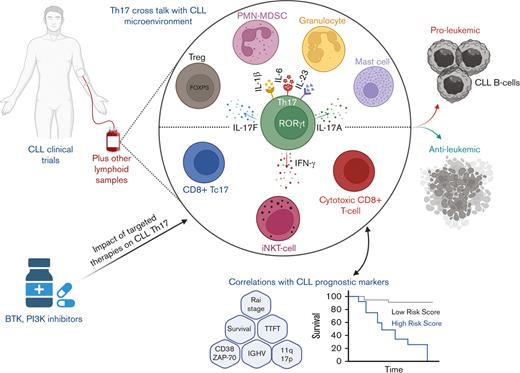
Abstract
T helper 17 (Th17) cells have a prominent role in autoimmune diseases. In contrast, the nature of these cells in cancer is controversial, with either pro- or antitumorigenic activities depending on various cancer settings. Chronic lymphocytic leukemia (CLL), a B-cell malignancy, is characterized by an imbalance in T-cell immune responses that contributes to disease progression and increased mortality. Many clinical reports indicate an increase in Th17 cells and/or interleukin 17 serum cytokine levels in patients with CLL compared with healthy individuals, which correlates with various prognostic markers and significant changes in the tumor microenvironment. The exact mechanisms by which Th17 cells might contribute to CLL progression remain poorly investigated. In this review, we provide an updated presentation of the clinical information related to the significance of Th17 cells in CLL and their interaction with the complex leukemic microenvironment, including various mediators, immune cells, and nonimmune cells. We also address the available data regarding the effects of CLL-targeted therapies on Th17 cells and the potential of using these cells in adoptive cell therapies. Having a sound understanding of the role played by Th17 cells in CLL is crucial for designing novel therapies that can achieve immune homeostasis and maximize clinical benefits.
Introduction
Chronic lymphocytic leukemia (CLL) is a B-cell malignancy that represents the most predominant leukemia in the western world.1,2 Besides genetic lesions, the survival of malignant B cells is supported by a complex cross talk in the microenvironment that includes constitutive B-cell receptor signaling,3 survival cues from T cells, monocyte-derived nurse-like cells, and mesenchymal stromal cells.4-7 Dysfunctions in the T-cell compartment have been well established several decades ago as 1 of the characteristic hallmarks of CLL. As a consequence of this dysfunction, T cells are skewed toward a terminally differentiated phenotype displaying exhaustion features, low proliferation capacity, impaired cytotoxicity,8-11 and a hindered ability to form normal immune synapses with CLL B cells.12-14 This compromise in the CLL T-cell immune response is mainly responsible for the secondary malignancies and infections, which account for the increased morbidity and mortality in this disease.15,16
Despite this knowledge, there is still debate about whether T cells exert pro- or antileukemic activity in CLL. In contrast to the more defined antileukemic role of CD8+ T cells in CLL,17-22 the role of CD4+ T helper (Th) cells remain controversial. This can be attributed to the plasticity and heterogeneity of Th cell differentiation phenotypes that can play diverse roles in the tumor microenvironment. Th cells are classified according to their function and secreted cytokines into either Th1 cells, Th2 cells, T follicular helper (Tfh) cells, Th17 cells, or regulatory T cells (Tregs).23 Th1 cells promote cell-mediated immune response against intracellular bacteria or viruses through the release of various cytokines such as interferon gamma (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin 2 (IL-2). In contrast, Th2 cells produce IL-4, IL-5, and IL-13 and are required for the humoral immune response against extracellular pathogens. Tfh cells are critical for supporting the survival and proliferation of germinal center B cells, whereas Th17 cells play an important inflammatory role against extracellular bacteria and fungi. The immune stimulatory functions of Th cell subsets are delicately balanced through the immunosuppressive activities of Tregs, which help maintain immune homeostasis and prevent the development of autoimmune diseases. Previous studies have described a predominance of Th2 cell phenotype in peripheral blood mononuclear cells (PBMCs) of patients with CLL, as well as in Eμ-TCL1 model, the well-established transgenic murine model of CLL. This skewing in CD4+ T-cell response has been correlated with abnormalities in the c-JUN N-terminal kinase and MAPK pathway–related gene expression, contributing to CLL progression.20,24-27 In contrast, multiple evidence suggests an increase in IFN-γ–producing Th1 cells in patients with CLL and adoptive transfer Eμ-TCL1 murine model, which might have a potential to induce activation and proliferation of CLL cells.26,28-31
Moreover, Tfh cells were shown to be enriched in the blood and lymph nodes (LNs) of patients with CLL, which supports CLL cell proliferation and disease progression through the secretion of IL-21 cytokine.32-35 Vaca et al have recently demonstrated a significant expansion of activated Tfh cells in CLL nurse-like cell cocultures from a cohort of 28 different patients.36 Tregs are known for their protumorigenic role in different cancer entities through inducing immune suppression and cancer immune escape.37 In CLL, many reports have demonstrated a significant abundance of Tregs in the blood of patients with CLL, which corresponded to worse prognosis and disease progression.38-46 Tregs from patients with CLL were found to have increased expression of immunosuppressive molecules, such as CTLA-4, which was also confirmed in the Eμ-TCL1 murine models.14,41,47-50 In addition, CLL Tregs had more capacity to produce immunosuppressive cytokines, such as IL-10 and transforming growth factor β (TGF-β).41,51
Th17 cells were first identified in 2005 as a unique Th cell lineage distinct from Th1 and Th2.52,53 These cells have a critical role in maintaining immune homeostasis and host protection against extracellular pathogens through promoting inflammatory responses.54-58 Despite the clearly defined role of Th17 cells in promoting autoimmune diseases,59-63 their role in cancer pathogenesis remains controversial.64-72 In CLL, clinical-based evidence suggests an increase in Th17 cell percentages, counts, and/or IL-17 cytokine levels in patients’ peripheral blood compared with healthy donors. This increase in Th17 cell signature is claimed to be associated with significantly improved clinical outcomes;73,74 however, the exact mechanisms are yet unclear. In contrast, autoimmune cytopenia (AIC) is a prevalent complication in patients with CLL, where autoimmune hemolytic anemia affects 5% to 10% of patients and autoimmune thrombocytopenia affects 2% to 5% of patients.75 Whether Th17 cells are involved in these autoimmune disorders associated with CLL remains an open question.
Given the increasing research focus on Th17 cells and their role in cancer-associated immune responses, it is vital to understand the role of these cells in the CLL microenvironment. This knowledge will be important to consider when designing new CLL therapies to harness the immune system effects for improving clinical benefits. Here, we review the current knowledge of the clinical significance of Th17 cells in patients with CLL and the interplay between Th17 cells and other immune cells and cytokines present in the complex CLL microenvironment. Moreover, we briefly discuss the effects of CLL-targeted therapies on Th17 cell levels and the potential of using Th17 cells as an immunotherapeutic tool in CLL. Finally, we conclude with recommendations and directions for future research work.
