Key Points
TIM-3/Gal-9 autocrine loop hijacks and constitutively activates canonical Wnt pathway in AML LSCs.
To hijack canonical Wnt pathway, HCK and p120-catenin, highly expressed on AML LSCs, play crucial roles in signal transduction of TIM-3.
Abstract
The activation of β-catenin plays critical roles in normal stem cell function, and, when aberrantly activated, the maintenance and enhancement of cancer stemness in many solid cancers. Aberrant β-catenin activation is also observed in acute myeloid leukemia (AML), and crucially contributes to self-renewal and propagation of leukemic stem cells (LSCs) regardless of mutations in contrast with such solid tumors. In this study, we showed that the AML-specific autocrine loop comprised of T-cell immunoglobulin mucin-3 (TIM-3) and its ligand, galectin-9 (Gal-9), drives the canonical Wnt pathway to stimulate self-renewal and propagation of LSCs, independent of Wnt ligands. Gal-9 ligation activates the cytoplasmic Src homology 2 domain of TIM-3 to recruit hematopoietic cell kinase (HCK), a Src family kinase highly expressed in LSCs but not in HSCs, and HCK phosphorylates p120-catenin to promote formation of the LDL receptor–related protein 6 (LRP6) signalosome, hijacking the canonical Wnt pathway. This TIM-3/HCK/p120-catenin axis is principally active in immature LSCs compared with TIM-3–expressed differentiated AML blasts and exhausted T cells. These data suggest that human AML LSCs constitutively activates β-catenin via autocrine TIM-3/HCK/p120-catenin signaling, and that molecules related to this signaling axis should be critical targets for selective eradication of LSCs without impairing normal HSCs.
Introduction
Acute myeloid leukemia (AML) is one of the most common hematologic malignancies. Previous studies have shown that AML is derived from a fraction of stem cells called leukemic stem cells (LSCs), which have self-renewal and high propagation activity. We have reported that the T-cell immunoglobulin mucin-3 (TIM-3) is expressed on the surface of LSCs in >90% of patients with AML but is never found within the normal hematopoietic stem cell (HSC) population.1-3 TIM-3 is a type 1 cell-surface glycoprotein,4 also expressed in a fraction of T cells, natural killer (NK) cells, monocytes, and dendritic cells (DCs), and its signaling generally plays immune modulatory roles in these cells.5,6 In contrast, the majority of AML LSCs express TIM-3,3,7,8 and a preclinical study in a xenograft model has shown that engrafted human AML cells were eradicated by administration of cytotoxic antihuman TIM-3 antibodies, whereas the engrafted normal human hematopoiesis was intact.2 These results suggest that surface TIM-3 is expressed in functional LSCs in AML, and that it is a good therapeutic target.
Galectin-9 (Gal-9), a ligand of TIM-3, binds to the N-terminal immunoglobulin variable (IgV) domain of TIM-3. Ligation of TIM-3 by Gal-9 has been shown to recruit Src family kinases (SFKs) to its Src homology 2 (SH2) binding motif of the cytoplasmic tail, phosphorylate tyrosine residues of SFKs, and transduce cell signaling.9,10 In a xenograft model, engrafted AML cells produced high concentrations of human Gal-9 into the mouse sera, and injection of neutralizing antibodies that blocks Gal-9 ligation to TIM-3 inhibited the reconstitution of LSCs,1,2 suggesting that the TIM-3/Gal-9 signaling cascade was maintained in an autocrine manner, and is essential for AML LSC self-renewal.
Interestingly, Gal-9 ligation to TIM-3 strongly promotes nucleus translocation and accumulation of β-catenin in AML LSCs, presumably to promote self-renewal and propagation.1 LSCs are highly dependent upon β-catenin signaling for their self-renewal and expansion in several murine AML models.11-13 In primary human AML, the accumulation of β-catenin is an adverse prognostic factor with high incidence of relapse.14-16 One of the most important pathways for regulation of β-catenin is the canonical Wnt pathway, whose signaling inhibits the β-catenin degradation complex that consists of glycogen synthase kinase 3β (GSK3β), casein kinase 1α (CK1α), adenomatous polyposis coli (APC), and scaffolding Axin proteins.17 Once Wnt ligand, secreted from tissue microenvironment, binds to its receptor Frizzled, p120-catenin is activated, and then phosphorylation of LDL receptor–related protein 6 (LRP6) occurs.18 The LRP6 signalosome, consisting of dishevelled (Dvl), CK1γ, GSK3β, and Axin19 strongly inhibits the formation of the β-catenin degradation complex, resulting in nucleus translocation of β-catenin and activation of its downstream transcription factors. The canonical Wnt pathway plays a critical role in organ development, maintenance of tissue homeostasis, and self-renewal and propagation of adult stem cells, including HSCs and intestinal stem cells.20,21
Deregulation of the Wnt/β-catenin pathway is pivotal for tumorigenesis and maintenance of cancer stem cells.22-24 It has been shown that genes related to the β-catenin degradation complex are frequently mutated in various solid cancers; APC mutation is involved in colorectal cancer,25,26 and CTNNB1 mutation in endometrial carcinoma26,27 and hepatocellular carcinoma.26,28 Such somatic mutations impair β-catenin degradation, resulting in the aberrant accumulation of β-catenin in cancer.29 In striking contrast, AML does not have mutations related to the canonical Wnt pathway or the β-catenin degradation complex, if any,26,30 suggesting that AML LSCs have mutation-independent machinery to activate the β-catenin pathway.31 Activation of PI3K/AKT and MEK/ERK signaling by TIM-3 ligation has been reported in AML cells,1 but because these pathways are used in many other cellular events, their activation may not be sufficient to explain the mechanism of the Wnt/β-catenin pathway activation in AML.
In this study, we sought to clarify AML-specific molecular mechanisms for β-catenin regulation induced by the TIM-3/Gal-9 autocrine cascade. We found that in AML LSCs, TIM-3/Gal-9 signaling uses the canonical Wnt pathway via activation of LRP6, independent of the Wnt-Frizzled ligation. Gal-9 ligation activates the cytoplasmic SH2 domain of TIM-3 to recruit hematopoietic cell kinase (HCK), an SFK highly expressed in LSCs but not in HSCs,32 and the HCK phosphorylates and activates p120-catenin to promote formation of the LRP6 signalosome, hijacking the canonical Wnt pathway. Thus, the TIM-3/Gal-9 autocrine loop represents an AML-specific molecular mechanism for constitutive activation of the canonical Wnt pathway, which should play a critical role in self-renewal and propagation of human AML LSCs.
Materials and methods
Clinical samples
Bone marrow (BM) and peripheral blood samples of patients with AML diagnosed per the World Health Organization criteria were enrolled in this study. The characteristics of these patients are summarized in supplemental Table 1. Informed consent was provided by all patients and control participants in accordance with the Declaration of Helsinki. Cord blood cells were obtained from full-term deliveries provided by the Ishida ladies clinic (Fukuoka, Japan). The institutional review board of Kyushu University Hospital (Fukuoka, Japan) approved all research conducted on humans.
