TO THE EDITOR:
Prophylactic therapy has become the standard of care for patients with hemophilia A (HA); however, prophylaxis regimens that require 2 to 3 weekly infusions of drug are challenging. In the past decade, new factor VIII (FVIII) molecules with extended plasma half-life have been produced with the goal of reducing the number of infusions while maintaining higher trough levels of FVIII in plasma.1-4 One of the most established approaches to prolong the circulation time while preserving the biological activity is the covalent link of polyethylene glycol (PEG) chains to therapeutic proteins. To date, several PEGylated drugs are available for the treatment of a variety of chronic diseases including the recently marketed SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), containing PEGylated lipids.5-7 Moreover, PEG is widely used in the formulation of common personal care products.8 Concerning the treatment of HA, 3 novel PEGylated recombinant FVIII (rFVIII) products, rurioctocog alfa pegol, turoctocog alfa pegol, and damoctocog alfa pegol, have been authorized by the Food and Drug Administration and European Medicines Agency; these rFVIII molecules have a mean half-life 1.3 to 1.7 times longer than the standard rFVIII and show comparable safety and pharmacokinetic (PK) profiles.9
Although PEG is a nonimmunogenic polymer, pre-existing anti-PEG antibodies (APAs) have been reported in persons never treated with PEGylated biopharmaceutics with increasing incidence up to 72% over the years.10,11 Usually, pre-existing APAs have no clinical relevance but in few cases showed the potential to bind a PEGylated drug, enhancing its blood clearance and reducing therapeutic efficacy.12,13
In this report, we describe the poor plasma FVIII recovery observed in 2 patients with HA following their first infusion with the PEGylated rFVIII products associated with the presence of anti-PEGylated rFVIII antibodies after the BNT162b2 SARS-CoV-2 vaccination.
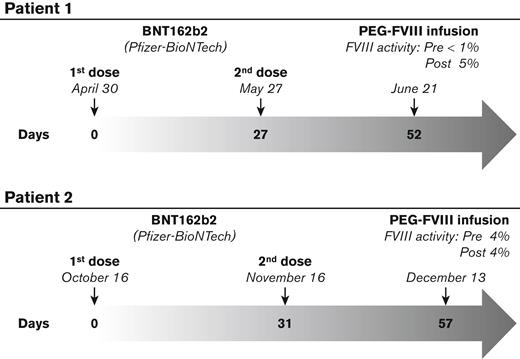
Patient 1 is a 42-year-old man on prophylaxis with 37 IU/kg every 3 days of a standard rFVIII product (NovoEight, Novo Nordisk A/S). In April and May 2021, the patient received his first and second dose of the BNT162b2 vaccine. In June 2021, 52 days after the first and 25 days after the second dose, before his switch to a novel PEGylated rFVIII, he underwent the PK study by the injection of 50 IU/kg of turoctocog alfa pegol (Figure 1).
Timeline of the events. Patient 1 received the first and the second dose of the SARS-CoV-2 vaccine (BNT162b2, Pfizer-BioNTech) in April and May, respectively. In June, he was infused with the PEG conjugates FVIII product turoctocog alfa pegol. Patient 2 received the first and the second dose of the SARS-CoV-2 vaccine (BNT162b2, Pfizer-BioNTech) in October and November, respectively. In December, he was infused with the PEGylated FVIII product damoctocog alfa pegol. A poor FVIII recovery after the PEGylated FVIII infusion was found for both patients. For patient 1, the pre- and postinfusion FVIII activity was <1% and 5%, respectively; for patient 2, the pre- and postinfusion FVIII activity was 4% unchanged.
Timeline of the events. Patient 1 received the first and the second dose of the SARS-CoV-2 vaccine (BNT162b2, Pfizer-BioNTech) in April and May, respectively. In June, he was infused with the PEG conjugates FVIII product turoctocog alfa pegol. Patient 2 received the first and the second dose of the SARS-CoV-2 vaccine (BNT162b2, Pfizer-BioNTech) in October and November, respectively. In December, he was infused with the PEGylated FVIII product damoctocog alfa pegol. A poor FVIII recovery after the PEGylated FVIII infusion was found for both patients. For patient 1, the pre- and postinfusion FVIII activity was <1% and 5%, respectively; for patient 2, the pre- and postinfusion FVIII activity was 4% unchanged.
