Key Points
Virus-specific T cells are safe in patients with SCD following hematopoietic stem cell transplant.
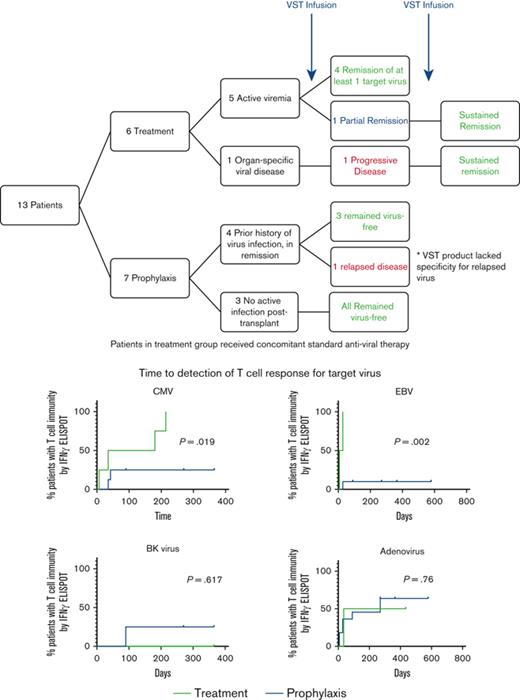
In patients with active viremia, 80% achieved remission of at least 1 target virus; 85.7% of the prophylaxis group remained virus-free.
Abstract
Hematopoietic stem cell transplantation (HSCT) is being increasingly used as a curative approach for sickle cell disease (SCD). With the risk of graft-versus-host disease (GVHD), especially in the human leukocyte antigen−mismatched donors, intense immunosuppression is required leading to an increased risk of viral infection. Post-HSCT, adoptive transfer of virus-specific T-cell (VST) therapies have not been well-studied in patients with SCD. Here, we report the outcomes of patients with SCD at a single-center who received VSTs after transplant to prevent or treat viral infections. Thirteen patients who received HSCT from human leukocyte antigen-matched (n = 9) or -mismatched (n = 4) donors for SCD were treated with a total of 15 VST products for the treatment or prophylaxis of multiple viruses (cytomegalovirus, Epstein-Barr virus, adenovirus, BK virus, human herpes virus 6 +/− human parainfluenza virus 3). Of the patients evaluated, 46.2% (n = 6)) received VSTs as treatment for viral infection. Eighty percent of patients with active viremia (n = 4/5) achieved remission of at least 1 target virus. Seven additional patients (53.8%) received VSTs prophylactically and 6 of 7 (85.7%) remained virus-free after infusion. No immediate infusion-related toxicities occurred, and severe de novo acute GVHD occurred in only 2 (15.4%) patients. Given the good safety profile, high-rate of clinical responses and sustained remissions when administered with standard antiviral treatments, the routine use of VSTs after HSCT as prophylaxis or treatment may improve the overall safety of transplant for patients with SCD.
Introduction
Hematopoietic stem cell transplantation (HSCT) remains the only curative option for many patients with relapsed and/or high-risk malignant diseases, immunodeficiency disorders, and hemoglobinopathies. With the advent of newer conditioning regimens, in vivo and ex vivo T-cell depletion and posttransplant cyclophosphamide, human leukocyte antigen (HLA)-mismatched HSCT is an approach with broadened applicability as a curative strategy for patients with sickle cell disease (SCD) who have limited donor options. However, HLA-mismatch donor HSCT is associated with a relatively high risk of graft-versus-host disease (GVHD) necessitating the use of pre- and posttransplant immunosuppression which increases the risk of viral reactivation, infection, and disease.1
Patients with SCD have a predisposition to develop infectious complications with decreased viral immunity because of relative lymphopenia, impaired lymphocyte function, and cell-mediated immune responses.2,3 This predisposition increases as lymphodepletion is used routinely before HSCT to minimize the relatively increased risk of graft rejection in SCD, in part owing to allosensitization in this population because of the need for multiple blood transfusions.4 The risk of viral infection after HSCT and before T-cell immune reconstitution, is especially increased in those who are recipients of mismatched donor grafts because of the additional immunosuppression required for effective GVHD prophylaxis.1,5,6 Studies in recipients of haploidentical donor transplants for SCD have well-documented this risk of increased viral infection, reactivation, and disease.7,8 More critically, viral infection or reactivation in the posttransplant setting has been implicated as a factor that may contribute to graft failure. Antiviral drug therapies have variable efficacy, are associated with resistance, and have appreciable toxicity including myelosuppression and renal impairment which limits their use in this HSCT population.9
Administration of posttransplant virus-specific T cells (VSTs) is an appealing therapeutic for the treatment and prevention of viral infections in this population, particularly when standard antiviral therapy is limited owing to preexisting end-organ damage caused by underlying SCD. Prophylaxis with donor-derived VSTs to prevent cytomegalovirus (CMV) infection and Epstein-Barr virus posttransplant lymphoproliferative disease (EBV-PTLD) has paved the way for novel treatment options in patients after HSCT.10-13 Although VSTs were initially isolated from the patient’s own HSCT donor who had immunity against the virus of interest, current advances in cell manufacturing have enabled the generation of VSTs from naïve donor sources such as cord blood.14 In addition, there is increasing interest in the use of third-party, partially matched donors to manufacture and establish “banks” of VST products for “off the shelf” use. Further, the use of viral peptide-pulsed antigen presenting cells has facilitated the rapid generation of VSTs targeting various viruses, thereby extending the clinical applications of these products. Specifically, several groups have produced VSTs targeting numerous viruses including (but not limited to) CMV, EBV, adenovirus (Adv), BK virus (BKV), human herpes virus 6 (HHV6), respiratory syncytial virus, influenza, parainfluenza and established the safety and efficacy of VSTs as treatment and/or prophylaxis of viral infection in patients who underwent HSCT13,15,16 with minimal to no increased risk of GVHD.9
Although several studies have reported the clinical application of VSTs in different transplant settings, there have been no focused studies that specifically report the outcomes of patients with SCD who receive VST treatment after HSCT. Here, we present the data and outcomes from a single center of patients with SCD who received VST for prophylaxis and treatment after HSCT.
Methods
A retrospective review was performed to identify pediatric patients (age range, 0-18 years) who received VST therapy after allogeneic HSCT for the treatment of SCD. Patients received VST infusions between August 2014 and November 2020 in the context of 6 prospective institutional review board approved nationally registered clinical protocols held under Investigational New Drug (ACTCAT2, NCT01923766; ALCI2, NCT01956084; CHAPS, NCT02510417; CHEERS, NCT03594981; MUSTAT, NCT01945814; NATS, NCT03180216). In addition, patients who were consented to institutional review board approved protocols and received VSTs under expanded access for compassionate use during the same time period were included in this analysis. The data of 2 recipients have been previously published as part of the primary trial results.14 VSTs were administered as prophylaxis or treatment of viral reactivation or active infection as soon as the patient met eligibility criteria after enrollment. To maintain consistency across the multiple studies included, prophylaxis was defined here as any patient (with or without a history of viral infection) with a negative viral load in the blood and absence of clinical symptoms of infection at the time of infusion. All patients undergoing allogeneic HSCT for SCD during the study period were offered enrollment for prophylaxis, with an emphasis on patients who received T-cell depleted or mismatched transplants. Patients receiving T-cell suppressive therapy including alemtuzumab within 28 days of VST infusion, steroid dose >0.5 mg/kg per day, Karnofsky/Lansky performance scale <50, grade 3 hyperbilirubinemia, evidence of acute GVHD grade 2 or higher or other severe active infections were excluded based on criteria for VST infusion on the aforementioned studies.