Microarray analysis
Total RNA was extracted from short hairpin RNA (shRNA)-mediated TIM-3 knockdown (KD) KASUMI-3 cells (by sh hepatitis A virus cellular receptor 2 [shHAVCR2]-1 and shHAVCR2-2) and scrambled control cells using Isogen2 (NIPPON GENE). Gene expression profiling was performed using SurePrint G3 human GE microarray 8 × 60 k version 2.0 (Agilent) per the protocol provided by the manufacturer. Briefly, cyanine-3–labeled complementary RNA (cRNA) was synthesized using the Low Input Quick Amp Labeling kit (Agilent), single-color, and 600 ng of cRNA from each sample was fragmented and hybridized to the array using a Gene Expression Hybridization Kit (Agilent). The array was scanned using an Agilent SureScan microarray scanner, and raw microarray data were loaded into the Gene Spring GX software (version 14.5; Agilent). In accordance with the guided workflow for Agilent single-color experiment, the normalization algorithm of 75th percentile shift was used, and the preprocessing baseline was adjusted to the median of all samples. Statistical analysis for gene set enrichment analysis was performed as described.
Cell stimulation by Gal-9 and incubation with or without inhibitors
In the primary samples, CD34+ cells were enriched from mononuclear cells using the Indirect CD34 MicroBead Kit (Miltenyi Biotec) and preincubated in RPMI-1640 with 10% fetal calf serum (FCS) for 2 hours at 37°C. Preincubated primary samples and cell lines were washed using prewarmed phosphate-buffered saline and subsequently incubated in RPMI-1640 without FCS and stimulated by 10 ng/mL recombinant human Gal-9 protein (R&D Systems). For the assays with inhibitors, cells were incubated in the presence of A-419259 (Cayman Chemical), U0126 (Promega), LY294002 (Abcam), human dickkopf Wnt signaling pathway inhibitor 1 (DKK-1) recombinant protein (Peprotech), and a Src family inhibitors set including protein phosphatase 1 (PP1), PP2, and aminogenistein (AMGT) (proteinkinase.de) for 2 hours before stimulation with Gal-9.
Western blotting analysis
Cells were lysed in radioimmunoprecipitation assay lysis buffer (50 mM Tris pH 7.4, 150 mM sodium chloride, 10 mM tetrasodium pyrophosphate, 25 mM sodium β-glycerophosphate, 1 mM ethylenediaminetetraacetic acid, 1% weight/volume sodium dodecyl sulfate, and protease and phosphatase inhibitors). The lysates were then denatured in an equal volume of 2× Laemmli sample buffer (BIO-RAD), fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on SuperSep precast gels (Wako), and subsequently transferred onto a PVDF membrane (BIO-RAD). Before staining with primary antibodies, blots were blocked in 5% nonfat dry milk or in tris-buffered saline and Tween 20 for 1 hour. For protein detection, the following primary antibodies were used: LRP6 (Abcam), phospho-LRP6 (Thr1479) (Abnova), phospho-LRP6 (Ser1490) (Abcam), p120-catenin (Abcam), phospho-p120-catenin (Tyr228) (Abcam), β-catenin (Abcam), TIM-3 (CST), HCK (Abcam), phospho-Src family (Tyr416) (CST), β-actin (SIGMA), Axin1 (Abcam) and Dvl2 (CST), LCK (Santa Cruz), FYN (Santa Cruz), and c-SRC (Santa Cruz). Secondary antibodies were horseradish peroxidase–conjugated antirabbit or antimouse light chain–specific antibody (Jackson ImmunoResearch). The blots were visualized with Immobilon Forte Western horseradish peroxidase substrates (MERCK) and imaged using an LAS3000 image analyzer (Fuji Film) or iBright FL1000 (Thermo Fisher Scientific). The image data of western blotting were quantified using the ImageJ software (National Institutes of Health).
Immunoprecipitation (IP)
Before IP, binding of anti-HCK rabbit monoclonal antibody (ab75839; Abcam), anti-LRP6 mouse monoclonal antibody (ab75358; Abcam), or anti-TIM-3 rabbit monoclonal antibody (#45208; CST) to protein A/G agarose beads was performed as described in the Pierce Crosslink IP Kits. The same cross-linking procedures were performed in normal rabbit or mouse IgG (Santa Cruz) as isotype control. Cells were lysed in IP lysis buffer (1 × IP buffer, 140 mM sodium chloride, 1% Triton-X-100%, 1% protease inhibitor, and 1% phosphatase inhibitor). Subsequently, cell lysates were mixed and incubated for 3 hours with anti-HCK or anti-LRP6 antibody crosslinked to A/G agarose beads. The beads were washed twice with lysis buffer. Bound proteins were eluted using elution buffer and subjected to immunoblotting analysis.
Statistical analysis
All statistical analyses were performed with the JMP Pro (version 14.0.0) software. Data are presented as mean ± standard error of the mean (SEM). The significance of differences between 2 groups was determined using Student t test. P values < 0.05 denoted statistical significance. False discovery rate–adjusted P values (q values) were calculated using the 2-stage sharpened method.33
Results
KD of TIM-3 in AML identifies the canonical Wnt pathway as a major downstream signaling of TIM-3/Gal-9 autocrine loop
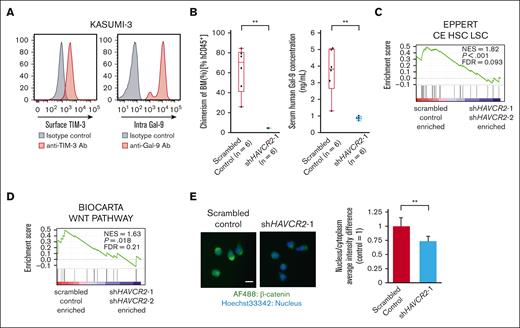
To verify the downstream signaling of TIM-3 in AML, we used KASUMI-3, an AML cell line expressing both functional surface TIM-3 and intracellular Gal-9 (Figure 1A; supplemental Figure 1A). The growth of this cell line is reduced in vitro by neutralization of Gal-9 using anti–Gal-9 antibodies, indicating that KASUMI-3 has an autocrine TIM3/Gal-9 system, like primary human AML LSCs (supplemental Figure 1B). We then performed shRNA-mediated knock down (KD) of TIM-3 in KASUMI-3 cells. Transfection of 2 independent shRNA targeting TIM-3 (shHAVCR2-1 and shHAVCR2-2) effectively suppressed the surface expression of TIM-3 in KASUMI-3 (supplemental Figures 1A-C), and significantly inhibited its in vitro proliferation (supplemental Figure 1D). TIM-3 KD also inhibited xenogeneic reconstitution of KASUMI-3 cells in NSG mice, suppressing their secretion of human Gal-9 into the sera (Figure 1B).
