Key Points
Bridging therapy and more lines of pre–CAR-T therapy were associated with inferior PFS and OS after CAR-T for aggressive B-cell lymphoma.
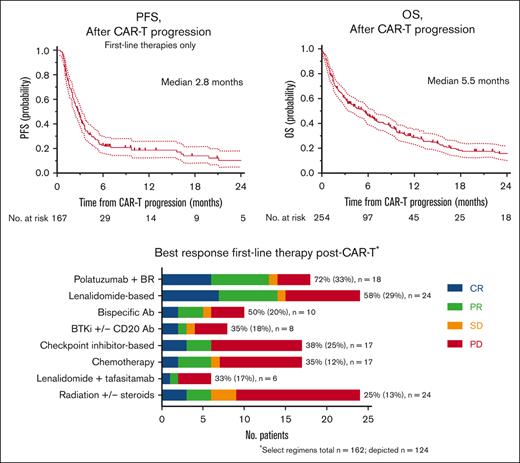
Post–CAR-T failure, the median PFS of first-line therapies was 2.8 months with the most active regimen, with a median PFS of only 5.5 months.
Abstract
Most patients receiving chimeric antigen receptor T-cell therapy (CAR-T) for aggressive B-cell non-Hodgkin lymphoma (B-NHL) do not experience a durable remission. Several novel agents are approved to treat relapsed, refractory aggressive B-NHL; however, it remains unclear how to sequence these therapies pre– and post–CAR-T. We conducted a multicenter retrospective analysis to describe peri–CAR-T practice patterns and survival predictors for patients receiving CD19-directed CAR-T. Patients (n = 514) from 13 centers treated with CAR-T for B-NHL between 2015-2021 were included in the study. Survival curves were constructed using Kaplan-Meier method. Multivariate Cox regression analysis was used to determine the impact of the variables on survival outcomes. For all patients receiving CAR-T, a greater number of lines of therapy pre-CAR-T apheresis and bridging therapy were predictive of inferior progression-free survival (PFS) and overall survival (OS). The median PFS and OS from the time of CAR-T cell infusion were 7.6 and 25.6 months, respectively. From the time of progression post–CAR-T, the median OS was 5.5 months. The median PFS of treatments administered in the first-line post–CAR-T failure was 2.8 months. Patients with refractory disease on day 30 had inferior OS and were less likely to receive subsequent treatment(s) than other patients with CAR-T failure. Allogeneic hematopoietic cell transplantation for selected patients at any time following CAR-T failure led to durable responses in over half of patients at 1 year. These data provide a benchmark for future clinical trials in patients with post–CAR-T cell progression, which remains an unmet clinical need.
Introduction
CD19-directed chimeric antigen receptor T-cell therapy (CAR-T) leads to high response rates in relapsed/refractory (R/R) aggressive B-cell non-Hodgkin lymphoma (B-NHL); nonetheless, only 30% to 40% of patients experience durable remission.1-4 Although there are now several novel agents approved for the treatment of R/R aggressive B-NHL,5-8 it is unclear how to sequence these therapies to optimize patient outcomes. Many patients receive treatment after apheresis and before CAR-T infusion (bridging therapy [BT]). However, the use of BT varies widely between trials and in clinical practice.1-4,9 There is a gap in the knowledge regarding which agents or modalities to prioritize for BT, especially in the era of novel therapies. Moreover, survival outcomes following CAR-T failure are poor, with a reported median overall survival (OS) from the time of progressive disease (PD) of <6 months.10,11 To date, data are lacking on the optimal sequencing of therapies for patients whose disease progresses following CD19-directed CAR-T. To address these critical knowledge gaps, we conducted a multicenter retrospective analysis to describe peri–CAR-T practice patterns (BT and first-line treatment post–CAR-T failure) and predictors of survival for all patients receiving CAR-T and after the failure of CAR-T.
Patients and methods
Study design and participants
Patients aged ≥18 years who received CD19-directed CAR-T (axicabtagene ciloleucel [axi-cel], tisagenlecleucel [tisa-cel], and lisocabtagene maraleucel [liso-cel]) either as a standard of care or as part of a clinical trial for R/R aggressive B-NHL from January 2015 to July 2021 were included from 13 academic medical centers in the United States (supplemental Table 1). Data were collected from the electronic medical records of each institution via chart review by the individual investigators. Demographic and clinical characteristics were collected along with CAR-T toxicities and treatment responses. Details regarding the treatment regimens administered pre– and post–CAR-T were collected, including dates of treatment, the best response to therapy, and the date of progression (if applicable). The histological subtype, fluorescence in situ hybridization, immunohistochemistry, and cell of origin (germinal center B-cell–like [GCB] and non-GCB) were determined at the time of diagnosis. Assessment of lactate dehydrogenase (LDH) levels, Eastern Cooperative Oncology Group (ECOG) performance status (PS), International Prognostic Index (IPI), central nervous system (CNS) disease, and stage of disease were determined at apheresis. Cytokine release syndrome (CRS) and immune effector cell neurotoxicity syndrome (ICANS) were retrospectively graded according to established consensus grading scales by individual investigators.12-15 Response to treatment was assessed using the Lugano criteria16 at individual institutions without centralized review. Each center obtained approval from an independent institutional review board for the study. The study was conducted following the Declaration of Helsinki.
Statistical analysis
Descriptive statistics were performed for the demographic, clinical, and treatment variables. Univariate outcome associations were derived using logistic regression and Fisher’s exact tests. The association between the continuous variables was assessed using the Wilcoxon rank-sum test. Survival outcomes were estimated using the Kaplan-Meier method and assessed from 2 main time points: from the time of CAR-T infusion and from the time of CAR-T progression. Progression-free survival (PFS) was estimated from each of these time points to the next disease relapse/progression or death due to any cause, with censoring of patients alive and progression-free survival at the last follow-up. OS was estimated from each of these time points to death due to any cause, with censoring of patients alive at the last follow-up. The log-rank test was used to explore the association between clinical characteristics and survival outcomes. Multivariate modeling using Cox regression was performed using the selected relevant demographic and clinical variables to determine the independent effect on survival outcomes. A P value < .05 was considered significant. All tests were 2-tailed.
Results
Patient characteristics
A total of 514 patients were included in this study, and their demographic and clinical characteristics are detailed in Table 1. Most patients had de novo DLBCL (69%), an ECOG PS of 0 to 1 (92%), elevated LDH at apheresis (68%), an IPI of ≥3 (51%), and had received ≥2 lines of therapy before CAR-T apheresis (88%). Thirty-two patients (6%) had secondary CNS involvement at the time of apheresis. Patients received a median of 2 lines (interquartile range 2-3) of therapy for aggressive B-cell lymphoma before CAR-T apheresis. Specific regimens for the treatment of R/R aggressive B-cell lymphoma before apheresis across multiple lines are listed in supplemental Table 2.