Th17 cell differentiation and plasticity
Th17 cells predominantly reside in the lamina propria of the small intestine; however, they can be induced at other epithelial and mucosal sites upon pathogenic insult.76 These cells are characterized by the production of IL-17A, IL-17F, IL-21, IL-22, and CCL20, and the expression of CCR6 and the master transcription factor RAR-related orphan receptor γt (RORγt).58,77 Other transcription factors, including STAT3,78 IRF4,79 and AHR80 have been shown to also play an important role in Th17 cell development. Differentiation of naïve Th into Th17 cells is governed by costimulatory signals provided by antigen-presenting cells, as well as the different cytokine combinations available in the microenvironment during antigen recognition. Both CD28- and inducible T-cell costimulator (ICOS)-induced costimulation have shown to be important for Th17 cell generation.53,81 Cytokines including IL-6, IL-1β, and TGF-β, are essential for initiating Th17 cell differentiation, which is maintained through the presence of IL-21 and IL-23.82,83
Th17 cells are characterized by a high degree of plasticity, which enables them to differentiate to other Th phenotypes based on the available microenvironmental cues. Based on this unique nature, there is still great ambiguity regarding the pro- or antitumoral role played by these cells in cancer settings. Th17 cells can be switched to Th1-like cells having the capacity to produce IFN-γ in the absence of TGF-β and in the presence of IL-12 and IL-23.84 Although these cells acquire a Th1-like phenotype, they remain distinguished from typical Th1 cells via the expression of unique markers, such as CCR6, CD161, and IL-17RE.70,85 The existence of Th17/Th1 cells has also been discussed in human tumors, such as ovarian and lung cancers.86,87 From a translational perspective, different studies have shown the potential of using in vitro polarized Th17 cells for adoptive cell therapy. The ability of Th17 cells to neutralize tumors was shown to be greater than that of Th1-polarized cells and was attributed to the capacity of Th17 cells to in vivo acquire a Th17/Th1 phenotype that secretes IFN-γ. Interestingly, Th17 cells were also shown to be able to gain a stem-like durable memory profile in vivo, which maintains the antitumor effects for longer periods of time.81,88-91
In contrast, Th17 cells and Tregs are known to share some aspects of their differentiation programs. TGF-β is critical for Treg differentiation; however, TGF-β promotes Th17 cell differentiation program in the presence of other cytokines such as IL-6 and IL-21.82 It has been previously described that Th17 cells can transdifferentiate into IL-17A− FOXP3+ cells in an allogeneic heart transplantation model.92 Regarding cancer, Downs-Canner et al have shown that suppressive IL-17A+Foxp3+ and ex-Th17 Foxp3+ cells (YFP+ IL-17A− Foxp3+) can be converted from Th17 cells in ovarian and colorectal cancer mouse models using IL-17aCreRosa26eYFP fate reporter mice.93 Furthermore, Ye et al have demonstrated that tumor-infiltrating lymphocytes derived from Th17 clones can be converted into FOXP3+ Tregs with in vitro suppressive functions after stimulation with OKT3 and allogeneic PBMCs.94
In conclusion, Th17 cell plasticity is vastly controlled by the complex nature of the tumor microenvironment, which is decided based on each specific tumor type and the progression stage of the tumor. Therefore, it is of critical importance to take these factors into consideration when investigating the tumor supportive or suppressive roles of Th17 cells.
Prognostic value of Th17 cells in CLL
Several clinical trials have shed some light on the biological levels of Th17 cells in CLL and their correlation with disease progression and prognostic markers. Although the mechanisms are not yet well studied, most of these studies agree on the potential beneficial role that might be played by Th17 cells in the disease progression in patients with CLL. Our aim in this section is to summarize all the current CLL findings correlating Th17 cells and/or their signature cytokine IL-17 with various disease prognostic markers. A summary of the discussed clinical trials is outlined in Table 1. For this review, the term “Th17” will be used to refer to CD4+ T cells producing IL-17 cytokine as indicated mainly by intracellular flow cytometry staining, unless stated otherwise. Moreover, differential plasma/serum levels of IL-17 cytokine in patients with CLL will be explored in different studies as a potential but not definitive indicator of Th17 cells because this cytokine can be produced by other immune cells.
Summary of clinical trials up to date, investigating the levels, prognostic value, and/or cellular interactions of Th17 cells and/or IL-17 cytokine in CLL
| Location . | No. . | Th17/IL-17 vs HD . | Th17/IL-17 correlation with: . | Key findings . | Reference (y) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease stage . | Survival . | CD38 . | ZAP-70 . | 11q/17p deletion . | Unmutated IGHV . | |||||
| Germany | 3 | — | — | — | — | — | — | — | Lenalidomide increases Th17 cell percenatge in patients with CLLand reduces Tregs | 165 (2010) |
| USA | 84 | Higher | — | ↑ | ↓ | — | — | ↑ | IL-17 belongs to a cytokine cluster in patients with CLL correlating with longer survival and TTFT | 97 (2011) |
| USA | 66 | Higher | ↓ | ↑ | ↓ | — | ↓ | ↓ | IL-17A+ cells of myeloid lineage present in the proliferation centers of spleens of patients with CLL | 100 (2012) |
| China | 72 | — | — | — | — | — | — | — | Tfh-Th17 (CXCR5+ CCR6+) is significantly elevated in CLL PBMCs, correlating with Binet stage C and mutated IGHV | 33 (2013) |
| Poland | 294 | Higher | ↓ | — | ↓ | ↓ | ↓ | — | Higher Th17 cell and IL-17A levels in patients with CLL are significantly correlated with better therapeutic outcomes and prognostic factors Th17 cell percentage in patients with CLL directly correlates with iNKT cells and inversely with Tregs | 73 (2013) |
| Iran | 40 | — | ↓ | — | NS | NS | — | ↓ | Frequency of Th17 and Tc17 cells is significantly low in patients with progressive CLL and associates with increased CD39+ Tregs | 74 (2013) |
| China | 50 | Higher | — | — | — | — | — | — | Progressive decline in Th17 cell percentage in patients with CLL during 1-year chlorambucil treatment | 99 (2014) |
| India | 40 | Higher | ↑ | — | NS | — | — | — | Th17 cell percentage, not Tregs, is remarkably higher in peripheral blood of patients with CLL who have AIC | 101 (2015) |
| USA | 74 | — | — | — | — | — | — | — | CLL CD4+ T cells have more capacity to differentiate into IL-17F–expressing Th17 cells in vitro Autologous CLL cells are significantly responsible for increasing IL-17F production by Th17 cells in vitro IL-17F activates NF-κB pathway in CLL CD8+ T cells and B cells but not in HD cells | 132 (2015) |
| China | 50 | Higher | ↓ | — | ↓ | ↓ | ↓ | NS | Treg/Th17 and IL-10/IL-17 ratios decrease along CLL progression and can be used as disease prognostic markers | 95 (2016) |
| Sweden | 23 | — | — | — | — | — | — | — | Peripheral blood Th17 cell (CCR6+ CXCR3−) percentage drops significantly starting wk 16 of lenalidomide/alemtuzumab combination therapy | 166 (2017) |
| Italy | 25 | Higher | — | — | — | — | — | — | CLL Tregs express Tbet, GATA-3, and RORγt indicating phenotypic switch to effector subsets Significant increase in IFN-γ/IL-10 and IL-4/IL-10 double-producing CD4+ T cells in peripheral blood of patients with CLL | 51 (2018) |
| Canada | 50 | Higher | NS | — | NS | — | NS | — | In vitro addition of rhIL-17A increases IL-6 expression in human CLL cells and BMMSCs when cultured either alone or in coculture IL-17 increases the engraftment of human CLL cells into NSG mice in peritoneal cavity, spleen, and BM through IL-6–dependent mechanism | 96 (2018) |
| Iran | 78 | Higher | NS | — | NS | ↓ | — | NS | Significant increase in mRNA expression and plasma IL-22 and IL-17 levels in patients with CLL High plasma IL-22 is positively correlated with CD38 and ZAP-70 expression | 98 (2018) |
| USA | 22 | Higher | — | — | — | — | — | — | Ex vivo treatment of PBMCs from patients with CLL with daratumumab increases proportion of Th17 cells In vivo treatment of PDX mice with daratumumab increases Th17 cell percentage | 110 (2020) |
| USA | 75 | — | — | — | — | — | — | — | PMN-MDSCs are more common than monocytic-MDSCs in blood of patients with CLL and correlate more with poor clinical course PMN-MDSCs are more efficient in vitro in inhibiting autologous T-cell proliferation PMN-MDSCs significantly promote in vitro IL-17F production by naïve CD4+ T cells from patients with CLL | 146 (2021) |