Of the 6 clinical protocols, 3 protocols evaluated VSTs targeting CMV, EBV, and Adv in a single product, 1 protocol used VSTs targeting EBV, 1 protocol used VSTs targeting CMV, EBV, Adv, and BKV, and 1 protocol administered VSTs targeting CMV, EBV, Adv, BKV, HHV6, and human parainfluenza virus 3 (HPIV3). Patients were evaluated for viral infection/reactivation before and after VST infusion by obtaining samples for polymerase chain reaction analysis from blood, stool, urine with or without nasopharyngeal and/or bronchoalveolar sampling as available or indicated. Polymerase chain reaction samples were collected for at least 3 months after VST infusion and were used to evaluate the antiviral response according to the protocol. Complete acute and chronic GVHD (cGVHD) staging and grading information and adverse events were reported as per the NCI Common Terminology Criteria for Adverse Events v4.03 (5 protocols) or v4.0 (1 protocol).
VST product generation
VST products were manufactured under Good Manufacturing Practice from the patient’s transplant donor including cord blood (CB) and peripheral blood (PB) donor sources. For patients whose transplant donor VST product was unavailable, VSTs manufactured from eligible third-party donors were used. VST expansion was performed as previously described.6,13 Briefly, donor PB or CB mononuclear cells were stimulated with antigen presenting cells either pulsed with virus-specific peptide libraries or transduced to express viral antigens. Resultant T-cell products were expanded in the presence of cytokines as previously described.12,14,17,18,19 VST products were generated to target 2 to 12 virus-specific antigens, depending on the protocol for CMV (PP65, IE1), EBV (eg, EBNA1, LMP2 +/− LMP1 +/− lymphoblastoid cell line [LCL]), Adv (hexon, penton), BKV (VP1, LgT), HHV6 (U54, V90) and/or HPIV3 (matrix, nucleoprotein). VST products were tested for sterility and nonalloreactivity through 51Cr release assay before infusion.
VST product functional analysis
VST product–specific activity against each of the target viral peptides (eg, PP65, IE1, LMP2, EBNA1, LMP1, hexon, penton, VP1, LgT, U54, V90, matrix and nucleoprotein; JPT Peptide Technologies, Berlin, Germany) was evaluated by anti-interferon gamma (IFN-γ) enzyme-linked immunospot (ELISpot) assay before infusion. Assays were read by Zellnet consulting (Fort Lee, NJ).
Clinical response definitions
Clinical response after VST infusion was categorized as sustained remission (SR; continued absence of detectable viral load in the patients’ PB, urine, or nasopharyngeal swab or organ involvement for systemic disease in patients treated prophylactically including patients with a previous history of infection who were virus-free at the time of VST infusion), complete response (CR; resolution of active targeted viral infections, as defined by clearance of detectable virus from body fluids assayed, and normalization of any clinical signs and symptoms attributed to the viral infection), partial response (PR; sustained decrease in viral load of at least 1 logarithm from baseline with improvement in clinical signs and symptoms attributed to the targeted viral infection [hepatitis, pneumonitis, diarrhea, fever curve, etc.]), mixed response (decrease in viral load of at least 1 logarithm from baseline for 1 targeted infection and an increase or no change in viral load for a second infection [only applicable for patients with ≥2 infections at baseline]), stable disease (changes insufficient to qualify as PR or progression), and progressive disease (PD; sustained increase in viral load of at least 1 logarithm from baseline or dissemination to other sites of disease). For patients with EBV lymphoma and measurable disease, response was assessed by response evaluation criteria in solid tumors (RECIST).16
Statistical analysis
Cumulative incidence curves were estimated using the GraphPad Prism 9 platform. Curve comparisons were calculated using the log-rank test. T-cell specificity to viral antigens was characterized as 10x, the upper limit of the 95% confidence interval for actin (negative control) and at least 10 spots forming units per 1 × 105 cells. For patients with baseline positivity (met above criteria before infusion), antigen response was defined as a two fold increase from baseline.
Results
Thirteen patients who received an HSCT for SCD were treated with VSTs after transplantation. One patient with SCD also had Wiskott-Aldrich syndrome. HSCT donors included matched sibling donor (MSD; n = 4), haploidentical donor (n = 4), matched unrelated donor (MUD; n = 2), and umbilical cord donors (UCB; n = 3; Table 1 and 2). Patients were allowed to receive multiple infusions of the same or different products. A total of 16 VST infusions were administered. Thirteen products were donor-derived from the HSCT donor and 3 products were derived from third-party donors. Median age at the time of receiving transplant was 13.3 years (range 0.95-18.2). VST infusions were administered at a median of 108 days (range 34-363 days) after HSCT. A total of 15 different products were administered to the 13 patients evaluated, 1 patient received the same product in 2 separate infusions. Dose of VST infusion varied by protocol, ranging from 5 × 106/m2 to 5 × 107/m2. Patients received VSTs targeting EBV alone (2 products); EBV, CMV, and Adv (8 products); EBV, CMV, Adv, and BKV (1 product) or all 6 viruses, EBV, CMV, Adv, BKV, HHV6, and HPIV3 (4 products).