Baseline patient characteristics, safety, and outcomes of patients with aggressive B-NHL receiving CD19-directed CAR-T
| Characteristic . | . | Number (%) . |
|---|---|---|
| Number of patients | 514 | |
| Age, y, median (range) | 59 (18-84) | |
| Sex | Male | 305 (59%) |
| ECOG PS at apheresis | 0-1 | 409 (80%) |
| 2 | 29 (6%) | |
| 3-4 | 6 (1%) | |
| Unknown | 70 (14%) | |
| Histologic subtype | De novo DLBCL | 357 (69%) |
| Transformed follicular lymphoma | 98 (19%) | |
| Richter’s transformation | 18 (3.5%) | |
| Other∗ | 12 (2.3%) | |
| Transformed marginal zone lymphoma | 10 (1.9%) | |
| PMBL | 9 (1.8%) | |
| PTLD | 5 (0.97%) | |
| Gray zone lymphoma | 3 (0.58%) | |
| Transformed mantle cell lymphoma | 2 (0.39%) | |
| Number of previous lines of therapy | 1 | 58 (11%) |
| 2 | 225 (44%) | |
| 3-4 | 203 (39%) | |
| 5-6 | 25 (5%) | |
| Unknown | 3 (1%) | |
| IPI at apheresis | 0-1 | 99 (19%) |
| 2 | 95 (18%) | |
| 3 | 128 (25%) | |
| 4-5 | 73 (14%) | |
| Unknown | 119 (23%) | |
| LDH elevation at apheresis | Yes | 248 (48%) |
| No | 115 (22%) | |
| Unknown | 151 (29%) | |
| Stage at apheresis | 1-2 | 84 (16%) |
| 3-4 | 418 (81%) | |
| Unknown | 12 (2%) | |
| Cell of origin | Germinal center B-cell like | 238 (46%) |
| Nongerminal center B-cell like | 163 (32%) | |
| Unknown | 113 (22%) | |
| Double expressor/double hit status | Double hit† | 81 (16%) |
| Double expressor‡ | 86 (17%) | |
| None | 267 (52%) | |
| Unknown | 80 (16%) | |
| Previous autologous transplant | Yes | 139 (27%) |
| No | 375 (73%) | |
| CNS involvement at apheresis | Yes | 32 (6%) |
| No | 470 (91%) | |
| Unknown | 12 (2%) | |
| CAR construct | Axi-cel | 330 (64%) |
| Tisa-cel | 138 (27%) | |
| Liso-cel | 45 (9%) | |
| Unknown | 1 (0.2%) | |
| CAR-T on clinical trial | Yes | 127 (25%) |
| No | 387 (75%) | |
| CRS | Any grade | 344 (67%) |
| Grade 3+ | 62 (12%) | |
| ICANS | Any grade | 236 (46%) |
| Grade 3+ | 87 (17%) | |
| Best response to CAR-T | ORR | 351 (68%) |
| Complete response rate | 251 (49%) | |
| Relapse | Late relapse (≥180 days) | 59 (11.5%) |
| CD19 antigen | CD19 loss post–CAR-T failure | 14 (17%) |
| No CD19 loss | 67 (83%) | |
| Missing/not assessed | 173 |
| Characteristic . | . | Number (%) . |
|---|---|---|
| Number of patients | 514 | |
| Age, y, median (range) | 59 (18-84) | |
| Sex | Male | 305 (59%) |
| ECOG PS at apheresis | 0-1 | 409 (80%) |
| 2 | 29 (6%) | |
| 3-4 | 6 (1%) | |
| Unknown | 70 (14%) | |
| Histologic subtype | De novo DLBCL | 357 (69%) |
| Transformed follicular lymphoma | 98 (19%) | |
| Richter’s transformation | 18 (3.5%) | |
| Other∗ | 12 (2.3%) | |
| Transformed marginal zone lymphoma | 10 (1.9%) | |
| PMBL | 9 (1.8%) | |
| PTLD | 5 (0.97%) | |
| Gray zone lymphoma | 3 (0.58%) | |
| Transformed mantle cell lymphoma | 2 (0.39%) | |
| Number of previous lines of therapy | 1 | 58 (11%) |
| 2 | 225 (44%) | |
| 3-4 | 203 (39%) | |
| 5-6 | 25 (5%) | |
| Unknown | 3 (1%) | |
| IPI at apheresis | 0-1 | 99 (19%) |
| 2 | 95 (18%) | |
| 3 | 128 (25%) | |
| 4-5 | 73 (14%) | |
| Unknown | 119 (23%) | |
| LDH elevation at apheresis | Yes | 248 (48%) |
| No | 115 (22%) | |
| Unknown | 151 (29%) | |
| Stage at apheresis | 1-2 | 84 (16%) |
| 3-4 | 418 (81%) | |
| Unknown | 12 (2%) | |
| Cell of origin | Germinal center B-cell like | 238 (46%) |
| Nongerminal center B-cell like | 163 (32%) | |
| Unknown | 113 (22%) | |
| Double expressor/double hit status | Double hit† | 81 (16%) |
| Double expressor‡ | 86 (17%) | |
| None | 267 (52%) | |
| Unknown | 80 (16%) | |
| Previous autologous transplant | Yes | 139 (27%) |
| No | 375 (73%) | |
| CNS involvement at apheresis | Yes | 32 (6%) |
| No | 470 (91%) | |
| Unknown | 12 (2%) | |
| CAR construct | Axi-cel | 330 (64%) |
| Tisa-cel | 138 (27%) | |
| Liso-cel | 45 (9%) | |
| Unknown | 1 (0.2%) | |
| CAR-T on clinical trial | Yes | 127 (25%) |
| No | 387 (75%) | |
| CRS | Any grade | 344 (67%) |
| Grade 3+ | 62 (12%) | |
| ICANS | Any grade | 236 (46%) |
| Grade 3+ | 87 (17%) | |
| Best response to CAR-T | ORR | 351 (68%) |
| Complete response rate | 251 (49%) | |
| Relapse | Late relapse (≥180 days) | 59 (11.5%) |
| CD19 antigen | CD19 loss post–CAR-T failure | 14 (17%) |
| No CD19 loss | 67 (83%) | |
| Missing/not assessed | 173 |
DLBCL, diffuse large B-cell lymphoma; ORR, overall response rate; PMBL, primary mediastinal large B-cell lymphoma; PTLD, posttransplant lymphoproliferative disorder.
Other histologies included: transformed lymphoplasmacytic lymphoma, T cell rich B-cell lymphoma, and Burkitt lymphoma.
MYC translocation with a concurrent BCL2 and/or BCL6 translocations.
Overexpression of MYC and BCL2 proteins.
Bridging practice patterns
Forty-seven percent (n = 240) of patients received BT. The median PFS from the time of CAR-T infusion for patients who received BT vs no BT was 4.2 months vs 18.4 months (P < .001) respectively and the median OS was 16.8 vs 69.3 months (P < .001) respectively (supplemental Figure 1). Patients receiving BT had a higher median IPI at apheresis (3 vs 2; P = .026), longer median time from apheresis to CAR-T infusion (34 vs 31 days; P = .003), and a higher median LDH at apheresis (265 vs 222 international units per liter [IU/L]). The incidence of extranodal disease (72% vs 65%; P = .12), stage ≥3 disease (85% vs 83%; P = .54), median age (62 vs 62; P = .26), and ECOG PS (1 vs 1; P = .39) at apheresis were similar among those receiving BT vs no BT. Eighty-nine percent (n = 213) of patients received 1 line of BT, 10% (n = 24) received 2 lines and 1% (n = 3) received 3 lines. The most common types of BT were chemotherapy (n = 102, 38%), followed by radiation therapy (RT) (n = 34, 13%), pola + BR (n = 33, 12%), immunomodulatory drug (IMiD)-based (n = 29, 11%), Bruton tyrosine kinase -inhibitor +/− anti-CD20 antibodies (n = 21, 8%), and steroids alone (n = 18, 7%) (supplemental Table 3). Patients who received RT alone vs systemic therapy (ST) +/− RT for bridging had similar median IPI (3 vs 3; P = .97), stage (4 vs 4; P = .87), and age (62 vs 64; P = .50), but were more likely to have a normal LDH (67% vs 42%; P = .04) and inferior ECOG PS (median 1 vs 1; P = .01) at apheresis.