| China | 40 | NS | — | — | — | — | — | — | Treg/Th17 and IL-10/IL-17 ratios are significantly higher in patients with CLL Significant increase in Tim-3 expression on CLL Tregs corresponding to disease progression and might be responsible for Treg/Th17 imbalance Galectin-9 serum levels significantly increase in CLL and correlate significantly with IL-10 and Tim-3+ Tregs In vitro blocking of Tim-3 increases CLL cell apoptosis and increases IFN-γ/TNF levels in Tim-3+ Treg/ Th1/CLL cells coculture media | 147 (2021) |
| USA | 30 | — | — | — | — | — | — | — | Activated CLL B cells increase miR-155 expression in T cells leading to increased IL-17F production in vitro which correlates with longer survival and TTFT in patients | 131 (2022) |
| Location . | No. . | Th17/IL-17 vs HD . | Th17/IL-17 correlation with: . | Key findings . | Reference (y) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease stage . | Survival . | CD38 . | ZAP-70 . | 11q/17p deletion . | Unmutated IGHV . | |||||
| Germany | 3 | — | — | — | — | — | — | — | Lenalidomide increases Th17 cell percenatge in patients with CLLand reduces Tregs | 165 (2010) |
| USA | 84 | Higher | — | ↑ | ↓ | — | — | ↑ | IL-17 belongs to a cytokine cluster in patients with CLL correlating with longer survival and TTFT | 97 (2011) |
| USA | 66 | Higher | ↓ | ↑ | ↓ | — | ↓ | ↓ | IL-17A+ cells of myeloid lineage present in the proliferation centers of spleens of patients with CLL | 100 (2012) |
| China | 72 | — | — | — | — | — | — | — | Tfh-Th17 (CXCR5+ CCR6+) is significantly elevated in CLL PBMCs, correlating with Binet stage C and mutated IGHV | 33 (2013) |
| Poland | 294 | Higher | ↓ | — | ↓ | ↓ | ↓ | — | Higher Th17 cell and IL-17A levels in patients with CLL are significantly correlated with better therapeutic outcomes and prognostic factors Th17 cell percentage in patients with CLL directly correlates with iNKT cells and inversely with Tregs | 73 (2013) |
| Iran | 40 | — | ↓ | — | NS | NS | — | ↓ | Frequency of Th17 and Tc17 cells is significantly low in patients with progressive CLL and associates with increased CD39+ Tregs | 74 (2013) |
| China | 50 | Higher | — | — | — | — | — | — | Progressive decline in Th17 cell percentage in patients with CLL during 1-year chlorambucil treatment | 99 (2014) |
| India | 40 | Higher | ↑ | — | NS | — | — | — | Th17 cell percentage, not Tregs, is remarkably higher in peripheral blood of patients with CLL who have AIC | 101 (2015) |
| USA | 74 | — | — | — | — | — | — | — | CLL CD4+ T cells have more capacity to differentiate into IL-17F–expressing Th17 cells in vitro Autologous CLL cells are significantly responsible for increasing IL-17F production by Th17 cells in vitro IL-17F activates NF-κB pathway in CLL CD8+ T cells and B cells but not in HD cells | 132 (2015) |
| China | 50 | Higher | ↓ | — | ↓ | ↓ | ↓ | NS | Treg/Th17 and IL-10/IL-17 ratios decrease along CLL progression and can be used as disease prognostic markers | 95 (2016) |
| Sweden | 23 | — | — | — | — | — | — | — | Peripheral blood Th17 cell (CCR6+ CXCR3−) percentage drops significantly starting wk 16 of lenalidomide/alemtuzumab combination therapy | 166 (2017) |
| Italy | 25 | Higher | — | — | — | — | — | — | CLL Tregs express Tbet, GATA-3, and RORγt indicating phenotypic switch to effector subsets Significant increase in IFN-γ/IL-10 and IL-4/IL-10 double-producing CD4+ T cells in peripheral blood of patients with CLL | 51 (2018) |
| Canada | 50 | Higher | NS | — | NS | — | NS | — | In vitro addition of rhIL-17A increases IL-6 expression in human CLL cells and BMMSCs when cultured either alone or in coculture IL-17 increases the engraftment of human CLL cells into NSG mice in peritoneal cavity, spleen, and BM through IL-6–dependent mechanism | 96 (2018) |
| Iran | 78 | Higher | NS | — | NS | ↓ | — | NS | Significant increase in mRNA expression and plasma IL-22 and IL-17 levels in patients with CLL High plasma IL-22 is positively correlated with CD38 and ZAP-70 expression | 98 (2018) |
| USA | 22 | Higher | — | — | — | — | — | — | Ex vivo treatment of PBMCs from patients with CLL with daratumumab increases proportion of Th17 cells In vivo treatment of PDX mice with daratumumab increases Th17 cell percentage | 110 (2020) |
| USA | 75 | — | — | — | — | — | — | — | PMN-MDSCs are more common than monocytic-MDSCs in blood of patients with CLL and correlate more with poor clinical course PMN-MDSCs are more efficient in vitro in inhibiting autologous T-cell proliferation PMN-MDSCs significantly promote in vitro IL-17F production by naïve CD4+ T cells from patients with CLL | 146 (2021) |
| China | 40 | NS | — | — | — | — | — | — | Treg/Th17 and IL-10/IL-17 ratios are significantly higher in patients with CLL Significant increase in Tim-3 expression on CLL Tregs corresponding to disease progression and might be responsible for Treg/Th17 imbalance Galectin-9 serum levels significantly increase in CLL and correlate significantly with IL-10 and Tim-3+ Tregs In vitro blocking of Tim-3 increases CLL cell apoptosis and increases IFN-γ/TNF levels in Tim-3+ Treg/ Th1/CLL cells coculture media | 147 (2021) |
| USA | 30 | — | — | — | — | — | — | — | Activated CLL B cells increase miR-155 expression in T cells leading to increased IL-17F production in vitro which correlates with longer survival and TTFT in patients | 131 (2022) |
↓, negative correlation; ↑, positive correlation; —, comparison not studied; HD, healthy donor; No., number of patients; NS, nonsignificant relation found; PDX, patient-derived xenograft.
Changes in Th17 and/or IL-17 levels in patients with CLL
Many research groups that aimed at understanding the questionable function of Th17 cells in CLL have used various measurement outcomes to detect the differential levels of IL-17–producing cells. They have shown that IL-17 plasma/serum levels were significantly higher in the peripheral blood of patients with CLL compared with that of healthy donors.73,95-99 Furthermore, intracellular staining of PBMCs or isolated CD4+ T cells showed a significant increase in the percentages and/or absolute numbers of IL-17–producing cells in patients with CLL.51,73,95,100,101 In line with this data, increased messenger RNA (mRNA) expression of IL-17A and RORγt in CLL CD4+ T cells and PBMCs was another confirmation of the upregulated Th17 cell programming in CLL.73,95,98 In contrast, 1 group showed no remarkable difference in IL-17 levels and percentages of Th17 cells in CLL bone marrow (BM) samples when compared with peripheral blood.73
Although IL-17A and IL-17F share similar biological activities, they can have distinct functions.102,103 Sherry et al have shown a significant increase in IL-17F+–expressing CD4+ T cells in PBMCs of patients with CLL compared with those of healthy donors after in vitro PBMC activation using CD3/CD28 for 7 days in the presence of a Th17-promoting cytokine cocktail containing recombinant human IL-6, IL-1β, and IL-23.
CLL cells develop and mature in secondary lymphoid organs, where they receive survival and proliferation signals.104 Jain et al have explored, for the first time, the existence of IL-17A–expressing T cells in the lymphoid tissues of patients with CLL, including splenic mononuclear cell suspension and fixed spleen and LN sections. These studies demonstrated an increase in Th17 cell percentages in splenic mononuclear cell suspensions from patients with CLL compared with those from healthy donors. In most patients, these percentages were higher than the peripheral blood Th17 cell percentages in paired samples. Using immunohistochemistry, IL-17A–expressing cells were found in CLL splenic sections, but none were found in healthy spleens. In contrast, no significant differences in Th17 cell percentages were detected between CLL and healthy LN sections. Immunofluorescence staining results recorded an accumulation of CD3+ IL-17A+ cells within proliferation centers in spleen samples, which were defined by high numbers of Ki-67+ CD20+ CLL B cells.100 These results shed light on the potential role played by the immune microenvironment surrounding proliferating CLL cells. This microenvironment can have a critical effect on the capacity of Th cells to differentiate into Th17 cells. From another perspective, the immune cells surrounding CLL cells might enhance the migration of Th17 cells into lymphoid organs through chemokine and cytokine effects.
Despite the agreement between the previously mentioned results, De Matteis et al have shown that RORC and IL-23A gene expression were downregulated in isolated CD4+ T cells from patients with CLL. This might suggest a negative regulation mechanism related to Th17-23 cells, a phenotype that requires the presence of IL-23 for Th17 differentiation in the absence of TGF-β, and might have a potential antitumor effector function.51 The discrepancy in this result was explained by the authors to be possibly related to the limited number of patients enrolled in this transcriptional profiling analysis.
