pneumonia (n = 3; 2%) and pneumonia fungal, cerebral hemorrhage, respiratory failure, sepsis (n = 2, 1% each), and death not otherwise specified. TEAEs leading to death assessed as treatment related were disease progression and DS in 1 patient each.
DS was reported as an AE among 22 (14%) patients, with grade ≥3 events in 14 (9%) patients. Concomitant leukocytosis was reported in 6 patients with DS events, all of whom had a baseline leukocyte count of <15 × 109/L. DS resulted in dose modifications (hold and/or reduction) in 12 patients, 11 of whom continued treatment with olutasidenib, including 1 at a reduced dose. Three patients permanently discontinued treatment because of DS, including 1 patient after a dose hold and subsequent dose reduction, and 2 patients who discontinued without prior dose modification. For most affected patients, DS occurred within the first 2 cycles of treatment, with a median time to the first event of 17.5 days (range, 1-561 days). Most patients with DS were treated with IV or oral dexamethasone. Other medications used to manage DS included oral hydroxycarbamide, furosemide, IV methylprednisolone, oral mercaptopurine, and IV antibiotics. One patient, a 70-year-old male with refractory AML, died on study-day 31 from complications of DS. This patient presented with hypoxia requiring oxygen supplementation on day 6 associated with pulmonary edema and renal decompensation and increasing leukocytes, neutrophils, and monocytes. Dosing was interrupted on day 8 and the patient received IV dexamethasone and hydroxyurea; however, leukocytosis progressed and the patient deteriorated to a fatal outcome 23 days after receiving the last dose of olutasidenib. Further details of the patient management guidelines for DS are available in the study protocol and supplemental Table 1.
Hepatic AEs of special interest (AESI) occurred in 38 (25%) patients, with grade 3 events reported in 19 (12%) and grade 4 events in 4 (3%) patients. The most frequent grade 3 or 4 hepatic AESI were increases in laboratory liver function parameters (Table 4). A summary of shifts in liver function parameters from baseline to the highest grade reported on study is provided in supplemental Table 2. Serious hepatic AESI were primarily reports of laboratory abnormalities and occurred in 11 (7%) patients, all of which were assessed as treatment related. Hepatic AESI were managed by treatment interruptions in 19 (12%) patients, dose reductions in 10 (7%) patients, and resulted in treatment discontinuation in 7 (5%) patients. No patients met the criteria for Hy’s law, and no hepatic AESI led to liver failure. Most events occurred early, within the first 2 months of treatment, and resolved within 2 to 4 weeks, with or without dose modifications.
Patients with treatment-emergent hepatic AESI (greater than 1%) of all grades, and of grade 3 or 4 severity
| Patients with hepatic AESI (>1%) . | All grades, n (%) . | Grade 3 or 4, n (%) . |
|---|---|---|
| Patients with at least 1 treatment-emergent hepatic AESI | 38 (25) | 23 (15) |
| Investigations | 33 (22) | 19 (12) |
| Alanine aminotransferase increased | 18 (12) | 7 (5) |
| Aspartate aminotransferase increased | 14 (9) | 4 (3) |
| Gamma-glutamyltransferase increased | 10 (7) | 8 (5) |
| Blood bilirubin increased | 8 (5) | 2 (1) |
| Hepatic enzyme increased | 7 (5) | 5 (3) |
| Liver function test deviated | 3 (2) | 3 (2) |
| Patients with hepatic AESI (>1%) . | All grades, n (%) . | Grade 3 or 4, n (%) . |
|---|---|---|
| Patients with at least 1 treatment-emergent hepatic AESI | 38 (25) | 23 (15) |
| Investigations | 33 (22) | 19 (12) |
| Alanine aminotransferase increased | 18 (12) | 7 (5) |
| Aspartate aminotransferase increased | 14 (9) | 4 (3) |
| Gamma-glutamyltransferase increased | 10 (7) | 8 (5) |
| Blood bilirubin increased | 8 (5) | 2 (1) |
| Hepatic enzyme increased | 7 (5) | 5 (3) |
| Liver function test deviated | 3 (2) | 3 (2) |
QT interval prolongation occurred in 12 (8%) patients; all events were of grade 1 or 2 severity, except for 1 grade 3 event assessed as unrelated to study treatment. All QT prolongation events were transient in nature. Interruption of dosing occurred in 2 patients, 1 of whom had experienced the grade 3 event assessed as unrelated to study treatment. Neither dose reduction nor treatment discontinuation was required for any patient because of QT prolongation. Of the 12 patients who experienced this AE, 6 had previously commenced treatment with a medication associated with QT prolongation either on or before the date of the event; 3 of the 12 patients had received levofloxacin, and 1 patient each had received amiodarone, fluconazole, and ondansetron.
