TO THE EDITOR:
Acute promyelocytic leukemia (APL) is a unique subtype of acute myeloid leukemia (AML) characterized by t(15;17) translocation, PML::RARA rearrangement, a high risk of fatal hemorrhage, and sensitivity to anthracyclines, all-trans retinoic acid (ATRA) and arsenic-based therapy.1 The white blood cell (WBC) count at presentation is the most important predictor of failure due to early death (ED) or relapse2 and is used to stratify patients into standard-risk (WBC ≤ 10×109/L) and high-risk (WBC > 10 × 109/L) subgroups.3
Almost 100% of patients with APL classified as standard-risk1 are cured when treated with ATRA and arsenic trioxide (ATO); of 77 evaluable patients, 100% achieved complete remission, and 97% were event-free at 2 years.4 Although the optimal management of patients with high-risk disease has not been conclusively established,1 an overall survival (OS) of 85%-95% can be expected with the combination of ATRA, ATO, and limited anthracycline or gemtuzumab ozogamicin, and this strategy minimizes the complications of regimens based on more intensive chemotherapy without ATO.5-8 In contrast, little is known about the rare occurrence of extreme hyperleukocytosis at presentation, defined here as an initial WBC count of ≥100×109/L. For example, extreme hyperleukocytosis was present in only 27 (1.5%) of 1757 patients in the APL93, APL2000, and APL2012 trials.8,9 To gain insight into this phenomenon, data were sought from hematologists in Australia, United Kingdom, and North America, regardless of management, clinical trial participation, or outcome. The inclusion of patients who succumbed before the initiation of therapy was also encouraged to minimize the selection bias that inevitably accompanies referral to a major leukemia center. The protocol was approved by the Human Research & Ethics Committee of the Sydney Local Health District and conducted according to the Declaration of Helsinki. Submitted data were deposited and managed using research electronic data capture tools.10
Data for 37 patients with extreme hyperleukocytosis (median WBC count, 124×109/L; range, 96.7-297.0) who presented between May 1996 and July 2020 were collected retrospectively from 17 institutions in 5 countries. Because the data spanned a 25-year period, determinations of hematological complete remission (hCR), molecular complete remission (mCR), and relapse status assigned by local institutions were accepted without further interrogation. Analysis was performed in January 2022 and an arbitrary landmark was established at 10 years before the analysis to segregate patients into early and late era cohorts as a surrogate for the intensity of hemostatic supportive care.
The end points included (i) ED, death within 30 days of presentation; (ii) relapse-free survival (RFS), time from achieving hCR to molecular or hematological relapse; (iii) disease-free survival (DFS), time from achieving hCR to death, relapse or development of therapy-related myelodysplasia/acute myeloid leukemia (t-MDS/AML); and (iv) OS, time from presentation to death. RFS, DFS, and OS curves were estimated using the Kaplan-Meier product limit method. Patients were stratified according to (i) presentation in the early vs late era, (ii) treatment paradigm (± inclusion of ATO in induction and/or consolidation), (iii) differentiation syndrome (DS) prophylaxis (± corticosteroids), and (iv) age using a cut point of 47 years. Time-to-event outcomes were compared with the log-rank test (SAS Version 9.4), and the age cut point was selected by classification and regression tree analysis (rpart function of R v4.03).
