Key Points
The best response rates were observed with pembrolizumab before tisagenlecleucel; however, definitive conclusions cannot be made.
Pembrolizumab did not augment the cellular expansion of tisagenlecleucel but delayed peak expansion in the day 1 cohort.
Abstract
Tisagenlecleucel demonstrated high response rates and a manageable safety profile in adults with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL) in the JULIET trial. However, lack of response and chimeric antigen receptor (CAR) T-cell exhaustion were observed in patients with programmed cell death protein 1 (PD-1) overexpression. Hence, pembrolizumab, a PD-1 inhibitor, was hypothesized to improve efficacy and cellular expansion of CAR T-cells in vivo. Here, we report the final analysis of the PORTIA trial in adult patients with r/r DLBCL who had ≥2 prior lines of therapy and had an Eastern Cooperative Oncology Group performance status of ≤1. Patients received 1 tisagenlecleucel infusion on day 1. Pembrolizumab (200 mg) was given every 21 days, for up to 6 doses. Three cohorts initiated pembrolizumab on days 15 (n = 4), 8 (n = 4), or –1 (n = 4). Safety, efficacy, cellular kinetics, and biomarker analyses were included. Tisagenlecleucel plus pembrolizumab was feasible and showed a manageable safety profile, without dose-limiting toxicities. Emerging efficacy with tisagenlecleucel was observed when pembrolizumab was given the day before tisagenlecleucel; however, the limited patient sample and short follow-up do not allow for definitive conclusions. Adding pembrolizumab to tisagenlecleucel did not augment the cellular expansion of tisagenlecleucel but delayed peak expansion if given the day before tisagenlecleucel (NCT03630159).
Introduction
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy with high response rates and a manageable safety profile among adults with relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) in JULIET, a multicenter, open-label, single-arm, phase 2 trial. The overall response rate (ORR) in JULIET is 53%; 39% of the patients achieved complete response (CR; 40.3-month median follow-up).1 Overexpression of the immune checkpoint receptor, programmed cell death protein 1 (PD-1), was associated with CAR T-cell exhaustion in JULIET,2 and lack of response or relapse was evident in some patients with the highest PD-1: programmed death ligand 1 (PD-L1) interaction scores in baseline tumors.3 Hence, blocking the PD-1 pathway with the PD-1 inhibitor, pembrolizumab, could enhance CAR T-cell efficacy.4 Here, we report the final analysis of PORTIA, a phase 1b trial that investigated the efficacy, safety, and cellular kinetics of tisagenlecleucel in combination with pembrolizumab in adult patients with r/r DLBCL.
Methods
Study design
PORTIA (NCT03630159) was a multicenter, open-label, phase 1b trial of tisagenlecleucel and pembrolizumab to evaluate their safety and efficacy in patients with r/r DLBCL.5 Eligibility criteria were similar to JULIET1 and included adult patients with r/r DLBCL who underwent ≥2 prior lines of therapy (supplemental Table 1). The institutional review board at each participating institution approved the study, which was performed according to the Declaration of Helsinki.
Treatment
Patients received tisagenlecleucel infusion on day 1. Each patient was assigned to 1 of the following 3 cohorts for initiating pembrolizumab: day 15 after infusion (D15), day 8 after infusion (D8), and 1 day before tisagenlecleucel (D–1). Pembrolizumab (200 mg) was administered IV every 3 weeks for up to 6 doses until progressive disease (PD) or unacceptable toxicity (supplemental Figure 1).
Study objectives and end points
The primary objective was to assess the feasibility, safety, and tolerability of pembrolizumab in combination with tisagenlecleucel and to determine the optimal time for pembrolizumab. The primary end point was incidence of dose-limiting toxicities (DLTs) during the 21 days after the first pembrolizumab infusion (supplemental Table 2). Secondary end points included ORR (CR and partial response [PR]), duration of response, progression-free survival, overall survival, safety, cellular kinetics, and immunogenicity.