Response and survival outcomes in all patients receiving CAR-T cell therapy
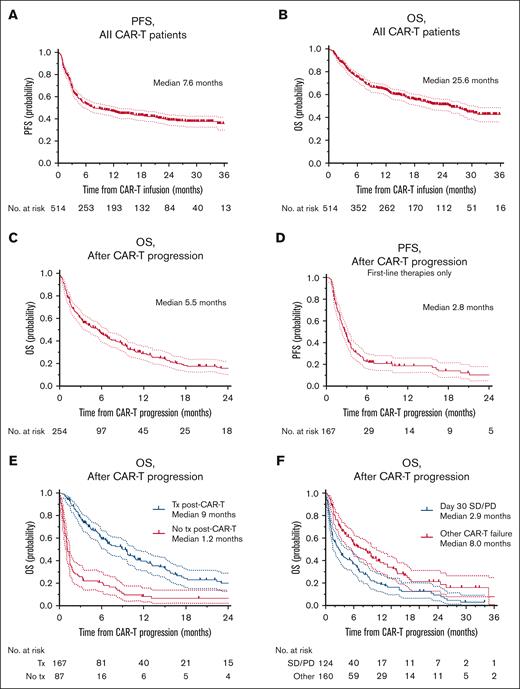
At a median follow-up of 17.9 months from the time of CAR-T, the median PFS was 7.6 months and OS was 25.6 months (n = 514) (Figure 1A-B). The highest ORR was 68% (complete response rate [CR], 49%). The ORR for axi-cel, tisa-cel, and liso-cel were 72% (CR, 53%), 57% (CR, 37%), and 73% (CR, 56%), respectively. Of the patients with an initial partial response on day 30, 23% (n = 34) converted to CR by day 90, and of the patients with SD on day 30 (n = 75), 8% (n = 6) converted to CR by day 90. The rates of CRS of any grade and ≥3 were 67% and 12%, respectively. The rates of any grade and ≥3 ICANS were 46% and 17%, respectively. The rates of CRS of any grade (and grade ≥3 CRS) by construct were as follows: axi-cel 74% (14%), tisa-cel 53% (11%), and liso-cel 53% (0%). The rates of any grade of ICANS (and grade ≥3 ICANS) by construct were as follows: axi-cel 55% (20%), tisa-cel 31% (10%), and liso-cel 22% (13%).
Survival curves for all patients receiving CAR-T and patients with CAR-T failure. Survival curves constructed using the Kaplan-Meier method. All curves include 95% CI and censored points are denoted with a mark on the curve. (A) PFS of all patients receiving CD19-directed CAR-T for aggressive B-NHL, from the time of CAR-T infusion. (B) OS of all patients receiving CD19-directed CAR-T for aggressive B-NHL, from the time of CAR-T infusion. (C) OS of all patients with CAR-T failure from time of post–CAR-T progression. (D) PFS of patients with CAR-T failure in patients who received subsequent therapies for aggressive B-NHL, from time of CAR-T progression. (E) OS of patients with CAR-T failure stratified by those who received subsequent therapy post–CAR-T vs those who did not. (F) OS from time of progression post–CAR-T for patients with SD or progressive at day 30 vs all other patients experiencing progression post–CAR-T. CI, confidence intervals; SD, stable disease; Tx, treatment.
Survival curves for all patients receiving CAR-T and patients with CAR-T failure. Survival curves constructed using the Kaplan-Meier method. All curves include 95% CI and censored points are denoted with a mark on the curve. (A) PFS of all patients receiving CD19-directed CAR-T for aggressive B-NHL, from the time of CAR-T infusion. (B) OS of all patients receiving CD19-directed CAR-T for aggressive B-NHL, from the time of CAR-T infusion. (C) OS of all patients with CAR-T failure from time of post–CAR-T progression. (D) PFS of patients with CAR-T failure in patients who received subsequent therapies for aggressive B-NHL, from time of CAR-T progression. (E) OS of patients with CAR-T failure stratified by those who received subsequent therapy post–CAR-T vs those who did not. (F) OS from time of progression post–CAR-T for patients with SD or progressive at day 30 vs all other patients experiencing progression post–CAR-T. CI, confidence intervals; SD, stable disease; Tx, treatment.
At the last follow-up, 284 patients (55%) showed disease progression or died. Thirty patients (6%) died without disease progression (range, 7-780 days; interquartile range 33-188 days). The most common cause of death in these patients was infection (n = 17, 57%), unknown (n = 9, 27%), neurotoxicity (n = 2, 7%), and respiratory failure (n = 2, 7%). Patients with progression or death at the last follow-up (n = 284) vs ongoing response (n = 230) were more likely to have an elevated LDH at apheresis (57% vs 42%; P < .01), extranodal disease at apheresis (75% vs 60%; P < .01), received BT (54% vs 37%; P < .01), higher IPI scores at apheresis (P = .02), received more lines of therapy pre–CAR-T (median 3 vs 2; P < .01), and not received tocilizumab (58% vs 45%; P < .01) (Table 2). Late relapse (relapse ≥180 days after CAR-T infusion) occurred in 59 patients (11.5% of all patients received CAR-T; 17% of patients responded to CAR-T). CD19-antigen expression was assessed in 81 patients (31%) following progression, and of these 14 (17%) had documented CD19 negativity.