Characteristics and clinical outcomes of patients with active viral infection treated with VSTs
| Patient ID . | Age at time of 1st infusion (y) . | Diagnosis . | BMT type . | Indication for VSTs . | VST donor . | Target viruses . | Day of infusion (after BMT) . | Virus status at time of infusion (blood) . | Viral treatment (before VSTs) . | VST dose . | Virus outcome (days post VST) . | Adverse events . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.86 | HbSS | MSD | EBV-PTLD | HSCT donor | CMV, EBV, adenovirus | Day +84 | EBV 3809 copies/mL | (Rituximab) | 2 × 107/m2 | PR PTLD resolved but recurred EBViremia (peak at 60 d) | Grade 3 transaminitis |
| HSCT donor | EBV∗ | Day +180, 194 | EBV undetectable | (Rituximab) | 2 × 107/m2 div in 2 doses | Sustained remission (low level detection <1000 copies/mL at 2 y not requiring treatment) Last follow-up: 723 d | None | |||||
| 3 | 9.41 | HbSS | 10/10 MUD | Adenovirus viremia and colitis, BK viruria, history of CMV reactivation | Third party (5/10 MMUD) | CMV, EBV, adenovirus | Day +293 | Adenovirus 25 900 copies/mL BKV 3200 copies/mL | Cidofovir (Brincidofovir) | 2 × 107/m2 | PR Transient improvement in viral load and colitis | None |
| Third party (5/10 MMUD) | CMV, EBV, adenovirus | Day +363 | Adenovirus 11 235 copies/mL BKV 10 300 copies/mL CMV undetectable | (Cidofovir) | 2 × 107/m2 | Adenovirus CR (42 d) BK virus PR (downtrending viral load) Last follow-up:154 d | None | |||||
| 4 | 3.68 | HbSS | MSD | CMV viremia, BK cystitis | HSCT donor | CMV, EBV, adenovirus, HHV6, HPIV3, BKV | Day +105 | CMV detectable <1000 copies/mL BKV 3000 copies/mL | Ganciclovir, (Cidofovir, Foscarnet) | 2 × 107/m2 | CMV CR (180 d) Resolution of BK cystitis with continued detectable viruria (peak > 1 × 108 at 27 d, downtrended without further antiviral treatment) Last follow-up:176 d | None |
| 5 | 14.03 | HbSC | MSD | EBV viremia, CMV viremia (below limit of detection) | HSCT donor | CMV, EBV, adenovirus | Day +34 | CMV low level detectable <1000 copies/mL EBV 231 787 copies/mL | Valganciclovir, (Rituximab) | 5 × 106/m2 | EBV CR (10 d) CMV CR (10 d) with intermittent low-level detection (<1000 copies/mL) not requiring treatment Last follow-up:1294 d | Chronic GVHD 2 months post-infusion |
| 7 | 8.94 | HbSS | 5/6 UCBT | Disseminated CMV (stool, urine) | HSCT donor | CMV, EBV, adenovirus | Day +71 | Undetectable | Ganciclovir (Foscarnet) | 5 × 106/m2 | PD initial clearance of CMV and symptoms but subsequently intermittently detectable up to 1376 copies/mL (90 d). Developed CMV retinitis 173 d after VST infusion. | Acute stage 3 skin and stage 1 GI GVHD, resolved with systemic and topical steroids |
| Recent history of CMV retinitis | Third party (3/10 MMUD) | CMV,∗EBV, adenovirus | Day +285 | Undetectable | Ganciclovir, valganciclovir (foscarnet, intravitreal ganciclovir, immunoglobulin) | 2 × 107/m2 | CMV sustained remission (90 d) Last follow-up: 436 d | None | ||||
| 11 | 3.32 | HbSS | 5/6 UCBT | CMV and adenoviremia | HSCT donor | CMV, EBV, adenovirus, BKV | Day +97 | CMV 10 778 copies/mL Adenovirus 358 copies/mL | Ganciclovir, cidofovir, valganciclovir (foscarnet) | 1 × 107/m2 | CR (90 d) on cidofovir. With intermittent low-level detection (less than quantifiable) for CMV not requiring further treatment Last follow-up: 384 d | None |
| Patient ID . | Age at time of 1st infusion (y) . | Diagnosis . | BMT type . | Indication for VSTs . | VST donor . | Target viruses . | Day of infusion (after BMT) . | Virus status at time of infusion (blood) . | Viral treatment (before VSTs) . | VST dose . | Virus outcome (days post VST) . | Adverse events . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.86 | HbSS | MSD | EBV-PTLD | HSCT donor | CMV, EBV, adenovirus | Day +84 | EBV 3809 copies/mL | (Rituximab) | 2 × 107/m2 | PR PTLD resolved but recurred EBViremia (peak at 60 d) | Grade 3 transaminitis |
| HSCT donor | EBV∗ | Day +180, 194 | EBV undetectable | (Rituximab) | 2 × 107/m2 div in 2 doses | Sustained remission (low level detection <1000 copies/mL at 2 y not requiring treatment) Last follow-up: 723 d | None | |||||
| 3 | 9.41 | HbSS | 10/10 MUD | Adenovirus viremia and colitis, BK viruria, history of CMV reactivation | Third party (5/10 MMUD) | CMV, EBV, adenovirus | Day +293 | Adenovirus 25 900 copies/mL BKV 3200 copies/mL | Cidofovir (Brincidofovir) | 2 × 107/m2 | PR Transient improvement in viral load and colitis | None |
| Third party (5/10 MMUD) | CMV, EBV, adenovirus | Day +363 | Adenovirus 11 235 copies/mL BKV 10 300 copies/mL CMV undetectable | (Cidofovir) | 2 × 107/m2 | Adenovirus CR (42 d) BK virus PR (downtrending viral load) Last follow-up:154 d | None | |||||
| 4 | 3.68 | HbSS | MSD | CMV viremia, BK cystitis | HSCT donor | CMV, EBV, adenovirus, HHV6, HPIV3, BKV | Day +105 | CMV detectable <1000 copies/mL BKV 3000 copies/mL | Ganciclovir, (Cidofovir, Foscarnet) | 2 × 107/m2 | CMV CR (180 d) Resolution of BK cystitis with continued detectable viruria (peak > 1 × 108 at 27 d, downtrended without further antiviral treatment) Last follow-up:176 d | None |
| 5 | 14.03 | HbSC | MSD | EBV viremia, CMV viremia (below limit of detection) | HSCT donor | CMV, EBV, adenovirus | Day +34 | CMV low level detectable <1000 copies/mL EBV 231 787 copies/mL | Valganciclovir, (Rituximab) | 5 × 106/m2 | EBV CR (10 d) CMV CR (10 d) with intermittent low-level detection (<1000 copies/mL) not requiring treatment Last follow-up:1294 d | Chronic GVHD 2 months post-infusion |
| 7 | 8.94 | HbSS | 5/6 UCBT | Disseminated CMV (stool, urine) | HSCT donor | CMV, EBV, adenovirus | Day +71 | Undetectable | Ganciclovir (Foscarnet) | 5 × 106/m2 | PD initial clearance of CMV and symptoms but subsequently intermittently detectable up to 1376 copies/mL (90 d). Developed CMV retinitis 173 d after VST infusion. | Acute stage 3 skin and stage 1 GI GVHD, resolved with systemic and topical steroids |
| Recent history of CMV retinitis | Third party (3/10 MMUD) | CMV,∗EBV, adenovirus | Day +285 | Undetectable | Ganciclovir, valganciclovir (foscarnet, intravitreal ganciclovir, immunoglobulin) | 2 × 107/m2 | CMV sustained remission (90 d) Last follow-up: 436 d | None | ||||
| 11 | 3.32 | HbSS | 5/6 UCBT | CMV and adenoviremia | HSCT donor | CMV, EBV, adenovirus, BKV | Day +97 | CMV 10 778 copies/mL Adenovirus 358 copies/mL | Ganciclovir, cidofovir, valganciclovir (foscarnet) | 1 × 107/m2 | CR (90 d) on cidofovir. With intermittent low-level detection (less than quantifiable) for CMV not requiring further treatment Last follow-up: 384 d | None |
Viruses in bold indicate product specificity for this target virus.
CR, complete remission; Haplo, haploidentical HLA match; PBSCT, peripheral blood stem cell transplant; PR, partial response; SR, sustained remission; UCBT, umbilical cord blood transplant.
Infusion of a different VST product from previous.