AEs
Adverse events (AEs) were reported using MedDRA (version 24.0) and Common Terminology Criteria for Adverse Events, version 5.0, except for cytokine release syndrome (CRS; Lee grading scale).6
Statistical methods
Incidence of DLTs was modeled using adaptive Bayesian logistic regression. Categorical data were summarized as frequency counts and percentages. Continuous data were summarized using descriptive statistics.
Cellular kinetics
Cellular kinetics were determined by transgene measurements (quantitative polymerase chain reaction) and tisagenlecleucel-transduced cells (flow cytometry) of CD3+ cells.
Cytokine/biomarker assessment
Circulating cytokines were investigated using longitudinal mean-time profiles. Baseline tumor biopsies were analyzed by a central laboratory using immunofluorescence immunohistochemistry to quantify PD-1/PD-L1 expression in both tumor and immune cells relative to total viable tumor cells (combined positivity score).
Results and discussion
Patient disposition
Baseline patient demographics are included in supplemental Table 3. Seventeen patients were screened, of whom 15 were enrolled. Of these, 3 patients discontinued before tisagenlecleucel: 2 because of AEs and 1 because of death. Three of 12 patients completed 6 cycles of pembrolizumab (2 in the D15 and 1 in the D–1 cohort). Discontinuation of pembrolizumab was due to PD in 5 (33.3%) patients and due to AE and patient decision, each, in 2 (13.3%) patients. Median follow-up from tisagenlecleucel infusion was 230 days.
Efficacy
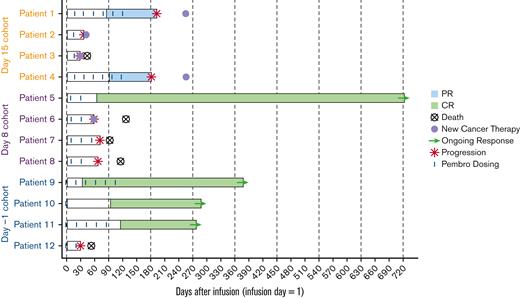
Among all patients, 4 (33.3%) had CR, 2 (16.7%) had PR, and 6 (50%) had PD. ORR was 50% (95% CI, 21.09-78.91). The D–1 cohort had the highest ORR (75%; 95% CI, 19.41-99.37); 3 patients achieved sustained CR. Two (50%) patients from the D15 cohort achieved PR (95% CI, 6.76-93.24), and 1 (25%) from the D8 cohort achieved CR (95% CI, 0.63-80.59) (Figure 1).
Swimmer plot analysis of response for all patients. Pembro, pembrolizumab.
Cellular kinetics
Cellular expansion was observed, with high variability in cellular kinetic parameters across cohorts (supplemental Table 4). Delayed tisagenlecleucel peak expansion was observed for the D–1 cohort (16 days vs 7.91 for D15 and 7.82 for D8). Clast and Tlast were higher for the D–1 cohort (557 copies per μg and 276 days compared with 104 copies per μg and 135 days for D15 and 259 copies per μg and 68.9 days for D8).
Pembrolizumab did not result in secondary expansion of tisagenlecleucel, regardless of the number of doses (supplemental Figure 2). Tisagenlecleucel had no effect on pembrolizumab pharmacokinetics.
Safety
All patients experienced ≥1 AE during the study. Treatment-related AEs are summarized in Table 1. No deaths were recorded ≤30 days after infusion; 5 of 12 patients died after 30 days because of PD. Two (16.7%) experienced treatment-related serious AEs (febrile neutropenia and CRS, each n = 1 [25%], from the D–1 and D8 cohorts, respectively). No patient discontinued study treatment because of AEs, and no DLTs were reported.