Th17 cells and clinical outcome in CLL
Rai staging is the most common classification system for the diagnosis of patients with CLL in the United States. It classifies the patients into 5 stages based on several criteria, including lymphocytosis, lymphadenopathy, splenomegaly, anemia, thrombocytopenia, and hepatomegaly.105 Several studies have shown that increased plasma IL-17 levels and the percentage of Th17 cells in peripheral blood are significantly correlated with lower Rai stages and indolent disease stages.73,74,95,100 Moreover, mRNA levels of RORγt have been found to be several folds higher in patients with indolent CLL vs progressive CLL.74 Jain et al have also found significant positive correlations between Th17 cell numbers in peripheral blood and overall survival, even in advanced-stage patients.100 Unsupervised hierarchical bicluster analysis revealed that IL-17 belongs to a cluster of cytokines in the serum of patients with CLL that strongly correlated with longer survival and time to first treatment (TTFT).97 This evidence suggests that Th17– or IL-17–producing cells might play an important antitumor role in CLL, which leads to improved survival and reduced disease progression. However, the molecular mechanisms are not yet clearly understood. In contrast, Kouzegaran et al did not find a significant correlation between Rai staging and increased IL-17 cytokine levels in patients with CLL.98 It should be noted that the results described here are from clinical trials across the globe, which makes ethnicity a critical factor in deciding the variability or disagreement between these results. In addition, differences in clinical trial conditions and statistical methods used for data analysis can all contribute to the discrepancies among different studies.
Th17 cells and CD38/ZAP-70 expression in CLL
CD38 and ZAP-70 expression by CLL cells have been used as prognostic indicators for patients with CLL, where increased expression of 1 or both markers correlates with shorter time to initial therapy and poor outcomes after chemoimmunotherapy. CD38 is an ectoenzymatic glycoprotein expressed on the surface of different immune cells, including B cells. Upregulation of CD38 on CLL cells has been correlated with increased survival and proliferation of leukemic cells in addition to chemotherapy resistance.106,107 ZAP-70 belongs to the Syk-ZAP-70 protein tyrosine kinase family that is normally expressed in T cells and natural killer cells and plays an important function in T-cell signaling initiation. ZAP-70 expression by CLL cells has been linked to upregulated immunoglobulin receptor signaling and immunoglobulin heavy chain (IGHV) mutational status.108,109
Various studies have shown that high circulation levels of IL-17 and the frequency of Th17 cells in patients with CLL are correlated with lower or negative expression of CD38 and/or ZAP-70 in CLL cells.73,97,98 On a genetic level, patients with CLL who showed detectable T-cell IL-17 mRNA had negative expression for both CD38 and ZAP-70.73 In addition, when patients with CLL were classified into CD38high and CD38low categories, Jain et al found a striking correlation between increased numbers of Th17 cells and better survival and clinical outcome in the CD38high group.100 In another study investigating the potential of using the IL-10/IL-17 cytokine ratio in the peripheral blood of patients with CLL as a prognostic disease marker, the authors found significant correlations between lower IL-10/IL-17 ratios and negative expression of CD38 and ZAP-70.95 However, other studies have found no correlation between elevated IL-17 plasma levels and CD38 expression.96,98,101 Using Th17 cell frequency as an outcome measurement, Jadidi-Niaragh et al have also found no correlation between CLL Th17 cells and the expression of either ZAP-70 or CD38; however, preenriched T cells were used for this study, which adds another layer of variability in the results.74
An interesting study by Manna et al has investigated the immune reconstitution effects of using CD38-targeted therapy in CLL. Ex vivo treatment of PBMCs from patients with CLL with daratumumab (monoclonal anti-CD38 antibody) displayed a significant increase in the proportion of Th17 cells (CXCR3− CCR6+). However, this effect was not noted in the PBMCs depleted from CLL cells, indicating that daratumumab effects on Th17 cells were most probably indirect, through the killing of the CLL cells. The immune modulatory effects of daratumumab also included depletion of CD38+ Tregs and increased percentages of IL-17+ and IFN-γ+ cytotoxic CD8+ T cells, which displayed enhanced cytotoxicity against autologous CLL cells. In addition, the authors were able to replicate similar results using an in vivo CLL patient-derived xenograft model.110
Th17 cells and genetic abnormalities in CLL
11q and 17p deletions are recurrent chromosomal abnormalities seen in patients with CLL that correspond to a poor prognosis. Moreover, the mutation status of IGHV gene is another known prognostic marker for CLL, where mutated genes correlate with better prognosis and less-advanced disease stages.111 Peripheral blood IL-17 levels were previously shown to be significantly lower in patients with 17p and/or 11q deletion.73,95 Besides plasma cytokine levels, Jain et al have found that patients with CLL with low Th17 cell absolute numbers in peripheral blood had less favorable karyotypes, 17p and 11q deletion. Although not significant, they also found that mutated IGHV/CD38low patients had higher Th17 cell numbers compared with the unmutated IGHV/CD38high group.100 Similar observation was recorded where Th17 cell frequency was increased in mutated vs unmutated CLL groups.74
Few studies have shown no correlation between IL-17 blood levels and IGHV mutation status of patients with CLL.95,98 Furthermore, Yan et al found that serum IL-17 strongly correlated with unmutated IGHV status in patients with CLL.97 However, these discrepancies can result from correlating disease severity with individual cytokine levels instead of clustered cytokine groups.
CLL cells as potential modulators of Th17 cell differentiation
Similar to most types of tumor cells, malignant CLL cells have the capacity to evade the immune system and hijack the surrounding microenvironment to favor their survival and proliferation. CLL-induced immune dysregulation has a wide range, affecting both innate and adaptive immune cells. For instance, CLL cells inhibit the cytotoxic activity of T cells through promoting the expression of checkpoint molecules on T cells, including PD-1, CTLA-4, TIGIT, LAG-3, and Tim-3.9,39,48,49,112,113 This effect is corresponded by an upregulation of inhibitory ligands on CLL cells, such as PD-L1, galectin-9, and CD276.112,114-116 Multiple evidence suggest the expansion of oligoclonal T cells in patients with CLL with highly similar T-cell receptor (TCR) clonotypes shared among different patients who belong to same stereotyped subset.22,117,118 These results suggest a chronic antigenic pressure posed by CLL cells on T cells in the microenvironment, which might lead to T-cell exhaustion. Furthermore, CLL cells have shown an interesting capacity to evade T-cell killing via formation of abnormal nonlytic immune synapses with cytotoxic T cells characterized by nonpolarized degranulation.12 The influence of CLL B cells on T cells is not only restricted to impairing cytotoxic capacities, but also disrupting T-cell metabolic fitness probably through mechanisms mediated by soluble factors.119
From another perspective, CLL-induced hypoxia, together with increased adenosine signaling and high IL-10 production by CLL cells, can promote the differentiation of macrophages into the protumor M2 phenotype.120 CLL cells have also been shown to promote the expression of indoleamine 2,3-dioxygenase in myeloid-derived suppressor cells (MDSCs) resulting in the suppression of T-cell activation and the induction of immunosuppressive Tregs.121 In addition, CLL cells can disturb normal dendritic cells (DCs) maturation, leading to impaired capacity for antigen presentation and T-cell activation to acquire cytotoxic phenotypes.122,123 This might be due to an effect of IL-6 produced by CLL cells122 and the increased levels of suppressor of cytokine signaling 5 inhibiting STAT6 activation in DCs.123