Identification of the canonical Wnt pathway as major downstream signaling of TIM-3/Gal-9 autocrine loop. (A) Fluorescence-activated cell sorting (FACS) analysis of surface TIM-3 expression (left) and intracellular Gal-9 expression (right) in KASUMI-3 cells (B) Percentage of BM chimerism in NSG mice with xenografts with TIM-3 KD KASUMI-3 cells (n = 6) and scrambled control cells (n = 6) (left panel), and human Gal-9 concentration in serum of mice with xenografts (right panel). ∗∗P < .01 vs scrambled control. These data were obtained at 13 weeks after xenotransplantation. (C-D) Enrichment plots for gene sets significantly enriched in scrambled control cells compared with KASUMI-3 cells transfected with shHAVCR2-1 and shHAVCR2-2. (C) HSC- and LSC-related genes.35 (D) Canonical Wnt pathway–related genes (BIOCARTA). (E) Quantification of nucleus translocation of β-catenin evaluated by ArrayScan system. Left panels show the representative images for localization of β-catenin in scrambled control and TIM-3 KD KASUMI-3 cells. Scale bar represents 10 μm. Right panel shows the quantification of β-catenin (green) translocation to the nucleus calculated from the fluorescence intensity and area overlapped with nucleus (blue), which were analyzed in TIM-3 KD KASUMI-3 cells and scrambled control cells. Data are presented as mean ± SEM, ∗∗P < .01 vs scrambled control.
Identification of the canonical Wnt pathway as major downstream signaling of TIM-3/Gal-9 autocrine loop. (A) Fluorescence-activated cell sorting (FACS) analysis of surface TIM-3 expression (left) and intracellular Gal-9 expression (right) in KASUMI-3 cells (B) Percentage of BM chimerism in NSG mice with xenografts with TIM-3 KD KASUMI-3 cells (n = 6) and scrambled control cells (n = 6) (left panel), and human Gal-9 concentration in serum of mice with xenografts (right panel). ∗∗P < .01 vs scrambled control. These data were obtained at 13 weeks after xenotransplantation. (C-D) Enrichment plots for gene sets significantly enriched in scrambled control cells compared with KASUMI-3 cells transfected with shHAVCR2-1 and shHAVCR2-2. (C) HSC- and LSC-related genes.35 (D) Canonical Wnt pathway–related genes (BIOCARTA). (E) Quantification of nucleus translocation of β-catenin evaluated by ArrayScan system. Left panels show the representative images for localization of β-catenin in scrambled control and TIM-3 KD KASUMI-3 cells. Scale bar represents 10 μm. Right panel shows the quantification of β-catenin (green) translocation to the nucleus calculated from the fluorescence intensity and area overlapped with nucleus (blue), which were analyzed in TIM-3 KD KASUMI-3 cells and scrambled control cells. Data are presented as mean ± SEM, ∗∗P < .01 vs scrambled control.
Global transcriptome analysis using gene set enrichment analysis34 of KASUMI-3 before and after the shHAVCR2 treatment revealed that TIM-3 KD significantly attenuated the expression of genes enriched in LSCs (Figure 1C: EPPERT CE HSC LSC35) as well as that in the canonical Wnt pathway (Figure 1D: BIOCARTA WNT PATHWAY), which was listed as top deregulate pathway of the Biocarta Pathways data set (supplemental Table 2; supplemental Figures 1E-F). Furthermore, the immunofluorescence assay using the ArrayScan system1 revealed that TIM-3 KD in KASUMI-3 cells significantly reduced the accumulation and translocation of β-catenin to the nucleus (Figure 1E).
TIM-3/Gal-9 signaling induces the formation of the LRP6 signalosome to activate the β-catenin pathway
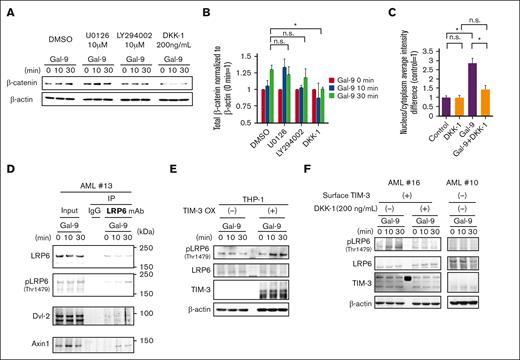
To test whether the canonical Wnt pathway activation was induced by TIM-3 signaling, we tested the effect of kinase inhibitors targeting MEK/ERK and PI3K/AKT pathways, both of which are activated by TIM-3/Gal-9 signaling in AML.1,36 As shown in Figures 2A-B, MEK1/2 inhibitor (U0126) or PI3K inhibitor (LY294002) could not inhibit the accumulation of β-catenin induced by TIM-3 signaling induced by Gal-9 ligation in KASUMI-3 cells. In contrast, Dickkopf-1 (DKK-1) (200 ng/mL), an inhibitor specific for the LRP6 signalosome formation, completely blocked β-catenin accumulation. Moreover, DKK-1 clearly canceled not only Gal-9–induced nucleus translocation of β-catenin in KASUMI-3 cells evaluated by ArrayScan (Figure 2C; supplemental Figure 2A) but also the transcription of AXIN2, a gene driven by β-catenin binding to the Tcf/LEF promoter in the nucleus37 (supplemental Figure 2B). These results suggest that LRP6 signalosome formation represents an indispensable process for β-catenin accumulation driven by TIM-3/Gal-9 signaling. Consistent with these results, IP assay with anti-LRP6 monoclonal antibodies revealed that LRP6 signalosome components including phosphorylated-LRP6, Dvl-2, and Axin1 were coimmunoprecipitated after Gal-9 stimulation in primary AML cells (Figure 2D). Thus, the TIM-3/Gal-9 signaling induces LRP6 signalosome formation and LRP6 phosphorylation, both of which are crucial steps of canonical Wnt pathway activation.19,38,39
Gal-9 ligation to TIM-3 induces LRP6 signalosome formation and subsequent β-catenin accumulation in AML. (A) WB analysis of β-catenin accumulation induced by stimulation with Gal-9 in the presence of inhibitors in KASUMI-3 cells. KASUMI-3 cells were stimulated with Gal-9 in the presence of dimethyl sulfoxide, 10 μM of U0126 (MEK1/2 inhibitor), 10 μM of LY294002 (PI3K inhibitor), or 200 ng/mL of DKK-1, and their total cell lysates subjected to WB analysis. (B) Summarized data of Gal-9 stimulation–induced changes in the accumulation of β-catenin from 3 independent experiments. (C) Extent of β-catenin translocation to the nucleus evaluated using the ArrayScan system compared with that observed in nonstimulated controls. Data in panels B and C are presented as mean ± SEM, ∗P < .05. (D) Immunoblotting analysis of total lysates (left) and IP lysates (right) of Gal-9–stimulated primary TIM-3+ AML samples. Cells were lysed and subjected to IP with an anti-LRP6 antibody or normal mouse IgG. A representative result of TIM-3+ primary AML cells out of 3 independent cases (AML#4, #9, and #13) is shown. (E) WB analysis of Gal-9 stimulation–induced phosphorylation of LRP6 at Thr1479 in mock-transfected and TIM-3-OX THP-1 cells. (F) WB analysis of cell lysates from TIM-3+ AML cells (left) and TIM-3− AML cells (right). TIM-3+ AML cells were stimulated with Gal-9 in the absence or presence of DKK-1 (200 ng/mL). Representative results out of 4 independent TIM-3+ AML cells (AML#1, #2, #4, and #16) and 2 TIM-3− AML cells (AML#10 and #11) are shown. n.s., no significant difference.