Discussion
Olutasidenib is a targeted, oral therapy in development for the treatment of patients with IDH1-mutated cancers. Inhibition of mIDH1 in tumor cells and the concomitant decrease in 2-HG can restore normal cellular differentiation and provide therapeutic benefit in patients harboring IDH1 mutations. IDH1 inhibitors have recently demonstrated efficacy in AML, with initial proof of concept in patients with MDS, solid tumor, and glioma.7,10,16-20
The pivotal cohort of this study comprised 153 patients with mIDH1 R/R AML. Baseline characteristics, including the age and overall health of the population, were generally comparable with populations enrolled in similar clinical trials for this indication. Olutasidenib demonstrated a clinically meaningful CR + CRh rate of 35%, with CR representing the majority of responses. Achievement of CR/CRh is associated with clinical benefit, both through the establishment of disease control and through a reduced risk of infection and bleeding after the resumption of hematopoiesis. The CR + CRh rate was defined as the primary efficacy end point, consistent with other Food and Drug Administration (FDA)-approved IDH or FLT3 inhibitors for the treatment of R/R AML.7,15,21-25 The CR + CRh reference rate of 15% was selected as the null hypothesis based on historical data in similar populations of patients at high risk, with rates of CR ranging from 12% to 16% and median OS from 3.3 to 6.3 months.26-28 The observed CR + CRh rate of 35% in our study thus represents clinically meaningful efficacy in the high-risk population evaluated. The majority of patients had co-occurring mutations, were in the intermediate-to-poor AML cytogenetic risk category, had received prior induction therapy, and had a median of 2, with up to 7, prior treatment regimens. These results are also encouraging in the context of outcomes recently reported for ivosidenib, an FDA-approved IDH1 inhibitor that achieved a CR + CRh rate of 33%, with median duration of CR + CRh of 8.2 months and a median DOR of 6.5 months in a similar population of patients with R/R AML.7 Although the CR + CRh rate of 35% in our study is similar to that reported for ivosidenib, median duration of CR + CRh for olutasidenib was 25.9 months and median DOR was 11.7 months. In this context, the efficacy of olutasidenib compares favorably with that of ivosidenib in patients with R/R AML, based on the data available to date. Furthermore, the median OS of 11.6 months for olutasidenib well exceeds that reported in historical studies, and is encouraging in the context of the 8.8 months reported for ivosidenib in a similar patient population.7 Although olutasidenib and ivosidenib are structurally distinct, apparent differences in the overall clinical profile of these agents remain unconfirmed in the absence of a head-to-head study. In addition, although patient and disease characteristics of the ivosidenib study population appear to be similar to those of the olutasidenib population described herein, there may be some small differences in latent variables that influence outcomes. Overall, the efficacy data we present here for olutasidenib appear to be encouraging in this poor-prognostic population, particularly the duration of the responses. Further data from ongoing studies may provide additional insights regarding differences between IDH1 inhibitors in the future.
Conversion to TI, another recognized indicator of clinical benefit, was achieved in 34% of patients (29 of 86) who received olutasidenib and had been classified as transfusion dependent at baseline. Moreover, 16 (11%) patients underwent allogeneic HSCT, indicating the potential of olutasidenib as a bridge to the only currently available therapy with curative potential. Most patients who underwent transplantation are still being followed-up for duration of response and survival, such that the median cannot yet be estimated, but long-term outcomes in this population will be explored in future reports. Results in 12 patients who received prior venetoclax were also promising; the remission rate was consistent with that of the broader study population, and most patients who achieved remission had an ongoing remission at the time of data cut-off.