Small extracellular vesicles (sEVs) release is a relatively recently identified mechanism by which CLL cells can reeducate the surrounding immune cells to promote disease progression. CD40/IL-4–stimulated CLL cells have been shown to release sEVs rich in specific microRNAs (miRNAs), including miR-363. Internalization of miR-363 sEVs by autologous CD4+ T cells promoted migration and immune synapse formation with CLL cells.124 Gargiulo et al have recently demonstrated for the first time the effect of sEVs directly isolated from murine Eμ-TCL1 spleens on the in vivo CLL progression in the murine model. Isolated sEVs were rich in specific miRNAs, such as miR-150, miR-155, miR-21, miR-210, miR-146a, miR-378a, and miR-27a, and they carried immune checkpoint ligands, such as PD-L1, GAL9, B7-H2, VISTA, and major histocompability complexes I/II. CLL-derived sEVs were able to change CD8+ T-cell transcriptome, proteome, and metabolome, resulting in exhausted cells with impaired cytotoxic potential, leading to tumor progression.125
In the context of Th17 cells, CLL cells might have a critical role in either recruiting or modulating the differentiation of Th17 cells in the microenvironment. Activated CLL cells have the capacity to release high levels of IL-6 and IL-1β,126-128 2 of the key cytokines involved in Th17 cell differentiation. Interestingly, the release of IL-1β by CLL cells was more linked to an early disease stage,97,129,130 which might explain the increased abundance of Th17 cells in better outcome patients. Jung et al recently demonstrated a novel role for miR-155 on modulating Th17 cell differentiation in CLL. Coculturing naïve T cells with autologous CLL–activated B cells from patients with better clinical outcome, resulted in increased miR-155 expression in T cells, which correlated more significantly with the increase in IL-17F production than IL-17A production in the culture media. This increase in miR-155 was also significantly correlated with longer survival and TTFT in patients with CLL.131
Coculture experiments have shown that CLL B cells were responsible for enhancing IL-17F production by CD4+ T cells and that this effect was dependent on the cytokine combination used for Th17 cell polarization.132 Furthermore, CD5 expression on CLL cells could have a potential role in Th17 cell differentiation based on the evidence that CD5+ B1 cells can induce CD4+ T-cell differentiation into Th17 cells133 and that CD5+ B cells could be the normal cellular equivalent of CLL B cells.134 A previous study has suggested that CD5 costimulation can have a dramatic effect on inducing IL-23 receptor (IL-23R) expression on naïve CD4+ T cells and sustained STAT3 activation.135 Because CD5 has been shown before to be homophilic,136 it might be possible that homotypic CD5 interactions between CLL B cells and CD4+ T cells might promote Th17 cell differentiation through upregulation of IL-23R.132 From another perspective, McGuire et al have found that CD5 can be of critical importance in generating Th17 cells through activating casein kinase 2. CD5–casein kinase 2 signaling can enhance Akt activation downstream of TCR engagement and increase Th17 cell differentiation through 2 mechanisms. The first is the inhibition of glycogen synthase kinase 3 reducing IFN-γ–STAT1 activation. The second is the activation of mTOR, leading to an increase in RORγt nuclear transport.137
In contrast, CLL cells can inhibit Th17 cell differentiation and function. CLL cells produce high levels of IL-10, which can skew the differentiation of Th cells to Tregs, which also release IL-10. Because Th17 cells can express IL-10R,138 CLL cells might act directly or indirectly to inhibit Th17 cell function via IL-10. Moreover, Yang et al have shown that non-Hodgkin lymphoma B cells inhibited IL-17 production by CD4+ T cells through CD27-CD70 or CD28-CD80/86 interactions.139
Th17 and microenvironment immune cells
The survival and proliferation of CLL cells, and hence disease advancement is highly dependent on the surrounding immune and stromal cells in the microenvironment.4,5 Despite the scarcity of data, we will summarize in this section the current knowledge related to the relationship between Th17 cells and other immune cells in the CLL microenvironment and how this might affect leukemic progression.
In a cohort of peripheral blood samples from 100 patients with CLL, Hus et al found a significant positive correlation between the percentages of Th17 cells and invariant natural killer T (iNKT) cells.73 iNKT cells have been previously studied in patients with CLL and showed to have intact numbers and function in early-stage disease.140 iNKT cells have a key role to play in promoting antitumor immunity,141 which might indicate a positive impact of Th17 cells in promoting antitumor effects through recruiting other cytotoxic immune cells. Knowing the role of nuclear factor κB (NF-κB) signaling in antitumor CD8+ T-cell function, IL-17F was found to have a significant effect on triggering the pNF-κBp105 pathway in CLL donor CD8+ T cells but not in healthy donor cells.132 This demonstrates another potential mechanism through which Th17-produced cytokines can stimulate cytotoxic immune responses in the CLL microenvironment. Preliminary experiments done by Jain et al demonstrated the expression of different members of the IL-17Rs (RA, RB, RC, and RD) in increasing order on CLL cells, normal B cells, T cells, and monocytes. This might also point to the ability of Th17 cells to affect CLL cell function and proliferation directly or indirectly via interaction with IL-17Rs on either CLL cells or neighboring immune cells like T cells and monocytes.100
Although IL-17 is known to be mainly produced by CD4+ Th17 cells, other types of cells have the capacity to produce this cytokine, including CD8+ T cells (Tc17), γδ T cells, polymorphonuclear (PMN) granulocytes, and mast cells.142 Multiple evidence suggest a role for Tc17 in antitumor immunity.143-145 Adoptive transfer of in vitro polarized Tc17 cells into a B16F10 melanoma murine model demonstrated an antitumor activity that can be attributed to the in vivo conversion of Tc17 into IFN-γ–producing cells, with increased expression of IL-7Rα and reduced KLRG-1 expression.144 In context of CLL, Jadidi et al have shown that frequencies of Tc17 decreased in patients with progressive CLL vs indolent CLL, indicating a potential mechanism of antileukemic activity mediated by these cells in CLL.74 Interestingly, Jain et al have reported for the first time the presence of IL-17A+ cells of myeloid lineage in the proliferation centers of the spleens of patients with CLL and not healthy donors. Most of these cells were maturing granulocytes (IL-17+ MPO+ and either CD13+ or CD15+ or CD13+ CD15+), and few were mature mast cells (MPO− CD117+ IL-17+ or MPO− MCT+ IL-17+). These cells were located near blood vessels, which may be because IL-17 promotes angiogenesis. In LNs, most of the non–Th17 IL-17+ cells were noncycling mast cells, with only a few cells of the granulocytic lineage.100
Tfh cells are known as strong inducers of B-cell activation and antibody production in secondary lymphoid organs. Cha et al have found a significant increase in CD4+ CXCR5+ Tfh cells in the peripheral blood of patients with CLL compared with healthy volunteers and in Binet stage C (late stage) compared with stage A (early stage). Out of the different Tfh cell subsets, Tfh-Th2 (CXCR5+ CXCR3− CCR6−) and Tfh-Th17 (CXCR5+ CCR6+) cells were elevated significantly in PBMCs of patients with CLL. Tfh-Th17 were markedly elevated in patients with Binet stage C, indicating possible involvement of this subset in CLL progression.33
A recent study was performed to investigate the significance of MDSCs in patients with CLL. PMN-MDSCs were the main population in the blood of patients with CLL that correlated more with poor clinical course compared with monocytic-MDSCs. In addition, PMN-MDSCs were more efficient in vitro in inhibiting autologous T-cell proliferation. The in vitro experiments showed that PMN-MDSCs significantly promoted IL-17F production by naïve CD4+ T cells from patients with CLL vs coculture with monocytic-MDSCs.146 This study might provide a possible explanation for the increase in Th17F cells in CLL via interaction with PMN-MDSCs in the microenvironment.