Gal-9 ligation to TIM-3 induces LRP6 signalosome formation and subsequent β-catenin accumulation in AML. (A) WB analysis of β-catenin accumulation induced by stimulation with Gal-9 in the presence of inhibitors in KASUMI-3 cells. KASUMI-3 cells were stimulated with Gal-9 in the presence of dimethyl sulfoxide, 10 μM of U0126 (MEK1/2 inhibitor), 10 μM of LY294002 (PI3K inhibitor), or 200 ng/mL of DKK-1, and their total cell lysates subjected to WB analysis. (B) Summarized data of Gal-9 stimulation–induced changes in the accumulation of β-catenin from 3 independent experiments. (C) Extent of β-catenin translocation to the nucleus evaluated using the ArrayScan system compared with that observed in nonstimulated controls. Data in panels B and C are presented as mean ± SEM, ∗P < .05. (D) Immunoblotting analysis of total lysates (left) and IP lysates (right) of Gal-9–stimulated primary TIM-3+ AML samples. Cells were lysed and subjected to IP with an anti-LRP6 antibody or normal mouse IgG. A representative result of TIM-3+ primary AML cells out of 3 independent cases (AML#4, #9, and #13) is shown. (E) WB analysis of Gal-9 stimulation–induced phosphorylation of LRP6 at Thr1479 in mock-transfected and TIM-3-OX THP-1 cells. (F) WB analysis of cell lysates from TIM-3+ AML cells (left) and TIM-3− AML cells (right). TIM-3+ AML cells were stimulated with Gal-9 in the absence or presence of DKK-1 (200 ng/mL). Representative results out of 4 independent TIM-3+ AML cells (AML#1, #2, #4, and #16) and 2 TIM-3− AML cells (AML#10 and #11) are shown. n.s., no significant difference.
Next, we tested whether TIM-3 is a specific receptor for Gal-9 in the context of canonical Wnt signaling. THP-1 is an AML cell line but does not express surface TIM-3.40 As shown in Figure 2E (left), the addition of Gal-9 did not affect the phosphorylation status of LRP6 at Thr1479, a critical phosphorylation site for the canonical Wnt pathway activation. However, when the TIM-3 was transfected in THP-1 cells (supplemental Figure 2C), the enforced expression of TIM-3 made THP-1 cells responsive to Gal-9, inducing progressive phosphorylation of LRP6 (Figure 2E, right).
Moreover, in primary AML cells, Gal-9 stimulation–induced LRP6 phosphorylation in CD34+ cells from TIM-3+ AML but not from TIM-3− AML (Figure 2F; supplemental Figure 2D). DKK-1 again completely abrogated phosphorylation of LRP6 by Gal-9 in TIM-3+ primary AML (Figure 2F). In contrast with DKK-1, U0126 and LY294002 could not attenuate Gal-9–induced LRP6 phosphorylation in KASUMI-3 cells (supplemental Figure 2E). These results collectively suggest that Gal-9 ligation to TIM-3 induces LRP6 signalosome formation to activate the canonical Wnt pathway in primary human AML cells.
HCK is a critical signal transducing molecule involved in AML to activate LRP6 phosphorylation
TIM-3 signaling is mediated by SFKs recruited to the cytoplasmic tail.9,41 We sought to identify the SFKs involved in AML-specific TIM-3 signaling. TIM-3+ AML cell lines, KASUMI-3 and KASUMI-6, were incubated with Gal-9 in the presence of 5 SFK inhibitors: AMGT specifically inhibits LCK; PP1 can inhibit LCK and FYN at a low concentration (10 nM) but at a higher concentration (200 nM) it further inhibits HCK and SRC;42 PP2 inhibits HCK, LCK ,and FYN at 10nM; and A-419259 potently inhibits HCK.43,44 As shown in Figure 3A, both AMGT and PP1 (10 nM) did not block LRP6 phosphorylation, or accumulation of β-catenin induced by Gal-9 (Figure 3A). In contrast, PP1 at a higher concentration (200 nM) and PP2 blocked the phosphorylation of LRP6 (Figure 3A). Finally, A-419259 inhibited LRP6 phosphorylation and β-catenin accumulation (Figure 3A). A-419259 blocked LRP6 phosphorylation and subsequent β-catenin accumulation also in primary AML cells (Figures 3B; supplemental Figure 3A). Consistent with these results, coimmunoprecipitation experiments using TIM-3–overexpressed (OX) THP-1 cells with antibody against TIM-3 showed that HCK was coimmunoprecipitated with TIM-3, but FYN, LCK, and SRC were not (supplemental Figure 3B). Furthermore, shRNA-mediated KD of SFKs revealed that KD of HCK strongly inhibited Gal-9–induced LRP6 phosphorylation and β-catenin accumulation, whereas KD of FYN and LCK did not (supplemental Figures 3C-F). These results suggested that HCK is a critical SFK for TIM-3 signaling for the activation of canonical Wnt pathway. Immunofluorescence staining of β-catenin (Figure 3C) and its measurement of nucleus/cytoplasm β-catenin intensity (Figure 3D) by the ArrayScan system1 clearly demonstrated that A-419259 significantly attenuated the accumulation and nucleus translocation of β-catenin in KASUMI-3 cells. Figure 3E shows the results of IP assays of primary TIM-3+ AML cell lysate with HCK monoclonal antibody. In primary AML cells, after incubation with Gal-9, HCK phosphorylated at Tyr410,45 an active form of HCK through the autophosphorylation, and TIM-3 protein were coimmunoprecipitated. These data suggest that TIM-3 recruits HCK to trigger the canonical Wnt pathway after Gal-9 ligation in AML.