Characteristics of patients with post–CAR-T progression/death vs ongoing response at last follow-up
| Characteristic . | Ongoing response, n = 230 . | Progression/death, n = 284 . | P value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Age at CAR-T | 58.5 (21-83) | 59 (18-84) | .42 |
| Female sex | 96 (41.7%) | 113 (39.8%) | .72 |
| Histologic subtype | <.01 | ||
| de novo DLBCL | 142 (62%) | 215 (76%) | |
| tFL | 62 (27%) | 36 (13%) | |
| PMBL | 4 (2%) | 5 (2%) | |
| Richter’s | 9 (4%) | 9 (3%) | |
| Other | 13 (6%) | 19 (7%) | |
| Subtype | .41 | ||
| Non-GCB | 66 (38%) | 131 (43%) | |
| GCB | 107 (62%) | 97 (57%) | |
| Lines of therapy before CAR-T | 2 (1-6) | 3 (1-6) | <.01 |
| LDH elevation at apheresis | 96 (42%) | 161 (57%) | <.01 |
| IPI at apheresis | .02 | ||
| 0-1 | 51 (27%) | 43 (18%) | |
| 2-3 | 113 (60%) | 147 (60%) | |
| 4-5 | 23 (12%) | 55 (22%) | |
| Extranodal disease at apheresis | 130 (60%) | 208 (75%) | <.01 |
| CNS disease at apheresis | 10 (4%) | 22 (8%) | .1 |
| Prior allogeneic HCT | 1 (0.4%) | 0 (0) | .45 |
| Prior autologous HCT | 64 (28%) | 75 (26%) | .76 |
| Double hit/double expressor | .01 | ||
| Double hit | 45 (20%) | 36 (13%) | |
| Double expressor | 30 (13%) | 56 (20%) | |
| None | 112 (49%) | 155 (55%) | |
| Unknown | 43 (19%) | 37 (13%) | |
| CAR-T product | .29 | ||
| Axi-cel | 155 (67%) | 175 (62%) | |
| Tisa-cel | 54 (23%) | 84 (30%) | |
| Liso-cel | 21 (9%) | 24 (8%) | |
| BT | 86 (37%) | 152 (54%) | <.01 |
| CRS | 157 (70%) | 185 (64%) | .16 |
| Tocilizumab use | 126 (55%) | 119 (42%) | <.01 |
| ICANS | 108 (47%) | 127 (45%) | .66 |
| Steroid use to treat CAR-T toxicities | 100 (45%) | 128 (47%) | .65 |
| Characteristic . | Ongoing response, n = 230 . | Progression/death, n = 284 . | P value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Age at CAR-T | 58.5 (21-83) | 59 (18-84) | .42 |
| Female sex | 96 (41.7%) | 113 (39.8%) | .72 |
| Histologic subtype | <.01 | ||
| de novo DLBCL | 142 (62%) | 215 (76%) | |
| tFL | 62 (27%) | 36 (13%) | |
| PMBL | 4 (2%) | 5 (2%) | |
| Richter’s | 9 (4%) | 9 (3%) | |
| Other | 13 (6%) | 19 (7%) | |
| Subtype | .41 | ||
| Non-GCB | 66 (38%) | 131 (43%) | |
| GCB | 107 (62%) | 97 (57%) | |
| Lines of therapy before CAR-T | 2 (1-6) | 3 (1-6) | <.01 |
| LDH elevation at apheresis | 96 (42%) | 161 (57%) | <.01 |
| IPI at apheresis | .02 | ||
| 0-1 | 51 (27%) | 43 (18%) | |
| 2-3 | 113 (60%) | 147 (60%) | |
| 4-5 | 23 (12%) | 55 (22%) | |
| Extranodal disease at apheresis | 130 (60%) | 208 (75%) | <.01 |
| CNS disease at apheresis | 10 (4%) | 22 (8%) | .1 |
| Prior allogeneic HCT | 1 (0.4%) | 0 (0) | .45 |
| Prior autologous HCT | 64 (28%) | 75 (26%) | .76 |
| Double hit/double expressor | .01 | ||
| Double hit | 45 (20%) | 36 (13%) | |
| Double expressor | 30 (13%) | 56 (20%) | |
| None | 112 (49%) | 155 (55%) | |
| Unknown | 43 (19%) | 37 (13%) | |
| CAR-T product | .29 | ||
| Axi-cel | 155 (67%) | 175 (62%) | |
| Tisa-cel | 54 (23%) | 84 (30%) | |
| Liso-cel | 21 (9%) | 24 (8%) | |
| BT | 86 (37%) | 152 (54%) | <.01 |
| CRS | 157 (70%) | 185 (64%) | .16 |
| Tocilizumab use | 126 (55%) | 119 (42%) | <.01 |
| ICANS | 108 (47%) | 127 (45%) | .66 |
| Steroid use to treat CAR-T toxicities | 100 (45%) | 128 (47%) | .65 |
Significant P values are shown in boldface.
HCT, hematopoietic cell transplantation; No., number; PMBL, primary mediastinal large B-cell lymphoma; tFL, transformed follicular lymphoma.
The median follow-up from the time of progression post–CAR-T was 15.9 months (range, 2.6-36.9). For all patients with post–CAR-T progression, the median OS was 5.5 months (Figure 1C). For those who received further treatment(s) post–CAR-T failure, from time of progression post–CAR-T, median PFS was 2.8 months (Figure 1D) and median OS was 9 months (Figure 1E).
Predictors of survival outcomes, all patients receiving CAR-T
On univariate analysis (UVA), predictors of inferior PFS and OS for all patients who received CAR-T (n = 514) were IPI ≥ 2 (P < .01 both), elevated LDH at apheresis (P < .01 both), no prior autologous HCT (P = .03 both), >2 lines of therapy pre–CAR-T (P < .01 both), CNS disease at apheresis (P = .01 and P < .01), and receipt of BT (P < .01 both) (supplemental Table 4). On multivariate analysis (MVA), predictors of inferior PFS included a greater number of lines of pre–CAR-T therapy (Hazard ratio [HR] 1.5; 95% CI, 1.2-1.9) and receipt of any BT (HR 1.7; 95% CI, 1.1-2.8) (Table 3). Among the patients who received BT on UVA, there were no significant differences in PFS between the selected bridging regimens (supplemental Figure 1). On MVA, inferior PFS was observed in those who received chemotherapy-based (HR, 5.1; 95% CI, 1.5-18.3) and IMiD-based bridging (HR, 5.2; 95% CI, 1.2-24.9) (Table 3). Predictors of inferior OS on MVA included IPI (HR, 1.4; 95% CI, 1.0-1.8), a greater number of lines of pre–CAR-T therapy (HR, 1.6; 95% CI, 1.3-2.0), and receipt of BT (HR, 1.7; 95% CI, 1.0-2.8) (supplemental Table 5). The specific CAR-T construct used was not associated with a difference in PFS or OS on MVA (Table 3 and supplemental Table 5).