Th17 cells and Tregs are 2 distinct populations of Th cells that can play 2 opposite immune modulatory roles, where Th17 cells promote immune inflammation and Tregs promote tolerance or immune suppression. Recent research data indicate that the disturbance in the delicate Th17-Treg balance can play a major role in the initiation of many autoimmune diseases or cancers. In context of CLL, different groups have noticed significant disturbances in this balance that correlated with different aspects of the disease. For instance, significant inverse correlations have been found between Th17 cells and Tregs in the peripheral blood of patients with CLL, where expansion of Tregs was linked to CLL progression.73,95,147 Pang et al used peripheral blood Treg/Th17 ratio as well as serum IL-10/IL-17 cytokine ratio as an indication of CLL progression, where they found increased ratios were associated with disease progression and positively correlated with Rai staging, CD38, ZAP-70 expression, and 17p chromosomal abnormality. Moreover, these ratios were significantly decreased after drug treatment in remission vs nonremission groups.95 Similarly, a significant increase in the IL-10/IL-17 ratio has been found in patients with advanced Binet stage CLL compared with those in early stages.147 Furthermore, reduction in peripheral blood Th17 cell proportion in patients with progressive CLL compared with those with indolent CLL and healthy donors was associated with a significant increase in CD39+ Tregs.74 This subset of Tregs has an inhibitory effect on Th17 cells, probably through physical cell contact and adenosine production.148 In general, CLL cells can have the potential to induce Treg expansion and inhibit Th17 cells through production of several factors, including TGF-β149 and high levels of IL-10.138,150
Maintaining the Treg/Th17 balance at equilibrium is very critical to prevent autoimmune diseases. In a cohort of 40 treatment-naïve patients with CLL, Lad et al found that percentages of Th17 cells and not Tregs were significantly higher in the peripheral blood of patients with CLL with AIC compared with those with advanced CLL with no AIC. In addition, they found that Treg/Th17 ratio was skewed toward Th17 in patients with AIC. Interestingly, LN aspirate samples showed a reduction in IL-17–expressing T cells compared with peripheral blood, which corresponded to a significant increase in IL-10–expressing Treg percentages.101 Previous work has described the ability of Th17 cells to express the IL-10R, which might give a possible explanation for the inverse Treg/Th17 relationship in CLL via the negative impact of IL-10 released by Tregs.138 These results highlight the significance of the delicate balance between Th cell differentiation subsets in preventing autoimmunity and malignancy progression.
Another possible explanation for Treg/Th17 imbalance in CLL was suggested by Pang et al, who studied the role of Tim-3/Galectin-9 expression in CLL. They found a significant increase in Tim-3 expression on CLL Tregs compared with healthy controls, as well as an increase in Tim-3+ Tregs along with Binet stage disease progression. Galectin-9 serum levels significantly increased in CLL and correlated significantly with IL-10 and Tim-3+ Tregs. In vitro antibody-mediated blocking of Tim-3 lead to an increase in CLL cell apoptosis and increase in IFN-γ and TNF-α supernatant levels when sorted Tim-3+ Treg, Th1 cells, and CLL cells were cocultured together.147 Because Galectin-9 has been previously suggested to inhibit Th17 cell differentiation,151 it might be possible that enhancement of Treg function via this pathway is partly responsible for disturbed Treg/Th17 balance in CLL.
An additional layer for the immune response complexity includes the interconversion among different Th cell phenotypes. De Matteis et al have shown a significant increase in the percentages of FOXP3+ Tregs expressing Tbet, GATA-3, and RORγt in the peripheral blood of patients with CLL compared with that of healthy volunteers. Using in vitro stimulation of patient cells, they found a significant increase in CD4+ T cells cosecreting IL-10/IFN-γ and IL-10/IL-4.51 This study highlights the potential of Tregs to differentiate into effector phenotypes in patients with CLL, potentially creating an inflammatory environment supporting malignant B-cell survival.
Cytokine disturbance and Th17 cells in CLL
Cytokine disturbance is an integral part of the complex CLL microenvironment, which plays a major role in promoting the survival of malignant B cells and disease progression.126,152-154 In this section, we will cover the recorded evidence of cytokine changes in CLL that might be involved in Th17 cell differentiation changes. An inverse correlation was found between IL-17A plasma levels and IL-10 and TNF levels in patients with CLL. These results were also confirmed by an inverse relation between percentages of Th17 cells and percentages of IL-4+ Th2 cells and TNF+ CD4+ T cells in the peripheral blood of patients with CLL.73 Evidence suggests that these cytokines can play a considerable role in disease progression, where IL-4 can inhibit CLL cell apoptosis by increasing Bcl-2 expression.155 Moreover, TNF can inhibit the apoptosis of malignant B cells by activating NF-κB.156 IL-10 is significantly increased in patients with CLL with progressive disease and is produced by malignant B cells to maintain their survival.126,157 Tregs are another important source of IL-10, which can complement the immune-suppressive microenvironment of CLL and inhibit Th17 cell function.158 These correlations might explain the reduced Th17 cell percentages in patients with CLL with a bad prognosis.
Yan et al have applied unsupervised hierarchical bicluster analysis to group the serum cytokines from patients with CLL into clusters associated with disease prognostic factors. Because the effect of cytokines is affected by the presence of other cytokines/chemokines in the disease milieu, it was expected that cytokine clustering would present a better prognostic indication of disease progression compared with individual cytokine analysis. Three clusters were identified, and IL-17 belonged to cluster 3 (CL3), which included IL-1β, IL-2, IL-4, IL-15, IL-17, and IFN-α. Patients with higher CL3 serum cytokine levels significantly correlated with longer overall survival and tended toward longer TTFT. IL-15 and IL-1β in CL3 can induce differentiation of Th17 cells and altogether correlate with better outcome in patients with CLL.97 In contrast, the authors described another cytokine cluster, CL1, which contained cytokines, such as the immune-suppressive IL-10 that are important for CLL cell survival. CL1 cytokines correlated significantly with more progressive disease and a short TTFT, and patients with CL1high/CL3low tended to die sooner than other patients. This result was in agreement with the previous finding that increased serum IL-10/IL-17 ratios were associated with more disease progression in CLL.95 IL-6, another important cytokine for Th17 cell differentiation, was assigned to CL2, which significantly correlated with longer TTFT only when combined with CL1 (CL1low/CL2high). Regarding individual cytokine levels, IL-6 and IL-1β were found to be significantly elevated in the serum of patients with CLL, which might be responsible for the significant increase in IL-17 levels in these patients. High IL-1β levels correlated with improved overall survival in patients and with CD38low expression.97 These results pinpoint to the clinical advantage of increased levels of IL-17 and Th17 cell–inducing cytokines in CLL.
IL-23 is 1 of the cytokines known to maintain Th17 cell differentiation. Previous trials have shown that there is a significant rise in serum/plasma IL-23 levels in patients with CLL.51,99,132 Combined with the data mentioned above, these results indicate that most of the cytokines required for Th17 cell differentiation (IL-6, IL-1β, and IL-23) are elevated in patients with CLL. CLL CD4+ T cells might be more primed to Th17 cell differentiation compared with normal Th cells. Sherry et al demonstrated this effect by showing a significant increase in supernatant IL-17F from cultures of CD4+ T cells derived from patients with CLL with autologous CLL B cells in the presence of IL-6, IL-1, and IL-23. They showed that addition of exogenous IL-6 leads to an increase in pSTAT3/pSTAT1 ratio in CLL CD4+ T cells compared with healthy CD4+ cells indicating more Th17 cell differentiation activity.132 The authors suggested that the higher potential of CLL CD4+ T cells for Th17 cell differentiation can also be attributed to CD5 expression by CLL cells not normal B cells as mentioned above.
IL-22 is another inflammatory mediator that has been initially described as being produced by Th17 cells through the IL-23 signaling mechanism.57 IL-22 is an important inducer of innate immune responses, especially at barrier surfaces such as the lungs, skin, and gut.159 The role of this cytokine in CLL is not well investigated; however, Kouzegaran et al found a significant increase in plasma IL-22 and IL-17 levels in patients with CLL, which was confirmed by a significant increase in the mRNA levels of both cytokines in PBMCs. High plasma IL-22 correlated with positive expression of CD38 and ZAP-70.98 IL-22 levels have been suggested to have a role in acute lymphocytic leukemia (ALL), myelodysplastic syndrome, and acute myeloid leukemia;160-162 however, its role in CLL and how it correlates with Th17 cell function in this disease need further investigation.