HCK is a critical signal transducing molecule involved in TIM-3–induced canonical Wnt pathway activation. (A) WB analysis of Gal-9–stimulated KASUMI-6 cells in the presence of SFK inhibitors: AMGT, PP1, PP2, and A-419259 (potent HCK inhibitor). Inhibitable members of SFKs at each concentration of inhibitors are listed. Because KASUMI-6 cells showed obvious response to SFKs inhibitors, we presented the representative data of KASUMI-6 cells. (B) WB analysis of cell lysates from Gal-9–stimulated TIM-3+ primary samples in the presence of 0, 1, and 10 nM of A-419259. Cells were preincubated with A-419259 for 2 hours before stimulation with Gal-9. Representative results out of 4 independent TIM-3+ AML cases (AML#6, #9, #14, and #17) are shown. (C) Images of KASUMI-3 cells captured from representative fields obtained by ArrayScan analysis. These cells were stimulated with or without Gal-9 in the presence or absence of A-419259 for 20 hours. Scale bar represents 10 μm. (D) Extent of β-catenin translocation to the nucleus evaluated using the ArrayScan system compared with nonstimulated control. Data are presented as mean ± SEM, ∗P < .05 and ∗∗P < .01. (E) Immunoblotting analysis with HCK, pSFK, and TIM-3 antibodies of total cell lysates (left) and immunoprecipitated lysates (right). Cells were stimulated with Gal-9 for 0, 5, and 10 minutes and subsequently lysed and subjected to IP with an HCK antibody or normal rabbit IgG. Representative results (AML#13) out of 3 independent experiments are shown here.
HCK is a critical signal transducing molecule involved in TIM-3–induced canonical Wnt pathway activation. (A) WB analysis of Gal-9–stimulated KASUMI-6 cells in the presence of SFK inhibitors: AMGT, PP1, PP2, and A-419259 (potent HCK inhibitor). Inhibitable members of SFKs at each concentration of inhibitors are listed. Because KASUMI-6 cells showed obvious response to SFKs inhibitors, we presented the representative data of KASUMI-6 cells. (B) WB analysis of cell lysates from Gal-9–stimulated TIM-3+ primary samples in the presence of 0, 1, and 10 nM of A-419259. Cells were preincubated with A-419259 for 2 hours before stimulation with Gal-9. Representative results out of 4 independent TIM-3+ AML cases (AML#6, #9, #14, and #17) are shown. (C) Images of KASUMI-3 cells captured from representative fields obtained by ArrayScan analysis. These cells were stimulated with or without Gal-9 in the presence or absence of A-419259 for 20 hours. Scale bar represents 10 μm. (D) Extent of β-catenin translocation to the nucleus evaluated using the ArrayScan system compared with nonstimulated control. Data are presented as mean ± SEM, ∗P < .05 and ∗∗P < .01. (E) Immunoblotting analysis with HCK, pSFK, and TIM-3 antibodies of total cell lysates (left) and immunoprecipitated lysates (right). Cells were stimulated with Gal-9 for 0, 5, and 10 minutes and subsequently lysed and subjected to IP with an HCK antibody or normal rabbit IgG. Representative results (AML#13) out of 3 independent experiments are shown here.
p120-catenin is a bridging molecule connecting TIM-3 signaling and the canonical Wnt pathway in AML LSCs
Next, we sought to identify the molecules involved in HCK-mediated canonical Wnt activation via TIM-3 signaling. p120-catenin, which is highly expressed in immature HSCs in normal hematopoiesis,46,47 is an indispensable molecule for the formation of the LRP6 signalosome,18,48 and is also known as a major substrate for SFKs including HCK.49 We knocked down gene expression of CTNND1 encoding p120-catenin using shRNA (shCTNND1-1 or shCTNND1-2) in KASUMI-3 cells, and found that p120-catenin KD significantly inhibited cellular growth (Figure 4A), and suppressed LRP6 phosphorylation and β-catenin accumulation in vitro (Figure 4B). In addition, p120-catenin KD led to a significant decrease in the engraftment efficiency of KASUMI-3 cells evaluated by serial transplantation experiments (supplemental Figure 4A-B). The forced expression of p120-catenin on p120-catenin KD KASUMI-3 cells (shCTNND1-1; target sites in the 3' untranslated region) rescued the canonical Wnt pathway activation (supplemental Figure 4C), leading to the recovery of the engraftment efficiency in both primary and secondary recipient mice (supplemental Figure 4A-B). Although the data are limited to the experiments using KASUMI-3 cells and not primary AML samples, these results may support the hypothesis that p120-catenin plays a role in the maintenance of self-renewal capacity of AML. Furthermore, incubation of primary TIM-3+ AML cells with Gal-9 resulted in increased phosphorylation of p120-catenin at Tyr228, a key phosphorylation site required for activation of p120-catenin50,51 (Figure 4C-D). The HCK inhibitor, A-419259, blocked the phosphorylation of p120-catenin induced by incubation with Gal-9 (Figure 4E-F). IP assay using anti-HCK monoclonal antibody revealed that HCK and p120-catenin were immediately coimmunoprecipitated after Gal-9 stimulation in primary CD34+ TIM-3+ AML cells, suggesting that interaction of HCK and p120-catenin occurred by TIM-3 ligation, and that HCK activated p120-catenin (Figure 4G). These results collectively suggest that p120-catenin is a bridging molecule connecting TIM-3 signaling and the canonical Wnt pathway in AML.
p120-catenin plays a crucial role for bridging TIM-3/Gal-9 signaling to the canonical Wnt pathway in AML cells. (A) Cellular growth of KASUMI-3 cells transfected with scrambled control and shRNA-mediated CTNND1 KD vectors. Two kinds of shRNA (shCTNND1-1: target sites in the 3' untranslated region, and shCTNND1-2: target sites in the coding sequence) were used. Data are presented as mean ± SEM, ∗P < .05 and ∗∗P < .01 vs scrambled control. (B) WB analysis of the canonical Wnt pathway–related protein using cell lysates from KASUMI-3 cells transfected with scrambled control and 2 kinds of shRNA-targeting CTNND1. (C) WB analysis of the phosphorylation status of p120-catenin at Tyr228 induced by stimulation with Gal-9 (AML#7). (D) Quantification of the levels of phosphorylated p120-catenin at Tyr228 by Gal-9 stimulation in KASUMI-3 cells. Results of 4 independent experiments (AML#1, #7, #9, and #16) are summarized. Data are presented as mean ± SEM, ∗P < .05 vs nonstimulated control. (E) WB analysis of the inhibitory effect of A-419259 (0, 1, or 10 nM) on Gal-9 stimulation–induced phosphorylation of p120-catenin using a primary AML sample (AML#9). (F) Quantification of the levels of phosphorylated p120-catenin induced by Gal-9 stimulation in the presence of each concentration of A-419259 (0, 1, and 10 nM). Data are presented as mean ± SEM, ∗P < .05 and ∗∗P < .01. Results from 4 independent AML experiments (AML#6, #9, #14, and #17) are summarized. (G) Immunoblotting analysis with total p120-catenin and phosphorylated p120-catenin (Tyr228) antibodies of total cell lysates (left), and immunoprecipitated lysates (right). Cells were stimulated with Gal-9 for 0, 5, and 10 minutes, and subsequently lysed and subjected to IP with an anti-HCK antibody or normal rabbit IgG. Representative results out of 3 independent experiments are shown here.