Grade ≥3 AEs-related to tisagenlecleucel and/or pembrolizumab
| AE . | Pembro D15 (n = 4), n (%) . | Pembro D8 (n = 4), n (%) . | Pembro D–1 (n = 4), n (%) . | Overall (N = 12), n (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| All grade . | Grade ≥3 . | All grade . | Grade ≥3 . | All grade . | Grade ≥3 . | All grade . | Grade ≥3 . | |
| Patients with at least 1 AE | 1 (25) | 0 (0) | 4 (100) | 2 (50) | 3 (75) | 2 (50) | 8 (66.7) | 4 (33.3) |
| Neutropenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (25) | 1 (25) | 2 (16.7) | 2 (16.7) |
| Decreased neutrophil count | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (25) | 0 (0) | 2 (16.7) | 1 (8.3) |
| CRS | 1 (25) | 0 (0) | 4 (100) | 0 (0) | 2 (50) | 1 (25) | 7 (58.3) | 1 (8.3) |
| Anemia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (25) | 0 (0) | 2 (16.7) | 1 (8.3) |
| Decreased lymphocyte count | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (25) | 1 (25) | 2 (16.7) | 1 (8.3) |
| Diarrhea | 3 (75) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (8.3) | 1 (8.3) |
| Decreased white blood cell count | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (25) | 0 (0) | 2 (16.7) | 0 (0) |
| Febrile neutropenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Malnutrition | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (8.3) | 1 (8.3) |
| Leukopenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Thrombocytopenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Hepatitis | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Increased liver function test | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (8.3) | 1 (8.3) |
| ICANS | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (8.3) | 0 (0) |
| AE . | Pembro D15 (n = 4), n (%) . | Pembro D8 (n = 4), n (%) . | Pembro D–1 (n = 4), n (%) . | Overall (N = 12), n (%) . | ||||
|---|---|---|---|---|---|---|---|---|
| All grade . | Grade ≥3 . | All grade . | Grade ≥3 . | All grade . | Grade ≥3 . | All grade . | Grade ≥3 . | |
| Patients with at least 1 AE | 1 (25) | 0 (0) | 4 (100) | 2 (50) | 3 (75) | 2 (50) | 8 (66.7) | 4 (33.3) |
| Neutropenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (25) | 1 (25) | 2 (16.7) | 2 (16.7) |
| Decreased neutrophil count | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (25) | 0 (0) | 2 (16.7) | 1 (8.3) |
| CRS | 1 (25) | 0 (0) | 4 (100) | 0 (0) | 2 (50) | 1 (25) | 7 (58.3) | 1 (8.3) |
| Anemia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (25) | 0 (0) | 2 (16.7) | 1 (8.3) |
| Decreased lymphocyte count | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (25) | 1 (25) | 2 (16.7) | 1 (8.3) |
| Diarrhea | 3 (75) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (8.3) | 1 (8.3) |
| Decreased white blood cell count | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (25) | 0 (0) | 2 (16.7) | 0 (0) |
| Febrile neutropenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Malnutrition | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (8.3) | 1 (8.3) |
| Leukopenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Thrombocytopenia | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Hepatitis | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) |
| Increased liver function test | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 1 (8.3) | 1 (8.3) |
| ICANS | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 1 (8.3) | 0 (0) |
D, day; ICANS, immune effector cell–associated neurotoxicity syndrome.
Seven of 12 patients across all cohorts experienced ≥1 CRS event; only 1 (D–1 cohort) experienced grade 3 CRS. The median time from infusion to onset of CRS was 2 days. Two patients required intensive care unit admission (median duration of 4 days). Two patients experienced CRS-related organ toxicities. Four patients required systemic anticytokine therapy. One patient experienced grade 2 ICANS (D–1 cohort), and none experienced grade ≥3 ICANS. There was no increase in typical inflammatory markers and cytokine profiles associated with severe CRS. No or minimal changes were observed for interleukin 1 beta (IL-1β), IL-2, IL-13, or tumor necrosis factor α. (supplemental Figure 3).
Baseline PD-L1 levels from 10 of 12 patients ranged from 3% to 70%; there was no obvious difference among the cohorts.
All 12 patients showed no detectable B-cell levels at all visits compared with baseline CD19+ B-cell levels.