From another perspective, IL-17–producing cells might play a role in CLL cytokine modulation, contributing to disease progression. Although IL-6 is a known inducer of Th17 cell differentiation, IL-17A can also induce the production of IL-6 through a variety of cells.163,164 To study the impact of bone marrow mesenchymal stem cells (BMMSCs) on CLL growth, Fang et al investigated the significance of IL-17/IL-6 axis. They reported a significant correlation between IL-17 and IL-6 in the peripheral blood of patients with CLL but not in healthy controls. In vitro addition of rhIL-17A increased IL-6 expression in human CLL cells and BMMSCs when cultured either alone or in coculture. In addition, in vitro coculture experiments showed that IL-6 produced by BMMSCs or CLL cells supported the survival of CLL cells. Therefore, they concluded that IL-17 might support the survival of CLL cells via inducing IL-6 expression. Using immunodeficient NSG mice, the same study showed that IL-17 increased the engraftment of human CLL cells into mice in the peritoneal cavity, spleen, and BM. Blocking IL-6 in vivo using tocilizumab (IL-6R antagonist) blocked this effect, indicating that IL-17 increased CLL engraftment through an IL-6–dependent mechanism.96
Th17 cells and CLL therapeutic strategies
In addition to the basic knowledge of the significance of Th17 cells in CLL, it is important to direct the research focus toward clinical applications. For instance, lenalidomide-mediated treatment effects in CLL were shown to be linked with a reduction in percentages of Tregs and increase in Th17 cells.165 However, more recently, a CLL phase 1/2 clinical trial of lenalidomide/alemtuzumab (CD52 monoclonal antibody) combination therapy showed a reduction in both Tregs and Th17 cells near the end of treatment, which might be caused by alemtuzumab addition.166 In contrast, small molecule inhibitors, such as ibrutinib (Bruton tyrosine kinase [BTK] inhibitor), idelalisib (phosphatidylinositol 3-kinase δ [PI3Kδ] inhibitor), and venetoclax (Bcl-2 inhibitor), have dramatically changed CLL treatment practice. In addition to their inhibitory effects on malignant CLL cell survival, modulatory effects on other immune cells have been documented.34,167-170 Few studies have started to investigate the modulatory effects of these inhibitors on Th17 cells in CLL (summarized in Table 2). Ibrutinib was the first Food and Drug Administration–approved irreversible BTK inhibitor to be used in CLL treatment, which also inhibits IL-2–inducible T-cell kinase (ITK).171 However, acalabrutinib is a more selective next-generation BTK inhibitor that lacks inhibitory activity against ITK. Stimulation of PBMCs from ibrutinib-treated patients with CLL with phorbol 12-myristate 13-acetate (PMA)/ionomycin showed a significant increase in the percentage of IL-17+ CD4+ T cells. In contrast, acalabrutinib-treated patients did not show a similar increase, indicating that the ITK inhibitory capacity of ibrutinib171 can be the culprit in Th17 cell expansion through blocking activation-induced cell death.167 Ibrutinib treatment in CLL has also demonstrated a transient increase in Th17F (IL-17F+) cells at the second month of treatment, which reverted to pretreatment levels at the third month.146 Furthermore, ibrutinib was shown to indirectly increase IL-17 production by T cells through modulation of DC function.172 In contrast, Puzzolo et al recently reported a significant decrease in Th17 cell percentages after 18 months of ibrutinib treatment and a reduction in absolute Th17 cell counts starting in month 8 and up to month 18.168 In line with this data, Niemann and colleagues also showed a significant drop in blood Th17 cells after 24 weeks of ibrutinib treatment in an investigator-initiated phase 2 trial. In vitro, ibrutinib displayed a capacity to reduce Th17 cell differentiation in a dose-dependent manner.173 These results agree with a previous study that demonstrated a disruption in Th17 cell differentiation from ITK knockout mice, claiming that ITK is critical for directing TCR signals to favor IL-17A production and Th17 cell differentiation via NFATc1.174
Summary of clinical trials that investigated the effect of B-cell receptor inhibitors on Th17 cell and/or IL-17 levels in patients with CLL
| Location . | No. . | Inhibitor investigated . | Key findings . | Reference (y) . |
|---|---|---|---|---|
| USA | 80 | Ibrutinib | Significant drop in blood Th17 cell (CD4+ CXCR3− CCR6+) frequency after 24 wk of ibrutinib treatment Ibrutinib displays an in vitro capacity to reduce murine Th17 cell differentiation in a dose-dependent manner | 173 (2016) |
| USA | 19 | IbrutinibAcalabrutinib | PMA/ionomycin stimulation of PBMCs from ibrutinib-treated patients with CLL shows a significant increase in percentage of Th17 cells Acalabrutinib-treated patients do not show similar increase in Th17 cells | 167 (2017) |
| USA | 10 | Idelalisib/ofatumumab | Idelalisib-ofatumumab therapy results in a significant increase in IL-17F–secreting CD8+ T cells in group of patients with CLL with high toxicity, which is associated with a reduction in Treg percentage In vitro, idelalisib and duvelisib can significantly increase Th17 cells and reduce Treg differentiation from healthy naïve CD4+ T cells | 170 (2019) |
| Germany | 9 | Idelalisib | Culturing CLL-derived CAR T cells in presence of idelalisib significantly increases Th17 (CCR4+ CCR6+) cell percentage on d 14 | 175 (2019) |
| USA | 20 | Ibrutinib | Ibrutinib treatment causes a transient increase in Th17F (IL-17F+) cells at second mo of treatment, which reverts to pretreatment levels at third mo | 146 (2021) |
| Italy | 71 | Ibrutinib/rituximab | Significant decrease in Th17 cell percentage after 18 mo of ibrutinib treatment and reduction in absolute Th17 cell counts starting mo 8 and up to mo 18 | 168 (2021) |
| USA | 26 | Duvelisib/FCR | Drug-induced toxicity is associated with an increase in CD4+ Th17 cells and related cytokines (IL-17A and IL-21) together with an increase in activated CD8+ T cells | 169 (2022) |
| Location . | No. . | Inhibitor investigated . | Key findings . | Reference (y) . |
|---|---|---|---|---|
| USA | 80 | Ibrutinib | Significant drop in blood Th17 cell (CD4+ CXCR3− CCR6+) frequency after 24 wk of ibrutinib treatment Ibrutinib displays an in vitro capacity to reduce murine Th17 cell differentiation in a dose-dependent manner | 173 (2016) |
| USA | 19 | IbrutinibAcalabrutinib | PMA/ionomycin stimulation of PBMCs from ibrutinib-treated patients with CLL shows a significant increase in percentage of Th17 cells Acalabrutinib-treated patients do not show similar increase in Th17 cells | 167 (2017) |
| USA | 10 | Idelalisib/ofatumumab | Idelalisib-ofatumumab therapy results in a significant increase in IL-17F–secreting CD8+ T cells in group of patients with CLL with high toxicity, which is associated with a reduction in Treg percentage In vitro, idelalisib and duvelisib can significantly increase Th17 cells and reduce Treg differentiation from healthy naïve CD4+ T cells | 170 (2019) |
| Germany | 9 | Idelalisib | Culturing CLL-derived CAR T cells in presence of idelalisib significantly increases Th17 (CCR4+ CCR6+) cell percentage on d 14 | 175 (2019) |
| USA | 20 | Ibrutinib | Ibrutinib treatment causes a transient increase in Th17F (IL-17F+) cells at second mo of treatment, which reverts to pretreatment levels at third mo | 146 (2021) |
| Italy | 71 | Ibrutinib/rituximab | Significant decrease in Th17 cell percentage after 18 mo of ibrutinib treatment and reduction in absolute Th17 cell counts starting mo 8 and up to mo 18 | 168 (2021) |
| USA | 26 | Duvelisib/FCR | Drug-induced toxicity is associated with an increase in CD4+ Th17 cells and related cytokines (IL-17A and IL-21) together with an increase in activated CD8+ T cells | 169 (2022) |
Regarding PI3K inhibitors, Gadi and colleagues recently investigated the immune-mediated toxicity associated with duvelisib-FCR (fludarabine, cyclophosphamide, and rituximab) treatment in CLL and were able to show that drug-induced toxicity was associated with an increase in CD4+ Th17 cells and related cytokines (IL-17A and IL-21) together with an increase in activated CD8+ T cells.169 In addition, treatment of patients with CLL with idelalisib-ofatumumab (CD20 monoclonal antibody) therapy resulted in a significant increase in IL-17F–secreting CD8+ T cells in the patient group that showed higher toxicity. This effect was associated with a reduction in Treg percentages. The authors confirmed the results by showing that both idelalisib and duvelisib can significantly increase Th17 cells and reduce Treg differentiation from healthy naïve CD4+ T cells in vitro.170 Furthermore, in vitro treatment of chimeric antigen receptor (CAR) T cells derived from patients with CLL with idelalisib resulted in a significant increase in Th17 cell (CCR4+ CCR6+) percentages on day 14 of culture.175
Despite these findings, more focus is still required to understand the mechanisms by which these small molecule inhibitors can modulate CLL Th17 cell function. This knowledge will be of potential benefit to help maintain the delicate balance between the autoimmune and antitumor roles of Th17 cells and to be able to maximize the clinical benefit.