Characteristics and clinical outcomes of patients receiving VSTs for viral prophylaxis
| Patient ID . | Age at first infusion (y) . | Diagnosis . | BMT type . | Indication for VSTs . | VST donor (donor/patient serostatus) . | Target viruses . | Day of infusion (post BMT) . | Virus status at time of infusion . | Viral treatment (completed before VSTs) . | VST dose . | Virus outcome (days post VST) . | Adverse events . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 16.55 | HbSS | MSD | History of CMV and EBV viremia, BK cystitis | HSCT donor CMV: −/+ | EBV, CMV, adenovirus | Day +111 | CMV, EBV, BKV undetectable | (Rituximab, cidofovir, valganciclovir) | 2 × 107/m2 | Sustained remission Last follow-up: 210 d | Acute grade 1 skin GVHD, resolved with topical steroids |
| 6 | 15.93 | HbSS | 8/8 MUD (2nd transplant) | Prophylaxis (history of EBV-PTLD after 1st transplant) | HSCT donor CMV: +/− EBV: +/+ | EBV, CMV, adenovirus | Day +39 | EBV undetectable | (Rituximab before 2nd transplant) | 2 × 107/m2 | Remains virus-free Last follow-up: 225 d | Chronic GVHD (skin) |
| 8 | 13.29 | HbSB0Thal | Haplo | Prophylaxis (donor EBV positive) | HSCT donor (EBV positive) CMV: −/− EBV: +/− | EBV | Day +114 | EBV undetectable | (Rituximab before transplant) | 2 × 107/m2 div into 2 doses | Remains virus-free Last follow-up: 713 d | Acute grade 3-4 skin GVHD responsive to systemic steroids. Later developed chronic skin GVHD |
| 9 | 3.96 | HbSS | 5/6 UCBT | History of adenoviremia | HSCT donor CMV: NA/− ADV: NA/+ | EBV, CMV, adenovirus | Day +118 | Adenovirus undetectable | (Cidofovir, immunoglobulin) | 1 × 107/m2 | Adenovirus relapsed disease 5 months post-VST Intermittent adenovirus reactivation below quantifiable levels <200 copies/mL and positive BAL treated with brincidofovir 5 months post-VST EBV, CMV negative Last follow-up: 399 d | Acute grade 2 skin GVHD, persisted with topical steroids developed into severe chronic skin GVHD resolved with tacrolimus, ECP |
| 10 | 13.38 | HbSS | Haplo | Prophylaxis (Donor EBV positive) | HSCT donor CMV: −/− EBV: +/− | EBV, CMV, adenovirus, HHV6, HPIV3, BKV | Day +80 | Undetectable | (Rituximab before transplant) | 1 × 107/m2 | Remains virus-free Last follow-up: 257 d | None |
| 12 | 18.5 | HbSS | Haplo (PBSCT) | Prophylaxis (Donor EBV, CMV positive) | HSCT donor CMV: +/− EBV: +/− | EBV, CMV, adenovirus, HHV6, HPIV3, BKV | Day +98 | Undetectable | (Rituximab before transplant) | 2 × 107/m2 | Remains virus-free Last follow-up: 180 d | None |
| 13 | 0.95 | WAS and HbSS | Haplo (PBSCT) | History of adenovirus before transplant, prophylaxis (donor EBV positive) | HSCT donor CMV: −/+ EBV: +/+ | EBV, CMV, adenovirus, HHV6, HPIV3, BKV | Day +149 | Undetectable | (Rituximab day +1 after transplant, Cidofovir before transplant) | 5 × 107/m2 | Remains virus-free Last follow-up: 202 d | None |
| Patient ID . | Age at first infusion (y) . | Diagnosis . | BMT type . | Indication for VSTs . | VST donor (donor/patient serostatus) . | Target viruses . | Day of infusion (post BMT) . | Virus status at time of infusion . | Viral treatment (completed before VSTs) . | VST dose . | Virus outcome (days post VST) . | Adverse events . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 16.55 | HbSS | MSD | History of CMV and EBV viremia, BK cystitis | HSCT donor CMV: −/+ | EBV, CMV, adenovirus | Day +111 | CMV, EBV, BKV undetectable | (Rituximab, cidofovir, valganciclovir) | 2 × 107/m2 | Sustained remission Last follow-up: 210 d | Acute grade 1 skin GVHD, resolved with topical steroids |
| 6 | 15.93 | HbSS | 8/8 MUD (2nd transplant) | Prophylaxis (history of EBV-PTLD after 1st transplant) | HSCT donor CMV: +/− EBV: +/+ | EBV, CMV, adenovirus | Day +39 | EBV undetectable | (Rituximab before 2nd transplant) | 2 × 107/m2 | Remains virus-free Last follow-up: 225 d | Chronic GVHD (skin) |
| 8 | 13.29 | HbSB0Thal | Haplo | Prophylaxis (donor EBV positive) | HSCT donor (EBV positive) CMV: −/− EBV: +/− | EBV | Day +114 | EBV undetectable | (Rituximab before transplant) | 2 × 107/m2 div into 2 doses | Remains virus-free Last follow-up: 713 d | Acute grade 3-4 skin GVHD responsive to systemic steroids. Later developed chronic skin GVHD |
| 9 | 3.96 | HbSS | 5/6 UCBT | History of adenoviremia | HSCT donor CMV: NA/− ADV: NA/+ | EBV, CMV, adenovirus | Day +118 | Adenovirus undetectable | (Cidofovir, immunoglobulin) | 1 × 107/m2 | Adenovirus relapsed disease 5 months post-VST Intermittent adenovirus reactivation below quantifiable levels <200 copies/mL and positive BAL treated with brincidofovir 5 months post-VST EBV, CMV negative Last follow-up: 399 d | Acute grade 2 skin GVHD, persisted with topical steroids developed into severe chronic skin GVHD resolved with tacrolimus, ECP |
| 10 | 13.38 | HbSS | Haplo | Prophylaxis (Donor EBV positive) | HSCT donor CMV: −/− EBV: +/− | EBV, CMV, adenovirus, HHV6, HPIV3, BKV | Day +80 | Undetectable | (Rituximab before transplant) | 1 × 107/m2 | Remains virus-free Last follow-up: 257 d | None |
| 12 | 18.5 | HbSS | Haplo (PBSCT) | Prophylaxis (Donor EBV, CMV positive) | HSCT donor CMV: +/− EBV: +/− | EBV, CMV, adenovirus, HHV6, HPIV3, BKV | Day +98 | Undetectable | (Rituximab before transplant) | 2 × 107/m2 | Remains virus-free Last follow-up: 180 d | None |
| 13 | 0.95 | WAS and HbSS | Haplo (PBSCT) | History of adenovirus before transplant, prophylaxis (donor EBV positive) | HSCT donor CMV: −/+ EBV: +/+ | EBV, CMV, adenovirus, HHV6, HPIV3, BKV | Day +149 | Undetectable | (Rituximab day +1 after transplant, Cidofovir before transplant) | 5 × 107/m2 | Remains virus-free Last follow-up: 202 d | None |
Viruses in bold indicate product specificity for this target virus.
BAL, bronchoalveolar lavage; CR, complete remission; Haplo, haploidentical HLA match; PBSCT, peripheral blood stem cell transplant; UCBT, umbilical cord blood transplant; WAS, Wiskott-Aldrich Syndrome.