Patient 2 is a 24-year-old male on prophylaxis with 32 IU/kg 2 times a week of a standard rFVIII product (Kovaltry, Bayer AG). In October and November 2021, he received his first and second dose of the BNT162b2 vaccine. In December 2021, 57 days after the first dose and 26 days after the second dose, he underwent the PK study with the injection of 50 IU/kg of damoctocog alfa pegol (Figure 1).
Plasma samples from both patients were collected before and after the PEGylated rFVIII infusion as well as before the first SARS-CoV-2 vaccination. For patient 1, a further plasma sample was obtained almost 1 year after the second dose of the SARS-CoV-2 vaccine. Written informed consent was obtained from patients, and the Ethics Committee of Milan Area 2 gave approval for the data analyses and publication.
FVIII coagulant activity, measured by using the Chromogenix Coamatic Factor VIII kit (Werfen Bedford, MA) before and after the infusion of the PEGylated rFVIII products, was less than 1% and 5% for patient 1, respectively, and 4% with no modification for patient 2 (Figure 1). In both cases, a very poor FVIII recovery after the PK infusion was obtained; therefore, the presence of FVIII inhibitor was ruled out and each patient returned to his original standard rFVIII product showing a normal FVIII recovery.
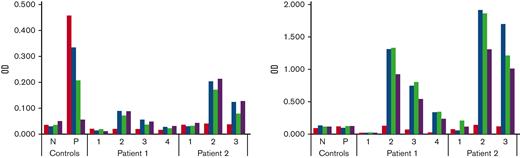
Following these results, a home-made enzyme-linked immunosorbent assay (ELISA) was used to search the anti-PEGylated rFVIII antibodies by using each of the licensed PEGylated rFVIII products (rurioctocog alfa pegol, turoctocog alfa pegol, damoctocog alfa pegol) as capturing substrates and a standard rFVIII (octocog alpha) control as well. The 4 rFVIII molecules, diluted at the concentration of 4 IU/mL were loaded, 3 rows for each product into the same immune-plate (NUNC, Thermo Fisher Scientific). Bound immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies were detected by using the anti-human IgG-HRP (GE Healthcare NA933) and the anti-human IgM-HRP (Sigma A4290). Plasma samples from 34 healthy subjects were pooled and used as negative control. Plasma samples from 5 patients with HA and anti-FVIII inhibitor were pooled and used as positive control for anti-FVIII antibodies. Both mixtures were prepared by using plasma samples, previously stored at −80°C, from subjects never treated with PEGylated drugs and collected before the messenger RNA (mRNA) SARS-CoV-2 vaccine diffusion. Patients and control samples were analyzed in ELISA at 1:10 dilution. The results of this assay (Figure 2) showed the presence of IgM antibodies reacting at similar extent against all PEGylated rFVIII products, but not against the standard rFVIII, in patients’ plasma collected before and after the PK infusion. In the patient’s plasma sample 1, obtained 1 year after the second dose of the SARS-CoV-2 vaccine, a signal although at a lower level, was shown. A weak positivity for IgG antibodies was found against the PEGylated products in patients’ plasma collected before and after the PK infusion. No reactivity against any rFVIII molecule was found in patients’ plasma samples collected before the SARS-CoV-2 vaccination as well as in the negative control. The presence of IgG reacting against the non-PEGylated rFVIII and the FVIII part of the PEGylated products was found in the positive control.