Adoptive T-cell therapy (ACT), including CD19 CAR T cells, is a successful immunotherapeutic approach for the treatment of various hematologic malignancies, such as large B-cell lymphoma and ALL.176,177 Nevertheless, the therapeutic response of patients with CLL to CAR T-cell therapy remains significantly poor (complete response rate of 29%) compared with those with ALL.178 This lack of clinical success is largely attributed to the dysfunctional T cells in CLL. It would be of major interest to investigate how Th17 cells can be used for improving CAR T-cell therapies in CLL. Th17 cells have been previously described as a Th cell phenotype with a high degree of plasticity and stem cell–like properties.89,90,179 The antitumor capacity of adoptively transferred in vitro polarized Th17 cells has been studied by several groups in murine models. Muranski et al have shown that ex vivo polarized murine TRP-1–specific Th17 cells have stronger antitumor effects against melanoma compared with Th1 cells. The antitumor ability of these cells was mainly attributed to the durable in vivo persistence and the ability to switch to a unique subtype of Th17 cells with Th1-like behavior and high IFN-γ production after adoptive cell transfer.89 Bowers and colleagues found that TRP-1+ Th17 cells can massively expand using a 3-week expansion protocol without restimulation. These cells yielded a more robust antitumor effect against B16F10 melanoma in mice compared with Th1 cells. Analyses showed that these in vitro expanded Th17 cells had more resistance to apoptosis and less expression of exhaustion markers while maintaining Tcf7 expression at all time points during expansion. These cellular features resulted in prolonged in vivo persistence capacity compared with Th1 cells. In the same study, human CAR+ Th17 cells expanded for 2 weeks retained their antitumor effect against mesothelioma compared with Th1 cells.91 In addition, a previous study has demonstrated that dual inhibition of PI3Kδ and Wnt/β-catenin pathways can reprogram TRP-1 CD4+ T cells into central memory Th17 cells when ex vivo differentiated under Th17 polarization conditions. These reprogrammed cells have shown superior antitumor effects against B16F10 melanoma. The same study demonstrated the beneficial effects of ICOS costimulation on the polarization and antitumor function of Th17 cells.180
In CLL, it has been described that an increased STAT3/IL-6 signature, including increased IL-17 production by CAR T cells, corresponded to a patient complete response to CD19 CAR T-cell therapy.181 However, to date no studies have been performed to elucidate the impact of using Th17 cells as a tool to improve ACT in CLL, which might be of potential clinical significance.
Conclusions and future directions
CLL is characterized by a unique, complex immune microenvironment in which Th17 cells seem to play an integral role. In this review, we represented the published data regarding the role of Th17 cells in CLL microenvironment (summarized in Figure 1). Although clinical results are still somewhat controversial, there seems to be more agreement on the potential antitumor role of these cells in CLL. This is based on the findings that demonstrate reduced Th17 cell abundance along disease progression and their positive correlation with better prognostic markers and less immune-suppressive microenvironment. Mechanistically, the potential protective role of Th17 cells against CLL progression lacks in-depth investigation and requires more comprehensive and functional studies to uncover this potential role. However, rational conclusions should be made regarding the role of T-cell subsets such as Th17 cells in CLL because these cells usually constitute a very small fraction in a pool of massively expanded CLL B cells. Moreover, further focus might be required on investigating the immune cell and cytokine profiles in lymphoid tissues and BM, where CLL cells nourish and receive various cues critical for their persistence and propagation. Another possibility would be to consider profiling Th17 cells serially along the course of disease progression instead of single time point. This will consider the pleiotropic nature of these cells, either pro- or antitumor actions, caused by the wide range of cytokines produced besides IL-17.
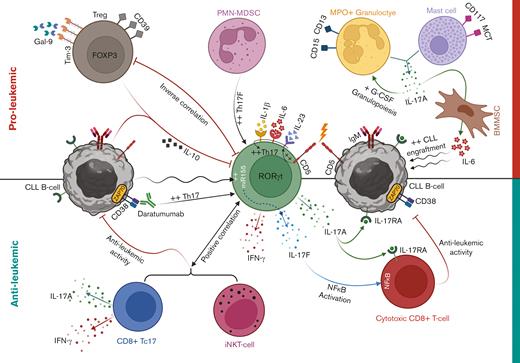
Th17 cell cross talk with CLL microenvironment. Th17 cell differentiation is promoted in CLL via augmented levels of differentiation cytokines, including IL-6, IL-23, and IL-1β. Homotypic CD5 interactions between naïve CD4+ T cells and CLL cells might also promote IL-23R expression and drive Th17 cell programming. A negative correlation exists between Th17 cells and CD38/ZAP-70 expression in CLL cells, and ex vivo monoclonal antibody blocking of CD38 increased Th17 cell proportions through killing CLL cells in patient PBMCs. CLL Th17 cells secrete high levels of IL-17A that might perform direct or indirect cytotoxic activity on CLL cells via interaction with IL-17RA. Activated CLL cells can increase miR-155 expression in Th17 cells, leading to increased IL-17F production, which can activate NF-κB signaling in CD8+ T cells, leading to increased cytotoxic potential. Antileukemic activity of CLL Th17 cells might include IFN-γ production and/or recruitment of cytotoxic iNKT- and IL-17–producing CD8+ T (Tc17) cells. In contrast, increased CD39+ Tregs in CLL with enhanced Tim-3/Galectin-9 (Gal-9) signaling can have immunosuppressive effects through the release of IL-10, which is also produced by CLL cells and can act on IL-10Rs expressed by Th17 cells. CLL-derived PMN-MDSCs can promote IL-17F production by Th17 cells, which might influence disease progression. Furthermore, cells of myeloid origin including mature granulocytes and mast cells can release IL-17A within CLL microenvironment, which might promote granulocyte colony stimulating factor (G-CSF) production and granulopoiesis for supporting CLL cell survival. Finally, IL-17A can promote IL-6 production by BMMSCs, which helps support CLL cell proliferation.
Th17 cell cross talk with CLL microenvironment. Th17 cell differentiation is promoted in CLL via augmented levels of differentiation cytokines, including IL-6, IL-23, and IL-1β. Homotypic CD5 interactions between naïve CD4+ T cells and CLL cells might also promote IL-23R expression and drive Th17 cell programming. A negative correlation exists between Th17 cells and CD38/ZAP-70 expression in CLL cells, and ex vivo monoclonal antibody blocking of CD38 increased Th17 cell proportions through killing CLL cells in patient PBMCs. CLL Th17 cells secrete high levels of IL-17A that might perform direct or indirect cytotoxic activity on CLL cells via interaction with IL-17RA. Activated CLL cells can increase miR-155 expression in Th17 cells, leading to increased IL-17F production, which can activate NF-κB signaling in CD8+ T cells, leading to increased cytotoxic potential. Antileukemic activity of CLL Th17 cells might include IFN-γ production and/or recruitment of cytotoxic iNKT- and IL-17–producing CD8+ T (Tc17) cells. In contrast, increased CD39+ Tregs in CLL with enhanced Tim-3/Galectin-9 (Gal-9) signaling can have immunosuppressive effects through the release of IL-10, which is also produced by CLL cells and can act on IL-10Rs expressed by Th17 cells. CLL-derived PMN-MDSCs can promote IL-17F production by Th17 cells, which might influence disease progression. Furthermore, cells of myeloid origin including mature granulocytes and mast cells can release IL-17A within CLL microenvironment, which might promote granulocyte colony stimulating factor (G-CSF) production and granulopoiesis for supporting CLL cell survival. Finally, IL-17A can promote IL-6 production by BMMSCs, which helps support CLL cell proliferation.
Acknowledgments
Figure 1 and the visual abstract were created with BioRender.com. The authors thank Jorge and Silvia Ferioli for the generous financial support they provided to help make this project possible.
Authorship
Contribution: W.G. performed all research and wrote the manuscript; and E.S. and J.P.-I. reviewed and assisted in content and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eva Sahakian, H. Lee Moffitt Cancer Center & Research Institute, Department of Immunology, Tampa, FL 33612; e-mail: eva.sahakian@moffitt.org.