VST infusions and product specificity
A total of 16 VST infusions were administered overall (13 donor-derived and 3 third-party). Eleven patients received 1 VST product. Two patients with minimal response or persistent disease after initial VST infusion received subsequent VST infusions from alternate third-party donors (patient 7 [P7]) or, in the case of P1, received a VST product derived from the same donor but generated using a different manufacturing protocol. The first product was generated to target EBV, Adv, and CMV, whereas the second product was generated to target only EBV for persistent EBV viremia. Antigen-specific responses of the VST products were evaluated by IFN-γ+ ELISpot assay as shown in Table 1 and 2. Products with positive responses to antigens for each of the target viruses are shown in Figures 1-5. Of the 15 products generated to target EBV, 9 (60%) were specific for LMP1, LMP2 and/or EBNA1. Of the 13 products generated to target CMV and Adv, 8 (61%) were specific for PP65 and/or IE1 and 11 (84.6%) were specific for hexon and/or penton. Two additional products (P8, P1.2) generated to target EBV using transduction with an Adv vector, were also specific for hexon and/or penton. Of the 5 products generated to target BKV, 3 (60%) were specific for VP1 and/or LgT. Of the 4 products generated to target HHV6 and HPIV3, 4 (100%) were specific for both matrix and NP and U54 and V90. Twelve of the 15 products were specific for >1 virus. One product that was generated to target EBV, CMV and Adv, was not specific for any targets on IFN-γ+ ELISpot assay (P9), another product generated to target EBV was only specific for Adv (P8).
Anti-CMV T-cell activity and clinical response in patients treated with CMV-specific VSTs for active CMV infection. (A) Anti-CMV antigen (phosphoprotein 65 [PP65], immediate early protein-1 [IE1]) T-cell response of the CMV-specific VST products as detected by IFN-γ ELISpot assay. Anti-CMV antigen T-cell response and CMV viral load (IU or copies per milliliter) in the recipient PB post-VST infusion for patient P4, P5, P7 treated with CMV infection (B-D) and timing of concomitant standard antiviral treatment. PCR, polymerase chain reaction; SFU, spot forming units.
Anti-CMV T-cell activity and clinical response in patients treated with CMV-specific VSTs for active CMV infection. (A) Anti-CMV antigen (phosphoprotein 65 [PP65], immediate early protein-1 [IE1]) T-cell response of the CMV-specific VST products as detected by IFN-γ ELISpot assay. Anti-CMV antigen T-cell response and CMV viral load (IU or copies per milliliter) in the recipient PB post-VST infusion for patient P4, P5, P7 treated with CMV infection (B-D) and timing of concomitant standard antiviral treatment. PCR, polymerase chain reaction; SFU, spot forming units.
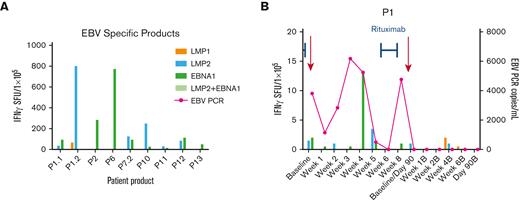
Anti-EBV T-cell activity and clinical response in patients treated with EBV-specific VSTs for active EBV infection. (A) Anti-EBV antigen (EBNA1, LMP1, LMP2) T-cell response of the EBV-specific VST products as detected by IFN-γ ELISpot assay. Anti-EBV antigen T-cell response and EBV viral load (copies per milliliter) in the recipient PB post-VST infusion for patient P1 treated with EBV infection (B) and timing of concomitant standard antiviral treatment. EBNA, Epstein-Barr nuclear antigen; LMP, latent membrane protein; PCR, polymerase chain reaction; SFU, spot forming units.
Anti-EBV T-cell activity and clinical response in patients treated with EBV-specific VSTs for active EBV infection. (A) Anti-EBV antigen (EBNA1, LMP1, LMP2) T-cell response of the EBV-specific VST products as detected by IFN-γ ELISpot assay. Anti-EBV antigen T-cell response and EBV viral load (copies per milliliter) in the recipient PB post-VST infusion for patient P1 treated with EBV infection (B) and timing of concomitant standard antiviral treatment. EBNA, Epstein-Barr nuclear antigen; LMP, latent membrane protein; PCR, polymerase chain reaction; SFU, spot forming units.
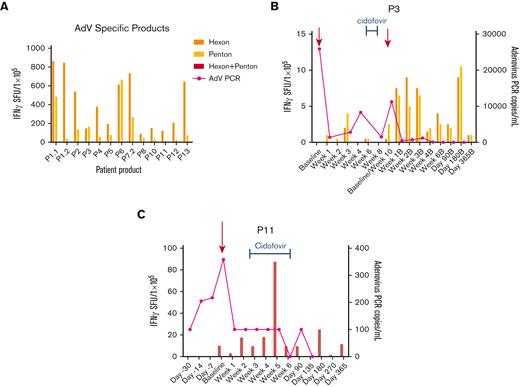
Anti-AdV T-cell activity and clinical response in patients treated with AdV-specific VSTs for active adenovirus (AdV) infection. (A) Anti-adenoviral antigen (hexon, penton) T-cell response of the AdV-specific VST products as detected by IFN-γ ELISpot assay. Anti-AdV antigen T-cell response and AdV viral load (copies per milliliter) in the recipient PB post-VST infusion for patient P3, P11 treated with AdV infection (B and C) and timing of concomitant standard antiviral treatment. PCR, polymerase chain reaction; SFU, spot forming units.
Anti-AdV T-cell activity and clinical response in patients treated with AdV-specific VSTs for active adenovirus (AdV) infection. (A) Anti-adenoviral antigen (hexon, penton) T-cell response of the AdV-specific VST products as detected by IFN-γ ELISpot assay. Anti-AdV antigen T-cell response and AdV viral load (copies per milliliter) in the recipient PB post-VST infusion for patient P3, P11 treated with AdV infection (B and C) and timing of concomitant standard antiviral treatment. PCR, polymerase chain reaction; SFU, spot forming units.
Anti-BKV T-cell activity and clinical response in patients treated with BKV-specific VSTs for active BKV infection. (A) Anti-BKV antigen (viral capsid protein 1 [VP1], large T [LgT]) T-cell response of the BKV-specific VST products as detected by IFN-γ ELISpot assay. Anti-BKV antigen T-cell response and BKV viral load (copies per milliliter) in the recipient PB post-VST infusion for patient P4 treated with BKV infection (B) and timing of concomitant standard antiviral treatment. BKV, BK virus; PCR, polymerase chain reaction; SFU, spot forming units.
Anti-BKV T-cell activity and clinical response in patients treated with BKV-specific VSTs for active BKV infection. (A) Anti-BKV antigen (viral capsid protein 1 [VP1], large T [LgT]) T-cell response of the BKV-specific VST products as detected by IFN-γ ELISpot assay. Anti-BKV antigen T-cell response and BKV viral load (copies per milliliter) in the recipient PB post-VST infusion for patient P4 treated with BKV infection (B) and timing of concomitant standard antiviral treatment. BKV, BK virus; PCR, polymerase chain reaction; SFU, spot forming units.