The patients in PORTIA had high-risk clinical characteristics (58.3% had lactate dehydrogenase greater than the upper limit of normal, 75% had extranodal involvement, and 100% had International Prognostic Index ≥2 at study entry). Limited data suggest that the combination of tisagenlecleucel and pembrolizumab was feasible and showed a manageable safety profile, with no DLTs or significant exacerbation of tisagenlecleucel-related AEs. The D–1 cohort showed delayed peak expansion, possibly due to extended cellular expansion by PD-1 inhibition or because of the high variability and limited patient numbers across cohorts. Similar time to peak expansion between responders and nonresponders was evident in the JULIET and BELINDA trials with tisagenlecleucel alone.1,7 Although PORTIA was not powered to evaluate efficacy, 3 of 4 patients in D–1 and 1 of 4 patients in the D8 cohorts achieved sustained CR; 2 patients in the D15 cohort achieved PR. Pembrolizumab did not augment tisagenlecleucel expansion. These results resemble other CAR T-cell combination trials with checkpoint inhibitors that demonstrate comparable clinical outcomes with CAR T-cell therapy alone.8-11 The study was terminated before the dose expansion part based on thorough data analysis from the dose timing selection part and in consideration of other therapies with similar efficacies. The enrollment halt was not due to safety concerns.
Acknowledgments
Medical writing assistance was provided by Janrick See, Healthcare Consultancy Group and supported by Novartis Pharmaceuticals Corporation. The study was funded and designed by Novartis Pharmaceuticals Corporation and was approved by the institutional review board at each participating institution. Data were analyzed and interpreted by the authors and sponsor.
Authorship
Contribution: U.J., N.W., J.P.M., P.A.R., E.K.W., and I.F. enrolled and treated the patients and gathered data; Y.D., X.H., D.P., and S.R. accessed and verified the data in the study; and all authors analyzed and interpreted the data, participated in the writing and review of this article, and approved the final submitted version.
Conflict-of-interest disclosure: U.J. received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement #945393. This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation program, supported by the Medical-Scientific Fonds of the City of Vienna, Cancer Research Fonds Project-Nr.21098; grant support from Novartis; and honoraria and advisory boards from/of Novartis, Gilead, Bristol Myers Squibb (BMS), and Miltenyi. N.W. reports grants and personal fees from Novartis, Gilead, Sanofi-Genzyme, and Therakos, supported by the Medical-Scientific Fonds of the City of Vienna, Cancer Research Fonds Project-Nr.21098. P.A.R. reports consultancy and/or advisory board membership for Novartis, Bayer, BMS, Kite/Gilead, Takeda, BeiGene, Janssen, and Karyopharm; speaker’s bureau for Kite/Gilead; honoraria from Novartis; and research funding from BMS, Kite/Gilead, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, and Tessa Therapeutics. I.F. is an advisor or consultant for AbbVie, AstraZeneca, BMS, BeiGene, Gilead, Janssen, Novartis, Roche, and Seattle Genetics, and reports honoraria from AbbVie, Gilead, Janssen, Novartis, Roche, and Seattle Genetics. Y.D., X.H., D.P., and S.R. are employees of Novartis. E.K.W. reports consultancy, honoraria, travel expenses, membership on an entity’s board of directors or advisory committees, and research funding from Novartis; reports membership on an entity’s board of directors or advisory committees from CRISPR; research funding from Juno; is a member of the NCI Leukemia Steering Committee; and is an advisor or consultant for and reports equity ownership in Cambium Medical Technologies and Cambium Oncology. J.P.M. declares no competing financial interests.
Correspondence: Ulrich Jäger, Division of Hematology and Hemostaseology and Comprehensive Cancer Center, Department of Medicine I, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail: ulrich.jaeger@meduniwien.ac.at.
References
Author notes
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data availability of these trials is according to the criteria and process described in www.clinicalstudydatarequest.com.
The full-text version of this article contains a data supplement.