p120-catenin plays a crucial role for bridging TIM-3/Gal-9 signaling to the canonical Wnt pathway in AML cells. (A) Cellular growth of KASUMI-3 cells transfected with scrambled control and shRNA-mediated CTNND1 KD vectors. Two kinds of shRNA (shCTNND1-1: target sites in the 3' untranslated region, and shCTNND1-2: target sites in the coding sequence) were used. Data are presented as mean ± SEM, ∗P < .05 and ∗∗P < .01 vs scrambled control. (B) WB analysis of the canonical Wnt pathway–related protein using cell lysates from KASUMI-3 cells transfected with scrambled control and 2 kinds of shRNA-targeting CTNND1. (C) WB analysis of the phosphorylation status of p120-catenin at Tyr228 induced by stimulation with Gal-9 (AML#7). (D) Quantification of the levels of phosphorylated p120-catenin at Tyr228 by Gal-9 stimulation in KASUMI-3 cells. Results of 4 independent experiments (AML#1, #7, #9, and #16) are summarized. Data are presented as mean ± SEM, ∗P < .05 vs nonstimulated control. (E) WB analysis of the inhibitory effect of A-419259 (0, 1, or 10 nM) on Gal-9 stimulation–induced phosphorylation of p120-catenin using a primary AML sample (AML#9). (F) Quantification of the levels of phosphorylated p120-catenin induced by Gal-9 stimulation in the presence of each concentration of A-419259 (0, 1, and 10 nM). Data are presented as mean ± SEM, ∗P < .05 and ∗∗P < .01. Results from 4 independent AML experiments (AML#6, #9, #14, and #17) are summarized. (G) Immunoblotting analysis with total p120-catenin and phosphorylated p120-catenin (Tyr228) antibodies of total cell lysates (left), and immunoprecipitated lysates (right). Cells were stimulated with Gal-9 for 0, 5, and 10 minutes, and subsequently lysed and subjected to IP with an anti-HCK antibody or normal rabbit IgG. Representative results out of 3 independent experiments are shown here.
p120-catenin is a key molecule that determines the signal intensity of canonical Wnt pathway activation driven by TIM-3 signaling
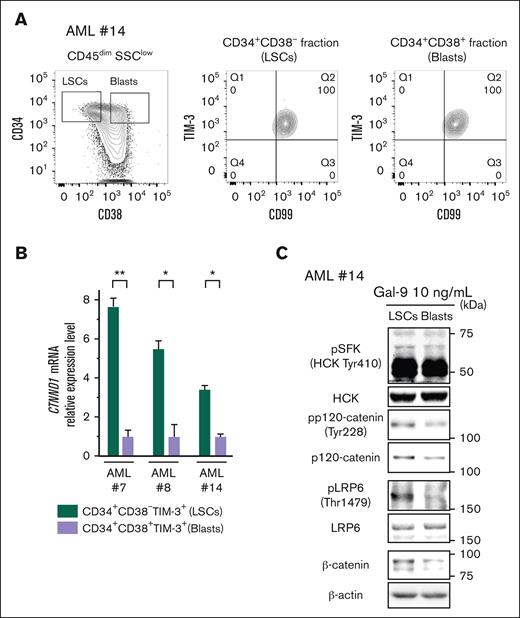
Considering that p120-catenin expression is preferentially observed in immature HSCs in normal hematopoiesis, we investigated whether TIM-3 signaling via p120-catenin activates the canonical Wnt pathway at different levels among AML subpopulations determined by maturity. In primary AML cells, both CD34+CD38− LSCs and CD34+CD38+ immature blasts exhibited a higher surface TIM-3 expression level than nonexhausted T cells in the identical BM specimen (Figure 5A; supplemental Figure 5A-C). In contrast, p120-catenin messenger RNA (mRNA) and protein were expressed in LSCs at higher levels, as compared with CD34+CD38+ AML blasts (Figure 5B-C). Furthermore, after Gal-9 ligation, LSCs had higher levels of LRP6 phosphorylated at Thr1479 and β-catenin accumulation than in CD34+CD38+ AML cells (Figure 5C). Thus, it is suggested that p120-catenin represents a key molecule that determines the signal intensity of TIM-3 signaling in AML.
TIM-3 signaling induces β-catenin accumulation in accordance with p120-catenin expression level in AML. (A) FACS analysis of the expression of TIM-3 on CD34+CD38– (middle; LSCs-enriched fraction) and CD34+CD38+ (right; main blasts–enriched fraction) AML cells from the CD45dimSSClow fraction. Representative FACS plots of 4 independent AML samples (AML#7, #8, #13, and #14) are shown. (B) RT-qPCR analysis of the expression of CTNND1 mRNA in LSCs compared with blasts from AML samples of 3 independent AML cases. Results were normalized to the mRNA expression of GAPDH. Data are presented as mean ± SEM, ∗∗P < .01 and ∗P < .05. (C) WB analysis of cell lysate from Gal-9–stimulated CD34+CD38– AML cells (LSCs) and CD34+CD38+ cells (blasts) (AML#14). Of note, LSCs with higher p120-catenin expression exhibited profound β-catenin accumulation.
TIM-3 signaling induces β-catenin accumulation in accordance with p120-catenin expression level in AML. (A) FACS analysis of the expression of TIM-3 on CD34+CD38– (middle; LSCs-enriched fraction) and CD34+CD38+ (right; main blasts–enriched fraction) AML cells from the CD45dimSSClow fraction. Representative FACS plots of 4 independent AML samples (AML#7, #8, #13, and #14) are shown. (B) RT-qPCR analysis of the expression of CTNND1 mRNA in LSCs compared with blasts from AML samples of 3 independent AML cases. Results were normalized to the mRNA expression of GAPDH. Data are presented as mean ± SEM, ∗∗P < .01 and ∗P < .05. (C) WB analysis of cell lysate from Gal-9–stimulated CD34+CD38– AML cells (LSCs) and CD34+CD38+ cells (blasts) (AML#14). Of note, LSCs with higher p120-catenin expression exhibited profound β-catenin accumulation.
Exhausted T cells do not express p120-catenin at sufficient levels to activate the β-catenin pathway by Gal-9 ligation
Gal-9–induced canonical Wnt pathway activation via HCK and p120-catenin activation was universally observed in TIM-3+AML cells independent of the subtypes and differentiation status of AML52 (supplemental Figures 6A and 6B). In contrast, cord blood–derived normal CD34+ hematopoietic stem progenitor cells (supplemental Figure 7A) lacked the expression of TIM-3, and Gal-9 stimulation did not induce canonical Wnt pathway activation (supplemental Figure 7B).