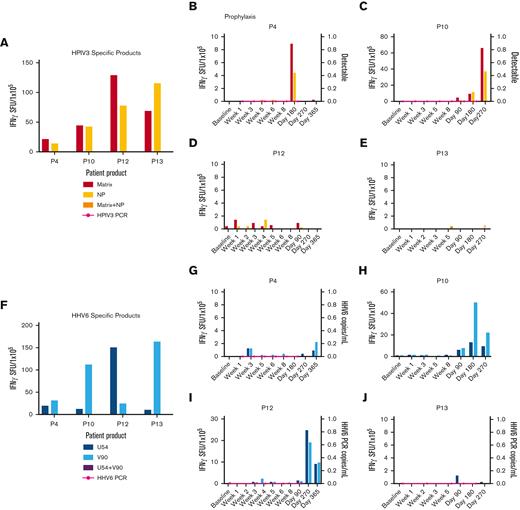
Anti-HPIV3 and HHV6 T-cell activity and clinical response. (A) Anti-HPIV3 antigen (matrix, NP) T-cell response of the HPIV3-specific VST products as detected by IFN-γ ELISpot assay. Anti-HPIV3 antigen T-cell response in the recipient PB and detection of HPIV3 by PCR of a nasal swab post-VST infusion for prophylaxis against HPIV3 (B-E). (F) Anti-HHV6 antigen (U54, V90) T-cell response of the HHV6-specific VST products as detected by IFN-γ ELISpot assay. (G-J) Anti-HHV6 antigen T-cell response and HHV6 viral load (copies per milliliter) in the recipient PB post-VST infusion for prophylaxis against HHV6. HHV6, human herpes virus 6; HPIV3, human parainfluenza virus 3; NP, nucleocapsid: PCR, polymerase chain reaction; SFU, spot forming units.
Anti-HPIV3 and HHV6 T-cell activity and clinical response. (A) Anti-HPIV3 antigen (matrix, NP) T-cell response of the HPIV3-specific VST products as detected by IFN-γ ELISpot assay. Anti-HPIV3 antigen T-cell response in the recipient PB and detection of HPIV3 by PCR of a nasal swab post-VST infusion for prophylaxis against HPIV3 (B-E). (F) Anti-HHV6 antigen (U54, V90) T-cell response of the HHV6-specific VST products as detected by IFN-γ ELISpot assay. (G-J) Anti-HHV6 antigen T-cell response and HHV6 viral load (copies per milliliter) in the recipient PB post-VST infusion for prophylaxis against HHV6. HHV6, human herpes virus 6; HPIV3, human parainfluenza virus 3; NP, nucleocapsid: PCR, polymerase chain reaction; SFU, spot forming units.
Viral infections and clinical responses
Of the 13 patients evaluated, 46.2% (n = 6) received VSTs as treatment for detectable viral reactivation or infection with CMV (n = 4), EBV (n = 2), Adv (n = 2) and/or BKV (n = 3) while receiving treatment with standard antiviral therapy (Table 1). Five patients had active viremia, 80% (n = 4/5) achieved complete remission of at least one of the target viruses for which they were positive after VST infusion. The remaining patient with active viremia demonstrated a PR. Patient P7, received VSTs at a time when they had disseminated CMV (with CMV in urine, stool, and nasopharynx) without active viremia. However, the infused CB-derived VST product lacked specificity against CMV. There was an initial sustained virus-free period after infusion but P7 subsequently developed CMV retinitis 173 days after VSTs and underwent a second VST infusion from an alternative donor source specific for CMV, followed by a long-term SR.
Seven patients (53.8%) received VSTs prophylactically to prevent viral infection or reactivation after receiving transplant (Table 2). Two of these patients (P9 and P2) had viral reactivation or infection after transplant but achieved an undetectable viral load with standard antiviral therapy before VST infusion. P9 who had a recent history of adenoviremia with a negative viral load at the time of VST infusion, received a CB-derived VST product that lacked specificity for Adv. This patient had a single positive Adv test from bronchoalveolar lavage 5 months after VSTs in the setting of cGVHD on immunosuppressive therapy and was treated with brincidofovir. The other patient (P2) remained virus-free after VST infusion. All remaining patients treated prophylactically remained free of detectable virus after VST infusion.
Time to detection of response to target viral antigens
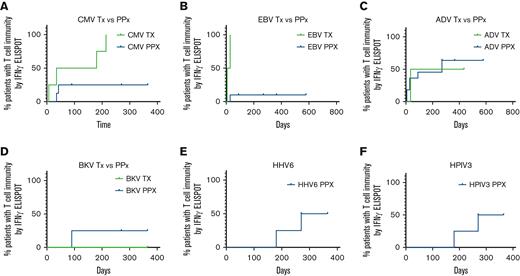
Probability of T-cell reactivity against targeted viral antigens over time is presented in Figure 6. The 4 patients treated for CMV infection with VSTs shown to be CMV-specific (n = 2/4) had detectable antiviral T-cell responses through IFN-γ+ ELISpot assay at a median of 180 days after VST infusion. Of the 8 patients treated prophylactically, 5 received VSTs shown to be CMV-specific and 2 of those patients had new detectable CMV-specific T-cell responses in the first 45 days after infusion. Time to detectable responses was statistically different between those treated for infection vs patients who received VSTs as prophylaxis (Figure 6A; P = .019). Two out of 13 patients evaluated for CMV had baseline T-cell reactivity in the PB before infusion, 1 was in the active infection group (P3) and 1 in the prophylaxis group (P2).
Time to detection of response to target viral antigens. (A) Time to detection of T-cell response to CMV for patients receiving VSTs as treatment (green; n = 4) and patients treated prophylactically (blue; n = 8). Time to detectable responses differed between those treated for infection vs prophylaxis (P = .019). Time to detection of T-cell response to EBV (B) for patients receiving VSTs as treatment (green; n = 2) and patients treated prophylactically (blue; n = 11). Time to detectable responses differed between those treated for infection vs prophylaxis (P = .002). Time to detection of T-cell response to Adv (C) for patients receiving VSTs as treatment (green; n = 2) and patients treated prophylactically (blue; n = 11). No difference in time to detectable response between treatment and prophylactic groups (P = .76). Time to detection of T-cell response to BKV (D) for patients receiving VSTs as treatment (green; n = 2) and patients treated prophylactically (blue; n = 3). No difference in time to detectable response between treatment and prophylactic groups (P = .617). Time to detection of T-cell response to HHV6 (E) and HPIV3 (F) for patients treated prophylactically (n = 4). Adv, adenovirus; BKV, BK virus; CMV, cytomegalovirus; HHV6, human herpes virus 6; HPIV3, human parainfluenza virus 3.
Time to detection of response to target viral antigens. (A) Time to detection of T-cell response to CMV for patients receiving VSTs as treatment (green; n = 4) and patients treated prophylactically (blue; n = 8). Time to detectable responses differed between those treated for infection vs prophylaxis (P = .019). Time to detection of T-cell response to EBV (B) for patients receiving VSTs as treatment (green; n = 2) and patients treated prophylactically (blue; n = 11). Time to detectable responses differed between those treated for infection vs prophylaxis (P = .002). Time to detection of T-cell response to Adv (C) for patients receiving VSTs as treatment (green; n = 2) and patients treated prophylactically (blue; n = 11). No difference in time to detectable response between treatment and prophylactic groups (P = .76). Time to detection of T-cell response to BKV (D) for patients receiving VSTs as treatment (green; n = 2) and patients treated prophylactically (blue; n = 3). No difference in time to detectable response between treatment and prophylactic groups (P = .617). Time to detection of T-cell response to HHV6 (E) and HPIV3 (F) for patients treated prophylactically (n = 4). Adv, adenovirus; BKV, BK virus; CMV, cytomegalovirus; HHV6, human herpes virus 6; HPIV3, human parainfluenza virus 3.