The demographics, clinical, and laboratory characteristics are summarized in Table 1. Cytogenetic and/or molecular confirmation of APL was available for 35 patients; an additional 2 patients were diagnosed by morphological and clinical criteria. A total of 33 (94%) of 35 patients for whom information was available had features of coagulopathy at presentation (clinical bleeding, hypofibrinogenemia, and/or elevated D-dimers). Eight (24%) of 34 patients had thrombotic manifestations compared with only 3 (10%) of 31 patients with APL in an Italian observational cohort study.11 There was no evidence that patients with extreme hyperleukocytosis were more likely to harbor additional karyotypic abnormalities compared with other patients with APL, where the incidence is frequently reported in the range of 30%.12-14 The majority of the current cohort for which karyotypic data were available had just t(15;17), whereas 1 patient had a normal karyotype, and only 10% had additional abnormalities. The skewed distribution of PML breakpoints (bcr3 in 66% of 29 patients) and the preponderance of FLT3-ITD (present in 73% of 15 patients) reflected the well-known association of bcr3, FLT3-ITD, and hyperleukocytosis.12,15
Demographics, clinical characteristics, and treatment components of patients with extreme hyperleukocytosis at presentation
| Characteristic . | Number of patients (%) . | Median (range) . |
|---|---|---|
| Total cohort | 37 (100) | |
| Age (y) | 37 (100) | 43 (1-73) |
| Sex | ||
| Male | 16 (43) | |
| Female | 21 (57) | |
| WBC count (×109/L) | 37 (100) | 124.0 (96.7-297.0) |
| Platelet count (×109/L) | 34 (92) | 33 (5-92) |
| Hemoglobin (g/L) | 34 (92) | 100 (36-142) |
| Fibrinogen (g/L) | 34 (92) | 1.3 (0.2-5.4) |
| D-dimers (mg/L)∗ | 22 (59) | 8.0 (>0.08-74.8) |
| Hemorrhagic manifestations | ||
| Yes† | 26 (70) | |
| No | 9 (24) | |
| Unknown | 2 (5) | |
| Thrombotic manifestations | ||
| Yes‡ | 8 (22) | |
| No | 26 (70) | |
| Unknown | 3 (8) | |
| Karyotype | ||
| t(15;17) only | 25 (68) | |
| t(15;17) + additional abnormalities | 3 (8) | |
| FISH only | 1 (3) | |
| Normal | 1 (3) | |
| Unknown | 7 (19) | |
| PML breakpoint | ||
| bcr1/2 | 10 (27) | |
| bcr3 | 19 (51) | |
| Unknown | 8 (22) | |
| FLT3 ITD | ||
| Yes | 11 (30) | |
| No | 4 (11) | |
| Unknown | 22 (59) | |
| FLT3 TKD | ||
| Yes | 1 (3) | |
| No | 12 (32) | |
| Unknown | 24 (65) | |
| Presentation | ||
| Early (>10 years before analysis) | 15 (41) | |
| Late (<10 years before analysis) | 22 (59) | |
| Induction | ||
| ATRA + anthracycline + cytarabine and/or hydroxyurea | 13 (35) | |
| ATRA + anthracycline | 12 (32) | |
| ATRA + anthracycline + ATO + cytarabine and/or hydroxyurea | 7 (19) | |
| ATRA + anthracycline + ATO | 4 (11) | |
| ATRA + hydroxyurea | 1 (3) | |
| Consolidation | ||
| ATRA + chemotherapy | 14 (50) | |
| ATRA + ATO | 13 (46) | |
| ATRA + ATO + chemotherapy | 1 (4) | |
| Maintenance | ||
| ATRA + 6MP + MTX | 12 (100) | |
| ATRA + ATO | 0 (0) |
| Characteristic . | Number of patients (%) . | Median (range) . |
|---|---|---|
| Total cohort | 37 (100) | |
| Age (y) | 37 (100) | 43 (1-73) |
| Sex | ||
| Male | 16 (43) | |
| Female | 21 (57) | |
| WBC count (×109/L) | 37 (100) | 124.0 (96.7-297.0) |
| Platelet count (×109/L) | 34 (92) | 33 (5-92) |
| Hemoglobin (g/L) | 34 (92) | 100 (36-142) |
| Fibrinogen (g/L) | 34 (92) | 1.3 (0.2-5.4) |
| D-dimers (mg/L)∗ | 22 (59) | 8.0 (>0.08-74.8) |
| Hemorrhagic manifestations | ||
| Yes† | 26 (70) | |
| No | 9 (24) | |
| Unknown | 2 (5) | |
| Thrombotic manifestations | ||
| Yes‡ | 8 (22) | |
| No | 26 (70) | |
| Unknown | 3 (8) | |
| Karyotype | ||
| t(15;17) only | 25 (68) | |
| t(15;17) + additional abnormalities | 3 (8) | |
| FISH only | 1 (3) | |
| Normal | 1 (3) | |
| Unknown | 7 (19) | |
| PML breakpoint | ||
| bcr1/2 | 10 (27) | |
| bcr3 | 19 (51) | |
| Unknown | 8 (22) | |
| FLT3 ITD | ||
| Yes | 11 (30) | |
| No | 4 (11) | |
| Unknown | 22 (59) | |
| FLT3 TKD | ||
| Yes | 1 (3) | |
| No | 12 (32) | |
| Unknown | 24 (65) | |
| Presentation | ||
| Early (>10 years before analysis) | 15 (41) | |
| Late (<10 years before analysis) | 22 (59) | |
| Induction | ||
| ATRA + anthracycline + cytarabine and/or hydroxyurea | 13 (35) | |
| ATRA + anthracycline | 12 (32) | |
| ATRA + anthracycline + ATO + cytarabine and/or hydroxyurea | 7 (19) | |
| ATRA + anthracycline + ATO | 4 (11) | |
| ATRA + hydroxyurea | 1 (3) | |
| Consolidation | ||
| ATRA + chemotherapy | 14 (50) | |
| ATRA + ATO | 13 (46) | |
| ATRA + ATO + chemotherapy | 1 (4) | |
| Maintenance | ||
| ATRA + 6MP + MTX | 12 (100) | |
| ATRA + ATO | 0 (0) |
ATO, arsenic trioxide; ATRA, all-trans retinoic acid; FISH, fluorescence in situ hybridization; ITD, internal tandem duplication; MTX, methotrexate; TKD, tyrosine kinase domain mutation; 6MP, 6-mercaptopurine.