Predictors of PFS on MVA
| All patients receiving CAR-T therapy . | |||
|---|---|---|---|
| Variable . | HR . | 95% CI . | P value . |
| Age, at apheresis, y∗ | 1.0 | 0.98-1.02 | .87 |
| Female sex (yes/no) | 1.06 | 0.66-1.70 | .81 |
| Histologic subtype (de novo DLBCL/all others) | 0.94 | 0.79-1.12 | .46 |
| Non-GCB cell of origin (yes/no) | 1.07 | 0.66-1.72 | .79 |
| LDH elevation, at apheresis (yes/no) | 1.65 | 0.96-2.85 | .07 |
| IPI score, at apheresis† | 1.23 | 0.94-1.62 | .13 |
| Stage, at apheresis† | 0.91 | 0.68-1.21 | .52 |
| CNS disease, at apheresis (yes/no) | 1.28 | 0.48-3.71 | .63 |
| Prior autologous HCT (yes/no) | 1.29 | 0.74 - 2.26 | .37 |
| Number of lines pre–CAR-T∗ | 1.47 | 1.16-1.89 | <.01 |
| CAR-T construct (axi-cel/tisa-cel)‡ | 1.17 | 0.82-1.69 | .38 |
| Double hit/double expressor status (DH/DE/other) | 0.85 | 0.63-1.14 | .29 |
| BT (yes/no) | 1.72 | 1.05-2.82 | .03 |
| Days between apheresis and CAR-T∗ | 1.0 | 0.99-1.01 | .98 |
| All patients receiving CAR-T therapy . | |||
|---|---|---|---|
| Variable . | HR . | 95% CI . | P value . |
| Age, at apheresis, y∗ | 1.0 | 0.98-1.02 | .87 |
| Female sex (yes/no) | 1.06 | 0.66-1.70 | .81 |
| Histologic subtype (de novo DLBCL/all others) | 0.94 | 0.79-1.12 | .46 |
| Non-GCB cell of origin (yes/no) | 1.07 | 0.66-1.72 | .79 |
| LDH elevation, at apheresis (yes/no) | 1.65 | 0.96-2.85 | .07 |
| IPI score, at apheresis† | 1.23 | 0.94-1.62 | .13 |
| Stage, at apheresis† | 0.91 | 0.68-1.21 | .52 |
| CNS disease, at apheresis (yes/no) | 1.28 | 0.48-3.71 | .63 |
| Prior autologous HCT (yes/no) | 1.29 | 0.74 - 2.26 | .37 |
| Number of lines pre–CAR-T∗ | 1.47 | 1.16-1.89 | <.01 |
| CAR-T construct (axi-cel/tisa-cel)‡ | 1.17 | 0.82-1.69 | .38 |
| Double hit/double expressor status (DH/DE/other) | 0.85 | 0.63-1.14 | .29 |
| BT (yes/no) | 1.72 | 1.05-2.82 | .03 |
| Days between apheresis and CAR-T∗ | 1.0 | 0.99-1.01 | .98 |
| Patients receiving BT . | |||
|---|---|---|---|
| Variable (yes/no) . | HR . | 95% CI . | P value . |
| Chemotherapy-based | 5.15 | 1.55-18.25 | <.01 |
| RT§ | 1.93 | 0.49-7.94 | .35 |
| Anti-CD20 monoclonal antibody | 3.91 | 0.50-40.40 | .21 |
| Steroids alone | 2.5 | 0.53-12.63 | .25 |
| IMiD-based | 5.24 | 1.22-24.91 | .03 |
| Polatuzumab + bendamustine + rituximab | 2.14 | 0.56-8.57 | .27 |
| BTK inhibitor +/− CD20 antibody | 2.21 | 0.49-10.57 | .31 |
| Patients receiving BT . | |||
|---|---|---|---|
| Variable (yes/no) . | HR . | 95% CI . | P value . |
| Chemotherapy-based | 5.15 | 1.55-18.25 | <.01 |
| RT§ | 1.93 | 0.49-7.94 | .35 |
| Anti-CD20 monoclonal antibody | 3.91 | 0.50-40.40 | .21 |
| Steroids alone | 2.5 | 0.53-12.63 | .25 |
| IMiD-based | 5.24 | 1.22-24.91 | .03 |
| Polatuzumab + bendamustine + rituximab | 2.14 | 0.56-8.57 | .27 |
| BTK inhibitor +/− CD20 antibody | 2.21 | 0.49-10.57 | .31 |
| Patients with CAR-T failure . | |||
|---|---|---|---|
| Variable . | HR . | 95% CI . | P value . |
| Age, y∗ | 1.02 | 0.97-1.06 | .50 |
| Female sex (yes/no) | 0.63 | 0.19-1.9 | .43 |
| IPI > 1 (yes/no) | 1.42 | 0.75-2.8 | .29 |
| Stage at diagnosis† | 1.25 | 0.72-2.2 | .42 |
| Non-GCB cell of origin (yes/no) | 2.94 | 0.97-10.1 | .07 |
| Elevated LDH at apheresis (yes/no) | 0.63 | 0.18-2.2 | .47 |
| >2 lines of therapy pre–CAR-T (yes/no) | 1.89 | 1.1-3.5 | .03 |
| Double hit/double expressor status (DH/DE/other) | 0.42 | 0.18-0.89 | .03 |
| CNS involvement (yes/no) | 0.18 | 0.02-1.5 | .11 |
| Prior autologous HCT (yes/no) | 0.70 | 0.2-2.6 | .58 |
| Extranodal disease at apheresis (yes/no) | 0.68 | 0.17-2.5 | .57 |
| CAR-T construct (axi-cel/tisa-cel) | 1.75 | 0.80-4.2 | .18 |
| Days between apheresis and CAR-T∗ | 1.02 | 0.99-1.06 | .34 |
| Patients with CAR-T failure . | |||
|---|---|---|---|
| Variable . | HR . | 95% CI . | P value . |
| Age, y∗ | 1.02 | 0.97-1.06 | .50 |
| Female sex (yes/no) | 0.63 | 0.19-1.9 | .43 |
| IPI > 1 (yes/no) | 1.42 | 0.75-2.8 | .29 |
| Stage at diagnosis† | 1.25 | 0.72-2.2 | .42 |
| Non-GCB cell of origin (yes/no) | 2.94 | 0.97-10.1 | .07 |
| Elevated LDH at apheresis (yes/no) | 0.63 | 0.18-2.2 | .47 |
| >2 lines of therapy pre–CAR-T (yes/no) | 1.89 | 1.1-3.5 | .03 |
| Double hit/double expressor status (DH/DE/other) | 0.42 | 0.18-0.89 | .03 |
| CNS involvement (yes/no) | 0.18 | 0.02-1.5 | .11 |
| Prior autologous HCT (yes/no) | 0.70 | 0.2-2.6 | .58 |
| Extranodal disease at apheresis (yes/no) | 0.68 | 0.17-2.5 | .57 |
| CAR-T construct (axi-cel/tisa-cel) | 1.75 | 0.80-4.2 | .18 |
| Days between apheresis and CAR-T∗ | 1.02 | 0.99-1.06 | .34 |
| Patients receiving first-line therapy after CAR-T failure . | |||
|---|---|---|---|
| Variable (yes/no) . | HR . | 95% CI . | P value . |
| Chemotherapy | 1.4 | 0.15-33.9 | .78 |
| Radiation +/− steroids | 0.21 | 0.028-1.15 | .11 |
| Lenalidomide-based | 0.15 | 0.026-0.76 | .03 |
| Polatuzumab + bendamustine + rituximab | 0.097 | 0.013-0.57 | .01 |
| Checkpoint inhibitor-based | 0.26 | 0.03-2.2 | .20 |
| Patients receiving first-line therapy after CAR-T failure . | |||
|---|---|---|---|
| Variable (yes/no) . | HR . | 95% CI . | P value . |
| Chemotherapy | 1.4 | 0.15-33.9 | .78 |
| Radiation +/− steroids | 0.21 | 0.028-1.15 | .11 |
| Lenalidomide-based | 0.15 | 0.026-0.76 | .03 |
| Polatuzumab + bendamustine + rituximab | 0.097 | 0.013-0.57 | .01 |
| Checkpoint inhibitor-based | 0.26 | 0.03-2.2 | .20 |
Multivariate modeling using Cox regression was constructed using demographic and clinical variables to determine the impact of variables on survival outcomes. Significant P values are shown in boldface.
BTK, Bruton tyrosine kinase; DE, double expressor; DH, double hit.
Linear.
Ordinal.
Axi-cel vs tisa-cel only was included in the MVA owing to small sample size of liso-cel.
Two the 34 patients who received RT as bridging received steroids concurrently with their radiation.