The median time to detection of an EBV-specific T-cell response in patients (n = 2) treated for EBV infection or reactivation was 17.5 days, whereas responses were detected in only 2 of 11 patients who received VSTs as prophylaxis (Figure 6B; P = .002).
Adv-specific T-cell responses, were detected at a median of 235 days after VST infusion in patients with active Adv infection (n = 2) vs 270 days for those who received VSTs as prophylaxis (n = 10). Two patients with a history of Adv infection had detectable Adv-specific T cells before VST infusion, therefore antiviral T-cell specificity was measured as two fold of the baseline response, though differences in the curves were not statistically significant (P = .76).
Of the patients treated with products generated to target BKV (n = 5), 3 patients were infused with BKV-specific T-cell products (P4, P12, and P13), 1 of whom was treated for active BKV cystitis. The 2 patients treated prophylactically with BKV-specific products had a detectable anti-BKV–specific T-cell response by 90 days after infusion. The remaining 2 patients (P10 and P11) were treated prophylactically for BKV and remained BKV-free after infusion but they did not develop detectable anti-BKV–specific responses up to 1 year after VST infusion. There was no statistically significant difference in time to detectable antigen response for patients treated for active disease (n = 1) and those treated prophylactically (n = 4) (P = .617).
Four patients were evaluated for HHV6 antiviral T-cell responses after receiving VSTs as prophylaxis. All 4 VST products were specific for the HHV6 target antigens (U54 and V90). Two patients had detectable HHV6-specific T-cell responses with an overall median time to detection of 317.5 days.
Finally, 4 patients who received VSTs as prophylaxis were evaluated for HPIV3-specific T-cell immunity. All 4 VST products were specific for the HPIV3 target antigens (matrix and nucleoprotein). Two of the 4 patients demonstrated detectable HPIV3-specific T-cell immunity with an overall median time to detection of 317.5 days.
Overall survival and adverse events after VST infusion
There were no deaths in patients receiving VST infusion after HSCT for SCD at 1 year after VST infusion. Moreover, all patients remain free from SCD at 1 year after HSCT. Four patients were diagnosed with acute GVHD after transplant, before VST infusion. One patient had stage 1 gastrointestinal (GI) GVHD after transplant that was quiescent at the time of first VST infusion. This patient later developed stage 3 skin and stage 1 GI GVHD that resolved with topical and systemic steroids and did not recur after a second VST infusion (P7). P9 had stage 1 to 2 GI GVHD and possible stage 2 skin GVHD perceived to likely be a drug reaction before VST infusion. He later developed acute grade 2 skin GVHD and severe chronic skin GVHD. P10 had stage 1 acute skin GVHD, resolved with topical steroids before VST infusion and did not develop GVHD after infusion. P13 had grade 2 acute skin GVHD, which resolved with oral steroids before VST and did not develop GVHD after infusion.
Acute GVHD after VST infusion was identified in 4 patients (30.7%; Tables 1 and 2), of whom 2 had grade 1 to 2 GVHD and 2 patients had grade 3 to 4 acute GVHD, both resolved initially with systemic and topical steroid treatment. One of these patients went on to develop chronic skin GVHD. In total, 4 patients (30.7%; Tables 1 and 2) developed cGVHD after transplant and VST infusion. One patient developed transaminitis after infusion of donor-derived VSTs targeting CMV, EBV, and Adv. The rise in liver enzymes coincided with EBV reactivation. The patient came off study because of EBV reactivation and was treated with rituximab, and the transaminitis resolved thereafter. The patient was subsequently enrolled on another VST protocol and infused with donor-derived EBV/LMP-specific VSTs with no further adverse events.
Discussion
The use of HLA-mismatched donors as a curative HSCT strategy for SCD has not only increased the number of patients who are eligible for transplant but also increases the risk of graft rejection and the need for immunosuppression to prevent GVHD1 putting these patients at increased risk for viral reactivation and infection.5,6,20 Recent studies evaluating patients with SCD who have received haploidentical transplants managed with posttransplant cyclophosphamide show that 70% of patients developed at least 1 viral infection or reactivation after transplant.21 The data presented here, suggest that VST administration is a safe and potentially effective approach for the prevention and treatment of viral infections after HSCT for patients with SCD. Furthermore, we show that the use of VSTs in this setting is associated with a high clinical response rate in patients who failed standard antiviral therapy. In addition, patients who had no detectable virus reactivation or infection at the time of VST infusion and received a target-specific product, remained virus-free after VSTs, even in patients who had a recent history of viral infection or reactivation.
Our study further validates previous reports14 demonstrating that in vivo VST expansion is increased in the presence of antigen. Specifically, our data show that the time to reconstitution of CMV and EBV-specific T-cell immunity was significantly shorter in patients with viral infection compared with those treated prophylactically as measured by IFN-γ ELISpot assay (P = .019 and P = .002, respectively). Although the difference did not reach statistical significance for other viruses targeted for treatment, Adv and BKV, this may be because, in part, of the small sample size. Furthermore, previous studies suggest that despite low IFN-γ responses, VSTs persist in the prophylactic setting and can subsequently respond on viral re-exposure.14,18,22 These results highlight the need for the development of more sensitive measures of VST persistence and assessment of their immunobiologic effects in the prophylaxis setting including using T-cell receptor and single-cell sequencing.
The use of multivirus–specific T-cell products is an attractive approach for treating and/or preventing multiple viruses with a single therapeutic. However, not all multi-VST products showed antiviral T-cell specificity for all target viruses which may also limit efficacy when used broadly for prophylaxis against multiple viruses. In this cohort, target virus specificity was demonstrated in 60% to 84.6% of products targeting CMV, EBV, Adv, and BKV. The 2 viruses targeted exclusively as prophylaxis, HPIV3 and HHV6 were part of hexavirus-specific T-cell product administered to 4 patients, all were specific for HPIV3 and HHV6. However, only 2 of the 4 patients had demonstrable antigen-specific T-cell immunity to these viruses as measured by IFN-γ ELISpot assay. Two patients who received products that were not uniformly multivirus specific, ultimately had reactivation of the virus that the VST products were not specific for. Improvements to VST manufacturing platforms are therefore required to limit product heterogeneity and ensure consistent multivirus functionality, as VST become more broadly available to HSCT recipients.