The median D-dimer is an approximation only and can be regarded as a minimum figure because some laboratories quoted results as greater than a particular threshold rather than providing a specific figure.
Sites of bleeding were intracranial (10, including intracerebral, subdural and subarachnoid), cutaneous (9, including bruising and bleeding from puncture sites), mucosal (7, including epistaxis, gastrointestinal, and urinary tract), intraocular or peri-orbital bleeding (3), intrapulmonary (1), and site not specified (1).
Sites of thromboses included intracerebral (3), deep venous thrombosis (1), superficial venous thrombosis (1), hepatic vein (1), pulmonary embolism (2), subclavian artery (1), and digital necrosis (1).
The median follow-up (reverse Kaplan-Meier method) was 4.5 years (0.6-13.2 years). All patients were commenced on ATRA-based treatment (Table 1), including an obtunded patient with a fronto-parietal intracerebral hemorrhage and midline shift. Leukapheresis was performed in only 3 patients and central nervous system prophylaxis was administered in 6 patients. Twenty-four of 36 patients received corticosteroid prophylaxis. Fifteen patients (41%) received ATO during induction and/or consolidation. ED occurred in 7 patients (19%); causes included progressive intracerebral bleeding (n = 3, days 1, 2, 7), multiterritorial stroke with concurrent subarachnoid bleeding and pulmonary embolism (n = 1, day 3), multifocal cerebral infarction with progressive intracerebral bleeding (n = 1, day 6), sepsis with bleeding (n = 1, day 16), and respiratory failure with DS (n = 1, day 22). Although we regard early initiation of ATRA as desirable,1 the limited data available do not suggest that delayed initiation was a major contributory factor in the early hemorrhagic deaths. An eighth patient died from aspergillus pneumonia and adult respiratory distress syndrome (day 46). The remaining 29 (78%) patients achieved hCR and 26 achieved mCR; no data were available for 3 patients. Most patients experienced one or more grade 3 to 5 complications during induction, most commonly sepsis, DS, respiratory failure, and intracranial hemorrhage (supplemental Table 1).
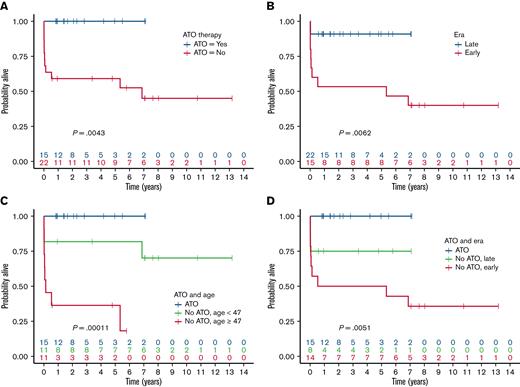
Outcomes at 5 years with 95% confidence intervals were 76% (58%-87%) for OS and 81% (55%-93%) for both DFS and RFS. When stratified by whether therapy included ATO (supplemental Table 2), there was a statistically significant difference in OS favoring ATO treatment (P = .0043; Figure 1A), and a similar difference was also evident when patients were stratified by late vs early era (P = .0062; Figure 1B). ATO and late era presentation were tightly correlated (Pearson’s r = 0.57, P < .001) because frontline use of ATO in high-risk patients with APL was only approved in Australia in 2015 and is still not formally approved for this indication in the United States or United Kingdom. OS was also associated with age, especially for patients who did not receive ATO (Figure 1C) but not with corticosteroid prophylaxis (supplemental Table 2). The classification and regression tree analysis importance measures confirmed treatment paradigm, then age (dichotomized), and then era of presentation as the binary factors most closely associated with OS (noting also the indicative P-values in Figure 1C,D).
OS of patients with APL with extreme hyperleukocytosis. Survival stratified by (A) ATO-based therapy, (B) early vs late era, (C) ATO-based therapy and age, (D) ATO-based therapy and early vs late era.
OS of patients with APL with extreme hyperleukocytosis. Survival stratified by (A) ATO-based therapy, (B) early vs late era, (C) ATO-based therapy and age, (D) ATO-based therapy and early vs late era.