Post–CAR-T failure practice patterns
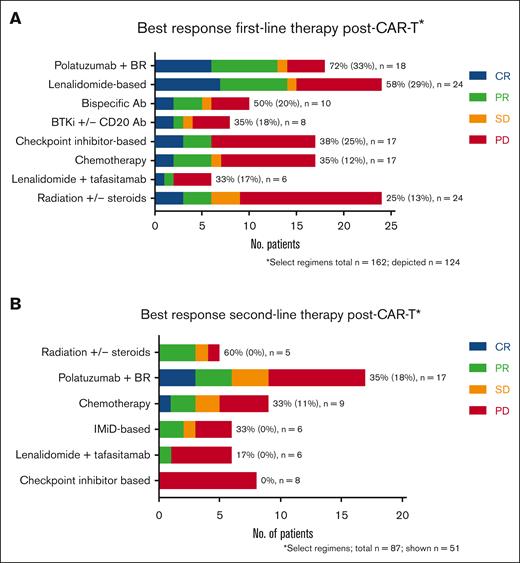
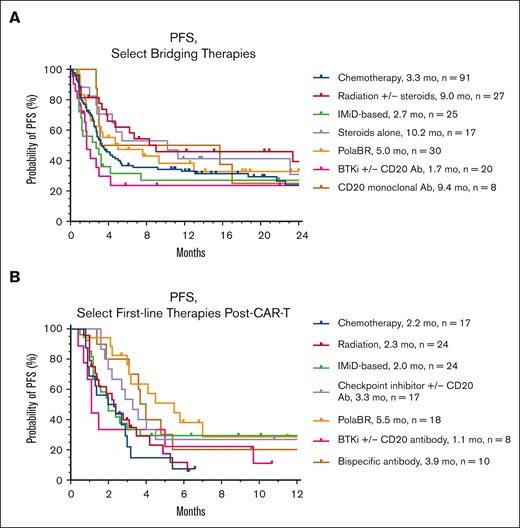
One-hundred sixty-seven patients (59%) received further treatment following CAR-T cell failure (162 evaluable responses). The most common treatment regimens in first-line post–CAR-T failure were radiation +/− steroids (n = 24, 15%), lenalidomide-based (n = 24, 15%), pola + BR (n = 18, 11%), checkpoint inhibitors +/− anti-CD20 antibodies (n = 17, 10%), and chemotherapy (n = 17, 10%). Thirty patients (18%) received first-line post–CAR-T treatment as part of a clinical trial. The details regarding the specific first-line treatment regimens after CAR-T failure are described in supplemental Table 6. Three patients received allogeneic HCT following CAR-T failure without intervening therapies (all had PD at the time of transplant). Overall and CR rates were highest with pola + BR (72% and 33%, respectively), lenalidomide-based regimens (58% and 29%), bispecific antibodies (50% and 20%), and ibrutinib +/− rituximab (38% and 25%, respectively) (Figure 2). For patients who received checkpoint inhibitors, chemotherapy, or lenalidomide + tafasitamab, the response rates ranged from 33% to 35% (Figure 2). PFS for first-line treatment regimens was highest for pola + BR (5.5 months), followed by bispecific antibodies (3.9 months), and checkpoint inhibitors (3.3 months) (Figure 3). Eighty-seven patients received second-line treatment following CAR-T failure. Overall, the response rates were lower with second-line therapy than with first-line therapy (Figure 2). The overall and CR rates for the most common second-line treatment regimens were radiation (60% and 0%, respectively), pola + BR (35% and 18%), chemotherapy (33% and 11%), lenalidomide-based regimens (17% and 0%), and checkpoint inhibitors (0% and 0%).
Best response to first- and second-line treatment regimens given after CAR-T failure. Regimens are listed in order of descending response. To right of each regimen in the ORR% (CR%). Lenalidomide-based regimens were analyzed separately from tafasitamab + lenalidomide. Lenalidomide-based regimens included either lenalidomide alone (6) or lenalidomide plus the following: rituximab (6), rituximab + radiation (1), rituximab + steroids (1), rituximab + intrathecal chemotherapy (1), intrathecal chemotherapy (1), steroids (1), venetoclax (1), obinutuzumab (2), and obinutuzumab + venetoclax (4) (supplemental Table 6). Ab, antibody; BR, bendamustine + rituximab; No., number; n, number; PR, partial response.
Best response to first- and second-line treatment regimens given after CAR-T failure. Regimens are listed in order of descending response. To right of each regimen in the ORR% (CR%). Lenalidomide-based regimens were analyzed separately from tafasitamab + lenalidomide. Lenalidomide-based regimens included either lenalidomide alone (6) or lenalidomide plus the following: rituximab (6), rituximab + radiation (1), rituximab + steroids (1), rituximab + intrathecal chemotherapy (1), intrathecal chemotherapy (1), steroids (1), venetoclax (1), obinutuzumab (2), and obinutuzumab + venetoclax (4) (supplemental Table 6). Ab, antibody; BR, bendamustine + rituximab; No., number; n, number; PR, partial response.
PFS of select bridging therapies and select first-line therapies after CAR-T failure. Median PFS is depicted to the right of each individual regimen. Ab, antibody; PolaBR, polatuzumab + bendamustine + rituximab.
PFS of select bridging therapies and select first-line therapies after CAR-T failure. Median PFS is depicted to the right of each individual regimen. Ab, antibody; PolaBR, polatuzumab + bendamustine + rituximab.
The time from CAR-T infusion to relapse/progression was longer in patients who received subsequent therapy than in those who received no therapy post–CAR-T failure (median 92 vs 62 days; P = .0002). Among patients receiving therapy post–CAR-T failure, either as the standard of care or in clinical trials, the time from CAR-T infusion to relapse/progression was similar (median 91 vs 94 days respectively; P = .5).
CAR-T patients with refractory disease at day 30
When evaluating patients with CAR-T failure with refractory disease (SD/PD) on day 30 assessment (n = 124, 44% of patients with CAR-T failure), only 47% (58/124) continued to receive further therapies vs 68% (109/160) of other CAR-T failure patients (P = .0004). A clinical trial as a first-line treatment after CAR-T was utilized in 14% (8/58) of patients with day 30 SD/PD vs 20% (22/109) of the remaining CAR-T failure patients (P = .4). Median OS from time of progression for patients with day 30 SD/PD vs other CAR-T failure patients was 2.9 months vs 8.0 months (P < .01, Figure 1F).
Allogeneic HCT post–CAR-T failure
Allogeneic HCT was performed in 16 patients (6%) who experienced CAR-T failure (supplemental Table 7). From the time of transplant, median PFS and OS were 14.3 and 16.2 months respectively, and estimated 1-year PFS and OS were 52% and 66% respectively (supplemental Figure 2). The median age at the time of transplant was 59.5 (range 22-68), 11 patients (69%) had extranodal disease, and none had CNS disease at the time of CAR-T. The median lines of therapy between CAR-T and allogeneic HCT were 1 (range, 0-4) and 7 patients (44%) were in CR at the time of transplant. One patient received RT, and the remainder received either polatuzumab-, lenalidomide-, or checkpoint inhibitor-based regimens (n = 6) to achieve CR before allogeneic HCT (supplemental Table 7). With a median follow-up of 25.3 months (range, 9.5-35.2), 9 patients (56%) remained alive and progression-free at the last follow-up, and 2 are alive following progression. Five patients died during the last follow-up (cause of death: PD, n = 4; sepsis, n = 1). Of the 6 patients who progressed following transplant, none were in CR at the time of transplant. Six of the 7 patients who were in CR at the time of allogeneic HCT remained in ongoing CR with a median follow-up of 24 months (range, 17-34).