The comutational profile at baseline was consistent with previous reports,22 suggesting that the population of our study is representative of patients with mIDH1 AML. To further explore the possibility that primary resistance to IDH inhibitors may be because of disease burden, as exemplified by comutations or VAF,15 analyses of clinical response were conducted by baseline genetics. Achievement of CR/CRh was associated with fewer comutations and with VAF clearance. Longitudinal analyses of 2-HG concentration were performed to explore potential mechanisms of acquired resistance. The sustained on-target activity of olutasidenib observed attributable to suppression of 2-HG was consistent with previous findings,12 indicating that acquired resistance is unlikely to be related to a loss of pharmacologic effect. Future reports will further investigate potential mutation-associated resistance pathways, including the role of IDH1 mutation subtypes and individual comutations in the response to olutasidenib.
In this pivotal cohort, olutasidenib was well tolerated with an AE profile largely characteristic of symptoms or conditions experienced by patients undergoing treatment for AML or of the underlying disease itself. The most common AEs were gastrointestinal and hematologic in nature. These events are expected, readily recognized, and managed with dose modifications and standard-of-care measures by physicians who provide care for patients with AML. Hepatic AESI occurred in the early cycles of treatment and were generally characterized by asymptomatic laboratory elevations and AEs presenting with a mixed cholestatic-hepatocellular injury pattern. These events were manageable with dose holds or modifications and did not result in liver failure. QT prolongation was infrequently observed, with only 1 patient experiencing a grade 3 event that resolved after treatment interruption. No QT prolongation AEs led to a dose reduction or treatment discontinuation. Furthermore, because of the class potential for QT prolongation with mIDH1 inhibitors, the safety profile was further investigated in a cardiac substudy in which Holter monitoring was performed; olutasidenib showed no effects on heart rate, AV conduction, or cardiac depolarization. No new clinically relevant morphologic changes demonstrated any signal of concern. There was also no evidence of a large effect of olutasidenib on cardiac repolarization, as evidenced by concentration-QTc modeling results and time-matched and categorical outlier analyses (Forma Therapeutics, Inc. data on file; July 2021). These observations are fully in accordance with the safety profile we describe herein.
DS is a significant and potentially life-threatening risk of novel, targeted AML therapies, including inhibitors of IDH1, IDH2, and FLT3. DS may occur when normal cellular differentiation is restored, thereby promoting the maturation of leukemic blast cells and their subsequent extravasation into the lungs, skin, and other soft tissues. This can result in diverse clinical manifestations of the syndrome, such that a careful differential diagnosis is required. The signs and symptoms are generally nonspecific and can resemble infections and other complications of AML. Most frequently, these include respiratory manifestations, with symptoms including dyspnea and pulmonary infiltrates or pleuropericardial effusion. Fever or leukocytosis in the absence of infection may also occur, in addition to the potential for edema-related weight gain, hypotension, rash, or acute renal failure in some patients. Familiarity with the clinical presentation of DS is critical because early recognition and treatment of suspected cases, especially rapid intervention with corticosteroids, have been shown to be effective in this syndrome, which can otherwise prove fatal if treatment is delayed. Early intervention for DS with targeted therapies is usually effective and rarely necessitates interruption or discontinuation of therapy.29 To characterize DS and establish practical recommendations for diagnosis and treatment more clearly, an independent review committee retrospectively evaluated 72 potential cases among 281 patients with R/R AML in a study of the FDA-approved IDH2 inhibitor enasidenib. It was concluded that DS occurred in ∼12% of patients treated with enasidenib. Of note, only 33 of the 72 suspected cases were confirmed as possible or probable DS by the review committee. The study also suggested that patients who experienced the syndrome were less likely to have <20% bone marrow blasts (6% vs 22%; P = .04) and more likely to have received fewer prior anticancer regimens (median, 1.0 [range, 1-4] vs 2.0 [1-14]; P = .05) at study entry than those who did not.30 More recently, the rate of DS was reportedly 11% for the IDH1 inhibitor ivosidenib in the registrational study in patients with R/R AML.7 In the pivotal cohort of this study, DS was reported in 14% of patients with R/R AML, the majority of whom were successfully managed with dose interruptions and supportive treatment with steroids, diuretics, and hydroxyurea. The incidence rate of DS for olutasidenib was thus comparable with that reported for FDA-approved IDH1 and IDH2 inhibitors in similar patient populations.