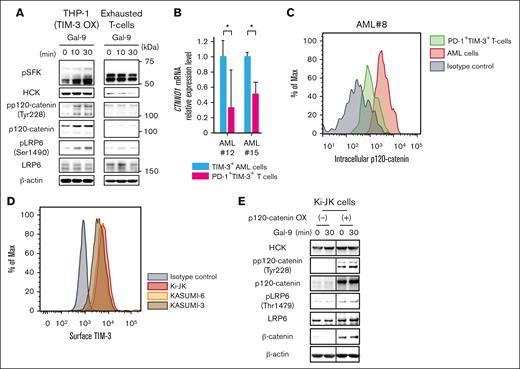
Exhausted T and NK cells express TIM-3, and Gal-9 ligation induces their immunosuppressive functions36,53 in an immune exhausted status.54,55 Human programmed cell death protein 1 (PD-1)+ TIM-3+ T cells were induced in vitro (supplemental Figure 8A), and incubated with Gal-9. As shown in Figure 6A, PD-1+ TIM-3+ exhausted T cells had almost undetectable levels of p120-catenin and HCK, and Gal-9 ligation did not induce LRP6 phosphorylation and subsequent β-catenin accumulation. In addition, quantitative reverse transcription polymerase chain reaction (RT-qPCR) and intracellular fluorescence analyses revealed that CD34+ TIM-3+ AML cells expressed significantly higher levels of p120-catenin as compared with TIM-3+ T cells (Figure 6B-C) obtained from identical patients (supplemental Figure 8B-C).
The expression of p120-catenin results in a striking difference in the signal transduction of TIM-3 between AML and exhausted T cells. (A) WB analysis of Gal-9–stimulated TIM-3-OX THP-1 and PD-1+TIM-3+ exhausted T cells. Of note, Gal-9 ligation to TIM-3 induced LRP6 phosphorylation in TIM-3-OX THP-1 cells but not in the exhausted T cells devoid of p120-catenin expression. (B) RT-qPCR analysis of CTNND1 mRNA in FACS-purified AML cells and the exhausted (PD-1+TIM-3+) T cells from 2 patients with AML (AML#12 and #15). Results were normalized to the mRNA expression of GAPDH. Data are presented as mean ± SEM, ∗P < .05. (C) Intracellular FACS analysis of p120-catenin expression in TIM-3+ AML cells and PD-1+TIM-3+ exhausted T cells in the identical patient with AML. A representative result out of 3 AML samples (AML#8, #12, and #15) is shown. (D) FACS analysis of surface TIM-3 expression in Ki-JK cells. TIM-3 expression of KASUMI-3 and KASUMI-6 are shown as positive controls. (E) The results of WB analysis using mock-transfected (left panels) and p120-catenin-OX Ki-JK cells (right panels) stimulated with Gal-9 are shown.
The expression of p120-catenin results in a striking difference in the signal transduction of TIM-3 between AML and exhausted T cells. (A) WB analysis of Gal-9–stimulated TIM-3-OX THP-1 and PD-1+TIM-3+ exhausted T cells. Of note, Gal-9 ligation to TIM-3 induced LRP6 phosphorylation in TIM-3-OX THP-1 cells but not in the exhausted T cells devoid of p120-catenin expression. (B) RT-qPCR analysis of CTNND1 mRNA in FACS-purified AML cells and the exhausted (PD-1+TIM-3+) T cells from 2 patients with AML (AML#12 and #15). Results were normalized to the mRNA expression of GAPDH. Data are presented as mean ± SEM, ∗P < .05. (C) Intracellular FACS analysis of p120-catenin expression in TIM-3+ AML cells and PD-1+TIM-3+ exhausted T cells in the identical patient with AML. A representative result out of 3 AML samples (AML#8, #12, and #15) is shown. (D) FACS analysis of surface TIM-3 expression in Ki-JK cells. TIM-3 expression of KASUMI-3 and KASUMI-6 are shown as positive controls. (E) The results of WB analysis using mock-transfected (left panels) and p120-catenin-OX Ki-JK cells (right panels) stimulated with Gal-9 are shown.
Next, we tested whether p120-catenin can alter TIM-3 signaling in Ki-JK cells, a human T-cell line that expresses TIM-3 (Figure 6D). Ki-JK cells expressed HCK but not p120-catenin. Incubation of Ki-JK cells with Gal-9 did not induce phosphorylation of LRP6 at Thr1479, nor accumulation of β-catenin (Figures 6E, left). When we enforced the expression of p120-catenin in Ki-JK cells, Gal-9 ligation induced phosphorylation of transduced p120-catenin as well as LRP6, resulting in β-catenin accumulation (Figure 6E, right). Thus, p120-catenin is critical for the activation of the canonical Wnt/β-catenin pathway by TIM-3 signaling in AML, but this activation does not occur in T cells, presumably because they lack p120-catenin expression.
Discussion
TIM-3 is an LSC-specific surface molecule expressed in the vast majority of AML cases, regardless of the subtypes of mutations, chromosomal abnormalities, or morphological classifications.2,3,7,8 TIM-3 is progressively upregulated in the CD34+CD38− stem cell fraction in association with leukemic transformation of chronic myelogenous leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes.1 Ligation of TIM-3 by Gal-9 induces β-catenin accumulation in the nucleus of AML LSCs.1 In murine MLL-AF9 AML models, self-renewing LSCs showed nucleus β-catenin accumulation, and conditional deletion of β-catenin wiped out LSCs in vivo.11 In human models, β-catenin is progressively accumulated in the LSC fraction of human chronic myelogenous leukemia in accordance with disease progression from chronic, accelerated phases, to blastic crisis,56 suggesting that the β-catenin is critical for LSCs, presumably to potentiate their self-renewal and propagation activities. These data suggest that TIM-3 signaling plays a critical role in the maintenance of malignant stemness in the vast majority of human myeloid leukemias.1,2
TIM-3 is generally recognized as an immune checkpoint molecule. Such checkpoint molecules usually harbor inhibitory signaling motifs in their cytoplasmic tails. TIM-3, however, lacks an inhibitory motif but can recruit SFKs for phosphorylation at its cytoplasmic tail, and, therefore, the nature of TIM-3 signaling may be dependent upon the type of recruited SFKs.5,57 In T-cell exhaustion, TIM-3 recruits FYN9,41,58,59 to produce inhibitory signaling. In contrast, TIM-3 in myeloid LSCs recruits HCK to phosphorylate p120-catenin that can directly activate the canonical Wnt pathway. p120-catenin, a critical component bridging TIM-3 signaling and the canonical Wnt pathway, is specifically and highly expressed in immature AML LSCs but not in TIM-3+ exhausted T cells. HCK, a member of the SFKs, is expressed in normal myeloid and B cells,45,60 but aberrant activation of HCK has been shown to be involved in the tumorigenesis of various types of cancers.61-64 AML LSCs express HCK at a higher level than normal HSCs,32 and the inhibition of HCK impairs the self-renewal of LSCs in vivo.43,44 Collectively, the TIM-3/Gal-9 autocrine loop and its AML-specific downstream components such as HCK and p120-catenin, constitute a novel molecular mechanism for activating the canonical Wnt signaling in AML. The AML-specific machinery for the constitutive activation of β-catenin driven by the TIM-3/Gal-9 autocrine loop is schematized in Figure 7. Although the extent of canonical Wnt pathway activation varied between patients, the phosphorylation of p120-catenin and LRP6 was commonly observed in various subtypes of AML samples after Gal-9 ligation to TIM-3. We therefore consider that Gal-9–mediated activation of HCK, p120-catenin, and the canonical Wnt pathway is a common phenomenon across different subtypes of TIM-3+ AML cells.