Predictors of survival outcomes after CAR-T failure
On UVA, inferior PFS was demonstrated in patients who received chemotherapy as first-line treatment post–CAR-T failure compared with nonchemotherapy regimens (2.2 vs 2.9 months; P = .047; Figure 3). There were no other statistically significant clinical predictors of inferior PFS on UVA after CAR-T failure.
On MVA, patients receiving >2 lines of therapy pre–CAR-T (HR, 1.89; 95% CI, 1.1-3.5; P = .03) had worse PFS. Patients without a double hit or double expressor disease (HR, 0.42; 95% CI, 0.18-0.89; P = .03) had superior PFS. When the 5 most common first-line treatment regimens post–CAR-T (range, 17-24 patients with each regimen) were compared, patients receiving either pola + BR or lenalidomide-based regimens as first-line therapy after CAR-T had better PFS (pola + BR HR, 0.097; 95% CI, 0.013-0.57; P = .01; lenalidomide-based HR, 0.15; 95% CI, 0.026-0.76; P = .03, respectively; Table 3). The characteristics of patients receiving first-line pola + BR or lenalidomide-based regimens post–CAR-T failure were similar to those of patients receiving other first-line treatments. Patients receiving pola + BR or lenalidomide-based regimens had similar response rates to CAR-T therapy (51% vs 60%; P = .3, respectively), time from CAR-T infusion to the start of subsequent therapy (median 105 vs 112 days; P = .2), incidence of moderate-severe neutropenia at the time of relapse (10% vs 10%; P = .99), rates of elevated LDH at apheresis (49% vs 57%; P = .4), and utilization rates of BT (53% vs 63%; P = .3).
Discussion
In this large, multicenter retrospective analysis of patients with aggressive B-NHL receiving CD19-directed CAR-T, the median PFS and OS for patients receiving CAR-T (7.6 and 25.6 months) were similar to those previously reported.2,4,9,17 Median OS from time of post–CAR-T progression in this analysis was just 5.5 months, similar to the previously reported OS of 5.3 to 5.9 months in smaller analyses.10,11
This analysis showed that a greater number of lines of therapy pre–CAR-T apheresis were associated with inferior PFS and OS for all CAR-T patients, and inferior PFS in patients experiencing CAR-T failure. This was not reported in smaller real-world analyses of CAR-T outcomes.2,4 Receipt of multiple lines of therapy pre–CAR-T apheresis could partly be reflective of a more aggressive disease phenotype that requires more lines of therapy to achieve enough disease control to safely receive CAR-T. However, it is also likely that these highly refractory patients would benefit from proceeding to CAR-T in earlier lines to limit the cumulative toxicities of systemic therapies and affect T-cell fitness. The ZUMA-7 trial, where patients received only 1 prior line of chemotherapy, was highly enriched for patients with highly aggressive and refractory disease biology, and the 24-month event-free survival was 41%, similar to CAR-T outcomes in third and later line trials,18 with quality of life outcomes favoring second-line CAR-T over the standard of care.19
Our analysis reaffirmed the negative association between BT and survival outcomes described in previous studies.2,4,20 Although there were no differences in PFS between the various bridging regimens on UVA, those receiving chemotherapy or IMiD-based BT appeared to have inferior PFS on MVA. Given the small numbers of patients receiving individual bridging regimens, the significance of this finding is unclear but warrants a prospective study. The negative association between BT and survival is likely driven, in part, by selection and site bias. Many patients may have received BT because the treating provider perceived their disease to be more active or aggressive. Furthermore, patients receiving BT with steroids alone or radiation may have had less active or aggressive disease than those receiving chemotherapy-based BT, which may have also impacted survival outcomes. Site bias may have also played a role, because at some centers, BT is standard for most patients irrespective of disease burden or clinical risk. Finally, patients receiving BT had a longer median time from apheresis to infusion, which may have also affected the outcomes.
Given the dismal outcomes for patients experiencing CAR-T failure, it is crucial to design clinical trials targeting these patients. It is important to recognize that patients with SD/PD at day 30 represent a significant proportion of patients with CAR-T failure (44% in our cohort). Trials should focus on enriching this patient population with more liberal inclusion criteria, accounting for anticipated cytopenias and poor functional status related to previous therapy and active disease. Current clinical trial designs often enriched for less refractory patients or mandate prolonged washout from cell therapies. In our cohort, patients with refractory disease at day 30 were significantly less likely to receive therapy after CAR-T, had low rates of clinical trial enrollment, and had worse OS than other patients experiencing CAR-T failure. Given that patients with early progression/relapse were less likely to receive standard care therapy, this highlights the need for more research into consolidation and/or maintenance strategies immediately post–CAR-T, especially for patients at the highest risk of relapse, such as those with high tumor bulk, aggressive disease requiring BT, or poor in-vivo CAR-T expansion.
In the first-line treatment following CAR-T relapse/progression, response rates were highest for pola + BR, lenalidomide-based regimens, and bispecific antibodies at ≥50% compared with 35% for chemotherapy. Response rates for checkpoint inhibitors in first-line treatment were similar to or slightly better than those reported in the literature in the post–CAR-T failure setting at 38%,21,22 although this finding is limited by a lack of correlative data on CAR-T persistence and markers of exhaustion. On MVA, pola + BR and lenalidomide-based treatment in the first-line post–CAR-T were associated with an improvement in PFS. These data suggest that outside of a clinical trial, a polatuzumab- or lenalidomide-based regimen may be preferable to standard chemotherapy regimens in the first-line post–CAR-T failure, although this finding is limited by the potential for selection bias, the small sample size in individual treatment groups, and lack of data on PS or disease bulk at the time of CAR-T relapse. The nonrandomized nature of the assignment of treatment regimens significantly limits the interpretation of the MVA. However, these data are the first to provide physicians with guidance on treatments that are feasible and may lead to better outcomes post–CAR-T failure. Prospective randomized trials targeting patients immediately post–CAR-T progression/relapse are severely lacking and are urgently needed, to guide patient care. Until such time, physicians must rely on observational data and single-arm clinical trials, which often enrich patients with less aggressive disease biology. To specifically inform treatment decisions in the immediate post–CAR-T failure setting, survival analyses were limited to specific treatment regimens utilized in first-line post–CAR-T failure. Other studies that have examined treatment regimens utilizing post–CAR-T failure have taken different approaches, grouping therapies given across multiple lines post–CAR-T failure, and grouping some treatment regimens together.23 For example, 1 study evaluated the use of polatuzumab vedotin-based regimens (polatuzumab vedotin alone and pola + BR) in the post–CAR-T failure setting and reported an ORR of 44% (CR, 14%) with a median PFS of 10 weeks, which is lower than the reported response rates and PFS in this analysis. However, in the aforementioned study, only 60% of patients received polatuzumab vedotin-based regimens as the first-line treatment, and ∼40% of patients had bendamustine omitted from their treatment regimen. The reason for the disparate findings of inferior PFS with IMiD-based BT and superior PFS with lenalidomide-based regimens as a first-line treatment after CAR-T warrants further study. However, preclinical data suggest enhanced antitumor function when lenalidomide is administered along with CAR-T-cell therapy.24,25 Moreover, in high-risk patients requiring BT, it is possible that lenalidomide may be less efficacious or slower to work than other therapies, although this requires validation in a larger, prospective series.