Overall, the efficacy data we present herein for olutasidenib appear to be encouraging in this poor-prognostic population, particularly the durability of the responses. Further data from studies currently in progress may provide additional insights into differences between IDH1 inhibitors. Investigation of olutasidenib in other mIDH1 hematologic malignancies is ongoing.31
In conclusion, olutasidenib monotherapy induced durable remissions and TI among patients with R/R mIDH1 AML with a well-characterized and manageable side effect profile. The observed efficacy is clinically meaningful and represents a therapeutic advance in this molecularly defined patient population that has a poor prognosis and limited treatment options.
Acknowledgments
Medical writing support was provided by Patrick Barry (Acumen Medical Communications) and was funded by Forma Therapeutics, Inc. This study was funded by Forma Therapeutics, Inc. D.C.T. is supported by the National Institute for Health Research Royal Marsden/Institute of Cancer Research Biomedical Research Centre.
The sponsor of this study (Forma Therapeutics, Inc.) collaborated with the clinical investigators in the study design, conduct, and data collection. The study sponsor analyzed the data and conducted the statistical analyses. All authors had access to, and had the opportunity to review, the study data.
Authorship
Contribution: O.P., J.B., and J.S. designed the study and developed the protocol; J.B. developed the statistical plan; S.d.B., P.F., K.Y., C.R., A.H.W., P.M., D.C.T., A.P., T.B., A.C., C.G., B.A.J., A.K., O.L., P.P., M.A., W.B., D.C., D.K.H., J.G.J., J.K., X.T., J.M.W., and J.Y. recruited the patients; S.d.B., P.F., K.Y., C.R., A.H.W., P.M., D.C.T., A.P., T.B., A.C., C.G., B.A.J., A.K., O.L., P.P., M.A., W.B., D.C., D.K.H., J.G.J., J.K., X.T., J.M.W., J.Y., J.C., O.P., J.B., J.S., and E.B. collected the data; O.P., J.B., J.S., and E.B. participated in the analysis and interpretation of pharmacokinetic and pharmacodynamic data; and all authors participated in the analysis and interpretation of the clinical data and the development of the manuscript and were responsible for the decision to submit the article for publication.
Conflict-of-interest disclosure: S.d.B. has received honoraria and research funding from Forma Therapeutics; has received honoraria and research funding from and is a consultant to Agios; has received honoraria from, is a consultant to, and is on the speakers’ bureau for Celgene; has received honoraria from and is a consult to Astellas, Daiichi Sankyo, Syros, AbbVie, Bayer, and Janssen; has received honoraria from Seattle Genetics; and is a consultant to Pierre Fabre, Novartis, Pfizer, and Servier. P.F. is a consultant to and has received honoraria and research funding from AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, and Bristol Myers Squibb (BMS). K.Y. is a consultant to and has received research funding from F. Hoffman-La-Roche and Jazz Pharmaceticals; is a consultant to and has received honoraria and research funding from Novartis; is a consultant to Astellas, BMS/Celgene, GlaxoSmithKline, Pfizer, Shattuck Labs, Taiho, and Takeda; has received research funding from Astex, Forma Therapeutics, Geron, Gilead, Janssen, Karyopharm, and Treadwell; and has received honoraria from AbbVie. C.R. is a consultant to, has received honoraria and research funding from, and is a board/advisory committee member for AbbVie, BMS, and Jazz Pharmaceuticals; has received honoraria and research funding from and is a board/advisory committee member for Amgen and Astellas; is a board/advisory committee member for Novartis, Pfizer, and Takeda; and is a consultant to, has received honoraria from, and is a board/advisory committee member for Servier. A.H.W. has received honoraria and research funding from, is a consultant to, is on the speakers’ bureau, and is a board/advisory committee member for Astellas; has received honoraria and research funding from, is on the speakers’ bureau, and is a board/advisory committee member for AbbVie/Genentech, Amgen, Celgene/BMS, and Novartis; has received research funding and honoraria from, is a consultant to, and is a board/advisory committee member for Servier and Syndax; has received honoraria from, is a consultant to, and is a board/advisory committee member for Janssen and Gilead; has received honoraria from and is a board/advisory committee member