Our cohort supports that VSTs are generally safe in this population, with severe (grade 3/4) acute GVHD limited to only 2 patients. Overall, the acute (any grade) and cGVHD rates were both 30.7%. In previous studies evaluating posttransplant rates of GVHD, in matched sibling donor transplants for SCD, grade 2 to 4 acute GVHD occurred in 14.8% of patients, with development of cGVHD reported as 14.3%.23 This is in contrast to patients after MUD transplant with grade 3 to 4 acute GVHD rate of 17% and cGVHD of 62%24 and posthaploidentical transplant followed by posttransplant cyclophosphamide with variable myelo- and nonmyeloablative conditioning reported acute GVHD rates of 0% to 33% and cGVHD rate of 0% to 75%.4,7,21,25,26 Although the heterogeneous transplant platforms and conditioning regimens in this cohort make it difficult to discern the relative contribution of VST administration to GVHD rates, our data appear to be consistent with reported experience in other disease settings in which GVHD rates in patients after VSTs were not appreciably different from expected rates after transplant.9,11,27
There are several limitations to this study because of the relatively small sample size and the heterogeneous group of patients with SCD evaluated with respect to transplant donor source, conditioning regimen, and degree of viral disease severity. Furthermore, because of the nature of the studies involved, there is wide variation on the concomitant and prior use of standard antiviral therapy for the treatment of active viral infections making definitive conclusions regarding efficacy challenging. However, these data are promising and provide support for the development of larger prospective trials to evaluate the efficacy of VST products for treatment of multiple viral infections in this setting. Furthermore, randomized trials are required to confirm VST potency in vivo and identify the most appropriate timing for VST administration in prophylaxis settings.
In summary, to our knowledge this is the first report that specifically evaluates the safety and antiviral outcomes in patients with SCD who received VST after HSCT. We show that VST therapy is feasible and safely elicit antiviral immunity in this patient population despite the use of multiple donor sources for VST product manufacture. With the expansion of HSCT donor eligibility for SCD, the number of patients receiving transplant will continue to grow. Given our encouraging data, advanced phase studies would now be timely to prospectively optimize posttransplant VST applications specifically for patients with SCD.
Acknowledgments
This work was supported by a Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant awarded to the Children’s Research Institute Hematology Training Program by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH), 5T32HL110841-08 (H.K.), a Hyundai Hope on Wheels Young Investigator Grant (H.K.), a Mark Foundation Momentum Fellowship (H.K.), and the Amos Medical Faculty Development Program cosponsored by the American Society of Hematology and the Robert Wood Johnson Foundation (A.A.). Funding for the individual trials in this analysis include for ATCAT2 and CHEERS - NIH/National Cancer Institute P01 CA148600; for NATS, CHAPS and MUSTAT–NIHLBI K23-HL136783-01, Jeffrey Modell Foundation, the Board of Visitors of the Children’s National Hospital; for ALCI2–Children’s Oncology Group grant FP00015221_SUB706_01 (C.M.B.), St. Baldrick’s Foundation grant 300001991 48-051-3 (C.M.B.).
Authorship
Contribution: H.K., M.M., C.M.B., M.D.K., and A.A. wrote the manuscript; M.D.K., A.A., C.M.B. developed the study trials included in the analysis; C.M.B., B.D.S., M.D.K., A.A. were the principal investigators on the clinical trials; C.M.B. held the Investigational New Drugs for all 6 studies; F.H. and E.J. provided programmatic oversight and quality assurance was provided; M.B., K.M.M., B.D.S., and A.A. delivered care for the patients who underwent transplantation enrolled on the trial and were responsible for data integrity and capture; P.J.H., J.T., C.D.M., S.O., S.B., and A.S. generated and evaluated the cells; F.H., E.J. provided regulatory oversight; H.K., H.L., M.J.-W. performed data analysis; H.K. performed statistical studies; and all authors reviewed the manuscript, made the decision to submit it for publication, and vouch for the accuracy and completeness of the data reported and fidelity to the protocol.
Conflicts-of-interest disclosure: M.D.K., A.A., P.J.H., and C.M.B. have intellectual property related to developing T-cell therapies for infectious diseases. C.M.B. has equity interest in Mana Therapeutics and stock or ownership in Cabaletta Bio, Catamaran Bio, Repertoire Immune Medicines, and Neximmune. P.J.H. is a cofounder and on the board of directors of Mana therapeutics, is an advisor to Cellenkos, is on the scientific advisory board of Cellevolve. The remaining authors declare no competing financial interests.
Correspondence: Hannah Kinoshita, Children’s National Medical Center, 111 Michigan Ave NW M5207, Washington, DC 20010; e-mail: hkinoshit2@childrensnational.org.
References
Author notes
Data are available on request from author, Hannah Kinoshita (hkinoshit2@childrensnational.org). Individual participant data will not be shared.
The full-text version of this article contains a data supplement.

![Anti-CMV T-cell activity and clinical response in patients treated with CMV-specific VSTs for active CMV infection. (A) Anti-CMV antigen (phosphoprotein 65 [PP65], immediate early protein-1 [IE1]) T-cell response of the CMV-specific VST products as detected by IFN-γ ELISpot assay. Anti-CMV antigen T-cell response and CMV viral load (IU or copies per milliliter) in the recipient PB post-VST infusion for patient P4, P5, P7 treated with CMV infection (B-D) and timing of concomitant standard antiviral treatment. PCR, polymerase chain reaction; SFU, spot forming units.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/10/10.1182_bloodadvances.2022008219/1/m_blooda_adv-2022-008219-gr1.jpeg?Expires=1767769702&Signature=2xf-Cud4xQYsPazkGuEUvn~pSdg0lWBpi4fq-jC1VTEngZ4V5r0e51Npmu-9gdlmTvLSD3uiiMsx9wXjf0AhlSfyYWtWOta9B31GGc7D4h-EIHj928YiCq~M7AUYnBs9SCsdypRvYYVrSPf176cI2lqXfuiqJZUYOikt31ClY7AG~9A80dX~18fzTgwhshMah0B5tnH7lYqAKP~SGJGXcNzGC1P3GpAstgAku2zlOlGzgX8k67SGXaoiCIxzG15qiHvSZ5viYvoriQ8q-hgcJ2QX4Of~NDX3ZglxTi-vqZUrWVDlvHr31VbUt6yxSOA0--XzzDadJHZHNQedYjGx5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Anti-BKV T-cell activity and clinical response in patients treated with BKV-specific VSTs for active BKV infection. (A) Anti-BKV antigen (viral capsid protein 1 [VP1], large T [LgT]) T-cell response of the BKV-specific VST products as detected by IFN-γ ELISpot assay. Anti-BKV antigen T-cell response and BKV viral load (copies per milliliter) in the recipient PB post-VST infusion for patient P4 treated with BKV infection (B) and timing of concomitant standard antiviral treatment. BKV, BK virus; PCR, polymerase chain reaction; SFU, spot forming units.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/10/10.1182_bloodadvances.2022008219/1/m_blooda_adv-2022-008219-gr4.jpeg?Expires=1767769702&Signature=J8Yq-D3VW~ycrq3Jf3CFxwtCHuVYZtYlMFPvtBXkoYrS6ui3SrRzCCWq5B0FMOyZL0mdNu1cWNp7nh5JOxtdeG5k-135dgcxypawkrsxVvFt8eAQuGx4fN~nIGYMEtgxLWihig0-tyfy4OzZkApTJt0sk570I7IfQ7YDwInJDE27LgYZcBkbqXDtbf9-mjF0-mnpbtvToS41nT9Qu5oKOXuMOZ4zv7NJ9TXGulFBhna9-HVEHMwdc6CWqkjLfs65uDcsdmgRSgSZB4AwdXLWJ8uIHsnUPuBPSsJGftMLDGoVTWDq7ApLnp7unZYdbebELXCF9YNOiA7FZ~hFtsrcsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)