Owing to a limited sample size, this analysis could not explore the role of CD19-directed antibodies, such as loncastuximab tesirine or tafasitamab, as a first-line treatment after CAR-T failure. However, among the 13 patients in the LOTIS-2 trial who experienced CAR-T failure before receiving loncastuximab, the ORR was 46%,8 suggesting that it may play a role in patients who retain CD19 expression. The rates of utilization of novel regimens pre–CAR-T apheresis regimens were low, with only 3% of patients receiving polatuzumab preapheresis. Given the improvement in PFS with the addition of polatuzumab to front-line treatment for DLBCL,26 many patients may soon be receiving polatuzumab as front-line treatment. The optimal way to manage post–CAR-T failure in these patients needs to be explored.
Allogeneic HCT is a potentially curative modality in selected patients with R/R aggressive B-NHL.27,28 However, until recently, there was a lack of knowledge on how often allogeneic HCT is utilized following CAR-T failure and on the efficacy of this approach.29,30 In our cohort, allogeneic HCT was utilized in only 10% of patients (16 of 167) who received treatment post–CAR-T failure. Over half of these patients remain alive and in an ongoing response at the last follow-up, showing that allogeneic HCT still has a role in selecting patients who can achieve adequate disease control after CAR-T failure. This is similar to a recent analysis reporting a 1-year PFS of 45% in 88 patients receiving allogeneic HCT post–CAR-T failure.30 Outcomes following allogeneic HCT post–CAR-T failure appear similar to allogeneic HCT outcomes in the pre–CAR-T era, suggesting curative potential after CAR-T failure.27 Nonetheless, our analysis was limited by the small sample size and lacked details regarding the conditioning regimen, graft type, type of donor, and incidence of graft-versus-host disease, making direct comparisons difficult. Six of 7 patients in CR at the time of allogeneic HCT remain in CR with at least 17 months of follow-up, reaffirming that disease control before transplant is important for long-term outcomes.27,28
There were several other limitations to this analysis. The protocol did not mandate a uniform toxicity grading systems from institution to institution, and the grading may have differed between institutions. There was a variable amount of missing data for several important clinical variables, including LDH and ECOG PS, at apheresis. The number of treatment cycles was not recorded for patients receiving BT, nor was the response to BT. Finally, response assessments were collected retrospectively by individual site investigators and did not undergo a centralized review. Nonetheless, there are currently little data on the feasibility and effectiveness of therapies after CAR-T failure. These data provide valuable guidance for treating clinicians and investigators designing clinical trials to improve outcomes for post–CAR-T failure patients, a population with unacceptably poor outcomes.
In conclusion, a greater number of lines of pre–CAR-T therapy and BT were predictive of inferior PFS and OS for aggressive B-cell lymphoma patients receiving CAR-T therapy. More than 2 lines of therapy, pre–CAR-T apheresis, were predictive of inferior PFS in patients experiencing CAR-T failure. Patients with refractory disease on day 30 had inferior OS and were less likely to receive treatment after CAR-T than patients with CAR-T failure. The median PFS of first-line treatment regimens administered after CAR-T was dismal at just 2.8 months. The median OS after CAR-T progression was just 5.5 months. These data provide a benchmark for future clinical trials in patients with disease progression after CD19-directed CAR-T therapy, which is an unmet clinical need.
Authorship
Contribution: R.K., J.Z., I.N., and I.R. conceived and designed the study and wrote the manuscript; J.Z. and I.R. completed the statistical analysis; and all authors collected clinical data, interpreted the data, approved the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: N.E. reports: advisory board: Pharmacyclics, BeiGene, and TG Therapeutics; consultancy: Novartis; speakers bureau: Incyte. J.B.C. reports: consultancy: Janssen, Adicet, AstraZeneca, Genentech, Aptitude Health, Cellectar, Kite/Gilead, Loxo, BeiGene, and Adaptive; research funding: Genentech, Bristol Myers Squibb (BMS)/Celgene, LAM, BioINvent, LOXO, AstraZeneca, Novartis, M2Gen, and Takeda. T.K.M. reports: advisory board: Seattle Genetics. B.H. reports: consultancy: ADC Therapeutics; speakers bureau: BMS. A.S.K. reports: consultancy: AbbVie, BeiGene, BMS, and Janssen. S.M. reports: honoraria and research funding: AbbVie; research funding and speakers bureau: Beigene, Janssen, and Pharmacyclics; research funding: Loxo, Juno Therapeutics, and TG Therapeutics; honoraria, research funding, and speakers bureau: AstraZeneca; J.W. reports: consultancy: Gilead, Other, Husband; consultancy: Janssen, Other, Husband; Data and Safety Monitoring Board: Ariad/Takeda, Other, Husband; Data and Safety Monitoring Board: Epizyme, Other, Husband; consultancy: Agios, Other, Husband; consultancy: Actinium Pharma; Data and Safety Monitoring Board: BMS, Other, Husband; consultancy, honoraria, and research funding: Merck; consultancy, data and safety monitoring board: Novartis, Other, Husband; honoraria: Karyopharm (Curio Science). L.I.G. reports: consultant: BMS; advisory board: Kite Pharma; co-founder: Zylem Therapeutics Inc. A.D. has received consulting fees from AstraZeneca, AbbVie, BeiGene, Genentech, TG Therapeutics, Bayer Oncology and Pharmacyclics, and has ongoing research funding from AstraZeneca, Takeda Oncology, Bayer Oncology, Genentech, SecuraBio, MEI, TG Therapeutics, and BMS. D.S. reports: membership on an entity’s board of directors or advisory committees: Adaptive, TG Therapeutics, BeiGene, Epizyme, and Innate Pharma; research funding: Juno Therapeutics, Mingsight, Novartis, and Arqule; consultancy: CSL Behring, AbbVie, AstraZeneca, and Celgene; membership on an entity’s board of directors or advisory committees and research funding: Karyopharm. N.N.S. reports participation on advisory boards and/or consultancy for Kite Pharma, TG Therapeutics, Miltenyi Biotec, Lilly, Epizyme, Legend, Incyte, Novartis, and Umoja; and speakers bureau with Incyte. He has research funding and honoraria from both Lilly and Miltenyi Biotec. V.K. reports research funding from Novartis, MEI, and Celgene/BMS. S.K.B. reports: honoraria: Acrotech, Daiichi Sankyo, Seagen, and Kyowa Kirin. P.T. reports: advisory board: ADC Therapeutics, Genentech, and TG Therapeutics. G.S. reports: speaker for Kite Pharma; and honoraria from BeiGene. R.K. reports: advisory board: Celgene Corporation, Gilead Sciences, Juno Therapeutics, Kite Pharma, Janssen, Karyopharm, Pharmacyclics, MorphoSys, Epizyme, Genentech/Roche, EUSA; grants/research support: Celgene Corporation, Juno Therapeutics, BMS, Takeda, BeiGene, Gilead Sciences, and Kite Pharma; speakers bureau: AstraZeneca, BeiGene, Gilead Sciences, and MorphoSys. The remaining authors declare no competing financial interests.
Correspondence: Reem Karmali, Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, 676 N St Clair St, Suite 850, Chicago, IL 60611; e-mail: reem.karmali@northwestern.edu.
References
Author notes
Data are available on request from the corresponding author, Reem Karmali (reem.karmali@northwestern.edu).
The full-text version of this article contains a data supplement.