for MacroGenetics and Pfizer; has received honoraria and research funding from and is a board/advisory committee member for AstraZeneca; has received research funding from Astex; is an employee of the Walter and Eliza Hall Institute; and is eligible for a fraction of the royalty stream related to venetoclax. P.M. is a consultant to, has received research funding from, and is on the speakers’ bureau for AbbVie, BMS, and Pfizer; is a consultant to and has received research funding from Menarini/Stemline, Novartis, and Takeda; is a consultant to and is on the speakers’ bureau for Jazz Pharmaceuticals and Astellas; and is a consultant to Otsuka, Kura Oncology, BeiGene, Incyte, Ryvu, and Nerviano. D.C.T. has received research funding from Bayer and from AbbVie to attend the American Society of Hematology meeting. A.P. has received honoraria from and is a consultant to AbbVie; is a consultant to Gilead; and has received honoraria from Astellas, Agios, Pfizer, and Jazz Pharmaceuticals. A.C. is a board/advisory committee member for Jazz Pharmaceuticals, Pfizer, Novartis, and AbbVie. C.G. is a board/advisory committee member for Astellas, AbbVie, and Otsuka; has received honoraria for her institution for participation in advisory boards for Astellas, AbbVie, and Otsuka, and for chairing an educational meeting for AbbVie. B.A.J. has received research funding for his institution, is a consultant to, and is a board/advisory committee member for, AbbVie, BMS, Genentech/F. Hoffman-La-Roche, Jazz Pharmaceuticals, Pfizer, and Treadwell; has received research funding for his institution, is a consultant to, and is a data monitoring committee member for Gilead; has received research funding for his institution, is a consultant to, and is a protocol steering committee member for GlycoMimetics; is a consultant to and a board/advisory committee member for Rigel, Servier, Takeda, and Tolero; has received travel reimbursement/expenses from AbbVie and Rigel; has received research funding for his institution from 47, Accelerated Medical Diagnostics, Amgen, Arog, Celgene, Daiichi Sankyo, F. Hoffman-La-Roche, Forma Therapeutics, Hanmi, Immune-Onc, Incyte, Loxo Oncology, LP Therapeutics, Pharmacyclics, and Sigma Tau. P.P. is a board/advisory committee member for Jazz Pharmaceuticals, Astellas, Celgene, and AbbVie. M.A. is a consultant to and a board/advisory committee member for BMS/Celgene and Novartis; and is a consultant to Astellas, Jazz Pharmaceuticals, and Pfizer. W.B. is a board/advisory committee member for Syndax; has received research funding from Xencor and Celyad; and has received honoraria from AmerisourceBergen. D.K.H. has received research funding from Novartis. J.G.J. is a consultant to, a board/advisory committee member for, and has received research funding from AbbVie, Daiichi Sankyo, and Celgene; is a consultant to, and a board/advisory committee member for Novartis; and has received research funding from Arog Pharmaceuticals, Astellas, Forma Therapeutics, Genentech, Kura Oncology, PTC Therapeutics, and Syros Pharmaceuticals. J.M.W. has received research funding from and is a board/advisory committee member for Takeda; has received research funding from Immune System Key Ltd and Takeda; and is a board/advisory committee member for Genentech, Rafael Pharma, Reven Pharma, and Celgene/BMS. J.Y. has received research funding from Seattle Genetics, Janssen, Arog, Loxo Oncology, and Agios. O.P., J.B., J.S., and E.B. are employees of and hold stock in Forma Therapeutics. J.C. has received research funding for his institution from and is a consultant to AbbVie, BMS, Novartis, Pfizer, Takeda, Daiichi, Jazz Pharmaceuticals, Merus, and Forma Therapeutics; has received research funding for his institution from Astellas and Amphivena; and is a consultant to Gilead, BioLineRx, and BioPath. The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, Georgia Cancer Center, Augusta University, 1410 Laney Walker Blvd, CN2222, Augusta, GA 30912; e-mail: jorge.cortes@augusta.edu.
References
Author notes
For deidentified data, requests may be sent to datasharing@rigel.com at least 24 months after clinical trial completion, provided a scientifically valid research proposal is made by qualified, academic researchers for data associated with interventions that have received regulatory approval in the US and Europe.
The full-text version of this article contains a data supplement.