The impact of the era of presentation on OS was primarily due to differences in the ED rate (Figure 1B), reflecting temporal improvements in supportive care, particularly the management of coagulopathy. The ED rate was 33% in the early-era cohort, despite treatment at institutions with considerable experience in the management of APL and consistent with population-based estimates of ED.16-18 Although it has been claimed that the ED rate has not improved with treatment advances,19 the ED rate in the late era was only 9%, similar to the ED rate observed in clinical trials where selection bias, immediate access to ATRA, and aggressive hemostatic support has contributed to much better outcomes.5,20,21 Our experience mirrors that reported by the European APL group in which the ED rate of patients with the WBC count of >50 × 109/L fell from 18.2% in the APL93 trial to 8.6% in the subsequent APL2000 trial.9 However, ATO-based therapy appears to also impact OS directly; within the subgroup of late-era patients, ED was 0% in those who received ATO compared with 25% in those that did not receive ATO. Furthermore, deaths that occurred more than 3 months after initial presentation, when supportive care is no longer of critical importance, were restricted to patients in the early-era cohort who did not receive initial ATO of whom 2 had relapsed APL, and 1 had t-MDS/AML. It is therefore conceivable that the inferior OS experienced by patients with extreme hyperleukocytosis who did not receive frontline ATO-based therapy was attributable to a combination of (i) era-dependent differences in supportive therapy, (ii) lower efficacy of non-ATO regimens, and (iii) the leukemogenic effects of reliance on anthracycline-based chemotherapy.22-25
The European APOLLO trial (#NCT02688140) is currently assessing the effect of ATO-based therapy in high-risk patients with APL but the target accrual of 280 patients suggests that <15 patients with extreme hyperleukocytosis will be enrolled. Therefore, guidance about how to treat this subgroup will likely remain anecdotal rather than evidentiary. Although, to our knowledge, this is the largest study of APL with extreme hyperleukocytosis ever reported, it has several limitations, primarily due to the retrospective design encompassing patients from multiple countries over 25 years during which treatment practices have evolved considerably. Details of supportive therapy were not collected and treatment regimens were only broadly categorized. The small sample size also limits statistical power; nevertheless, some interesting observations have been made about this extremely rare subgroup of patients with APL who present with extreme hyperleukocytosis. At least for those who presented less than 10 years before this analysis, their OS appears comparable with that of the broader subgroup of patients with high-risk APL whose WBC counts exceed 10×109/L (supplemental Table 3). The data reinforce the need for aggressive supportive care and the inclusion of ATO-based therapy.
Acknowledgment: D.C.T. is supported by the National Institute for Health Research Royal Marsden/Institute of Cancer Research Biomedical Research Centre.
Contribution: H.J.I. conceived and designed the study, analyzed the data, and wrote the manuscript; J.R. analyzed the data and wrote the manuscript; and all other authors collected and submitted patient data and edited and critically reviewed the manuscript.
Conflict-of-interest disclosure: H.J.I. served Phebra and Syros in an advisory capacity. A.H.W. served on the advisory boards for Novartis, AstraZeneca, Astellas, Janssen, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, Bristol Myers Squibb (BMS), Shoreline, Macrogenics and Agios; receives research funding (to the institution) from Novartis, AbbVie, Servier, Janssen, BMS, Syndax, Astex, AstraZeneca, Amgen; serves on the speaker's bureaus for AbbVie, Novartis, BMS, Servier, and Astellas; is an employee of the Walter and Eliza Hall Institute; and is eligible for financial benefits associated with payments which the Walter and Eliza Hall Institute receives in relation to venetoclax. J.K.A. serves GlycoMimetics, Kura Oncology, AbbVie, Astellas Pharma, Syros Pharmaceuticals, BioSight, Bluebird Bio, Stemline Therapeutics, and Curio Science in a consulting or advisory role; receives research funding from Astellas Pharma, Pfizer, Agios, BMS, Cyclacel, Celgene, Boehringer Ingelheim, BioSight, Kura Oncology, AbbVie, Amgen, Aprea AB, Amphivena, Fujifilm, Kartos Therapeutics, Aptose Biosciences, ALX Oncology, Immunogen, Kura Oncology, Loxo, and Telios; receives travel and accommodation expenses from BioSight, Astellas Pharma, and Daiichi Sankyo; and has a relationship with NCI, Oncology Learning Network. The remaining authors declare no competing financial interests.
Correspondence: Harry J. Iland, Institute of Haematology, Royal Prince Alfred Hospital, Camperdown, NSW 2050 Australia; e-mail: harryiland@gmail.com.
References
Author notes
Data are available on request from the corresponding author (subject to the approval of the Sydney Local Health District Human Research Ethics Committee), Harry J. Iland (harryiland@gmail.com).
The full-text version of this article contains a data supplement.