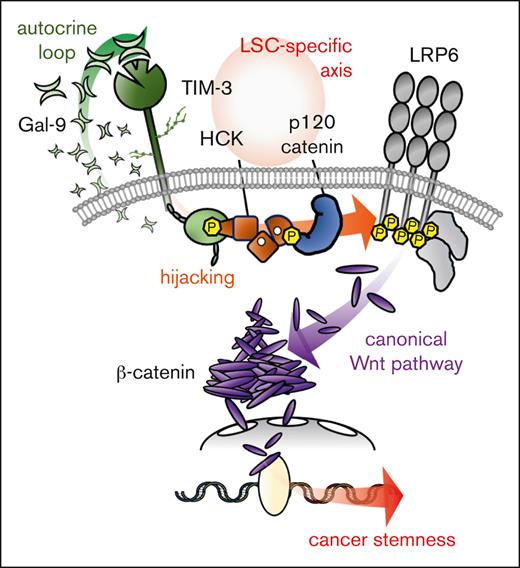
Schematic summary of a novel molecular mechanisms of the hijacking of the canonical Wnt pathway by TIM-3 signaling in AML LSCs. The schema shows how TIM-3 signaling induces constitutively canonical Wnt pathway activation and aberrant accumulation of β-catenin in AML-LSCs. As shown in the left panel, β-catenin is constantly destroyed by the β-catenin degradation complex in the absence of canonical Wnt pathway activation. As shown in the right panel, in AML, a TIM-3/Gal-9 autocrine loop constitutively recruits and activates HCK, leading to the induction of p120-catenin phosphorylation. The activated p120-catenin initiates LRP6 signalosome formation to inhibit the function of the β-catenin degradation complex, leading to the aberrant accumulation of β-catenin in AML cells. Through the use of LSCs-specific molecules such as TIM-3, HCK, and p120-catenin, the AML LSCs hijack the canonical Wnt pathway.
Schematic summary of a novel molecular mechanisms of the hijacking of the canonical Wnt pathway by TIM-3 signaling in AML LSCs. The schema shows how TIM-3 signaling induces constitutively canonical Wnt pathway activation and aberrant accumulation of β-catenin in AML-LSCs. As shown in the left panel, β-catenin is constantly destroyed by the β-catenin degradation complex in the absence of canonical Wnt pathway activation. As shown in the right panel, in AML, a TIM-3/Gal-9 autocrine loop constitutively recruits and activates HCK, leading to the induction of p120-catenin phosphorylation. The activated p120-catenin initiates LRP6 signalosome formation to inhibit the function of the β-catenin degradation complex, leading to the aberrant accumulation of β-catenin in AML cells. Through the use of LSCs-specific molecules such as TIM-3, HCK, and p120-catenin, the AML LSCs hijack the canonical Wnt pathway.
Our study provides a rationale for targeting TIM-3 signaling to treat human myeloid leukemias. Neutralizing anti–TIM-3 antibodies are being developed as “immune checkpoint inhibitors” for cancer treatment.65 Sabatolimab, an antihuman TIM-3 humanized IgG4 monoclonal antibody that can block ligation of Gal-9 to TIM-3, has been tested for the treatment of myeloid malignancies, including AML, high-risk myelodysplastic syndromes, and chronic myelomonocytic leukemia in combination with hypomethylating agents, and appears to show good efficacy with favorable tolerability profile.57,66 Our data strongly suggest that neutralizing anti–TIM-3 antibodies should be effective to treat human myeloid leukemias not only through its immune checkpoint inhibition, but through targeting the TIM-3/Gal-9 autocrine signaling that can hijack the canonical Wnt/β-catenin pathway.
TIM-3 is also expressed in myeloid cells including DCs6 and macrophage-monocytes,67 and lymphoid cells including NK cells55 and exhausted T cells,68 and TIM-3 generally suppresses their immunogenic activity.5 Although the immunosuppressive function of TIM-3 signaling is shared in myeloid and lymphoid cells, the outcome of β-catenin accumulation is totally different in these cells. Thus, canonical Wnt pathway activation downstream of TIM-3 was not observed in TIM-3+ exhausted T cells because of the absence of p120-catenin. In contrast, DCs and monocytes express p120-catenin,69 and, therefore, TIM-3 signaling might induce β-catenin accumulation in TIM-3–expressing DCs and monocytes. Because β-catenin induces the anti-inflammatory effect of macrophages70,71 and generates tolerogenic DCs,72,73 our study might contribute to the understanding of the immunosuppressive effect of TIM-3 in normal myeloid cells.
In conclusion, we identified that the TIM-3/Gal-9 autocrine loop constitutively activates β-catenin signaling of LSCs in AML, by hijacking the Wnt/β-catenin pathway through activation of HCK and p120-catenin. This cell-autonomous and mutation-independent machinery to maintain self-renewal and/or propagation of LSCs should be a clinically important target in treatment of human myeloid leukemias.
Acknowledgments
This study was supported, in part, by a Grant-in-Aid for Young Scientists (A) to Y.K. (number 16748470), a Grant-in-Aid for Scientific Research (S) to K.A. (number 16747244), and a Grant-in-Aid for Scientific Research (B) to T.M. (number 16674756) and to Y.K. (number 19109659), from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was also supported, in part, by a Grant-in-Aid for Japan Agency for Medical Research and Development to Y.K. (number 16768249) and to K.A. (number 16770576). This study was also supported, in part, by a Grant-in-Aid for the Shinnihon Foundation of Advanced Medical Treatment Research to Y.K.
Authorship
Contribution: H.I., K.H., T.H., Y. Kikushige, T.M., and T.S. designed and performed all experiments, analyzed the data, performed the statistical analysis, and wrote the manuscript; Y. Kikushige and K.A. approved the final version of the manuscript for publication; all other authors contributed to the interpretation of data and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshikane Kikushige, Kyushu University Graduate School of Medical Sciences, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; e-mail: kikushige.yoshikane.726@m.kyushu-u.ac.jp.
References
Author notes
Expression microarray data are available in the Gene Expression Omnibus database (accession number GSE130328). All materials are available commercially or can be derived using the methods described in this article.
Data are available on request from the corresponding author, Yoshikane Kikushige (kikushige.yoshikane.726@m.kyushu-u.ac.jp).
The full-text version of this article contains a data supplement.