ELISA detecting the anti-PEGylated FVIII antibodies. Antibodies (IgG on the left and IgM on the right) against the different FVIII molecules were detected by using each FVIII molecule as the capturing reagent. The bar colors indicate the used capturing molecule: red, octocog alfa; blue, rurioctocog alfa pegol; green, turoctocog alfa pegol; purple, damoctocog alfa pegol. Plasma samples were analyzed at different time points: 1, before SARS-CoV-2 vaccination; 2, before the PEGylated FVIII infusion (baseline); 3, after PEGylated FVIII infusion (30’); 4, 1 year after the second dose of the SARS-Co-V-2 vaccine. N indicates the pool of plasma from healthy subjects, and P indicates the pool of plasma from hemophilia A patients with anti-FVIII antibodies. Reactivity against the PEGylated FVIII products (higher with IgM than IgG) was found for both patients in plasma collected after the SARS-Co-V-2 vaccination but not in plasma obtained before. No significant signal against the non-PEGylated FVIII molecule was found. The pool of plasma containing anti-FVIII antibodies showed variable IgG reactivity against all molecules. No relevant signals were found with the pool plasma from healthy subjects.
ELISA detecting the anti-PEGylated FVIII antibodies. Antibodies (IgG on the left and IgM on the right) against the different FVIII molecules were detected by using each FVIII molecule as the capturing reagent. The bar colors indicate the used capturing molecule: red, octocog alfa; blue, rurioctocog alfa pegol; green, turoctocog alfa pegol; purple, damoctocog alfa pegol. Plasma samples were analyzed at different time points: 1, before SARS-CoV-2 vaccination; 2, before the PEGylated FVIII infusion (baseline); 3, after PEGylated FVIII infusion (30’); 4, 1 year after the second dose of the SARS-Co-V-2 vaccine. N indicates the pool of plasma from healthy subjects, and P indicates the pool of plasma from hemophilia A patients with anti-FVIII antibodies. Reactivity against the PEGylated FVIII products (higher with IgM than IgG) was found for both patients in plasma collected after the SARS-Co-V-2 vaccination but not in plasma obtained before. No significant signal against the non-PEGylated FVIII molecule was found. The pool of plasma containing anti-FVIII antibodies showed variable IgG reactivity against all molecules. No relevant signals were found with the pool plasma from healthy subjects.
Clinically relevant interference by APAs (pre-existing or boosted) with the PEGylated drugs has been reported for PEG-uricase, PEG-asparaginase, and PEGylated aptamers.14-17 Pertaining to PEGylated rFVIII, APAs were found in few patients with HA during treatment with damoctocog alfa pegol in the frame of the phase 2/3 PROTECT VIII trial, and with turoctocog alfa pegol in the frame of the pathfinder2 phase 5 trial (NCT01480180).18,19 In both cases, the antibodies were transient, at low titer, and had no clinical effect. Recently, transient anti-turoctocog alfa pegol–binding antibodies and anti-PEG IgM and IgG antibodies were described in previously untreated patients with severe HA treated with turoctocog alfa pegol in the frame of the ongoing pathfinder6 (NCT02137850) phase 3 trial. The authors found that the anti-PEG IgG titer was temporarily correlated with a decrease in FVIII recovery with no significant changes in the hemostatic response.20
In the context of the COVID-19 pandemic, both mRNA SARS-CoV-2 vaccines include PEGylated lipids in the nanoparticles containing the mRNA. The effect of vaccination with mRNA-1273 and BNT162b2 on APAs level has been recently studied in a group of healthy adults.21 The study found that PEG-specific antibodies can be boosted by vaccination and can be associated with higher systemic reactogenicity.
Based on the recently reported findings, we hypothesize that the poor FVIII recovery observed in our 2 patients with HA switched to the novel PEGylated rFVIII products was associated to increased levels of APAs following the second dose of BNT162b2 vaccine. These antibodies, by reacting with the PEGylated part of the FVIII molecule, significantly reduced the FVIII recovery in the PK study.
In conclusion, because of the increasing use of PEGylated drugs and the need of booster doses of anti-SARS-CoV-2 mRNA vaccines, clinicians should carefully monitor the PK and pharmacodynamics of the PEGylated rFVIII products when therapeutic efficacy is missing or inadequate.
Acknowledgments: The authors gratefully acknowledge Luigi Flaminio Ghilardini for his help in preparing the figures.
This work was partially supported by the Italian Ministry of Health - Bando Ricerca Corrente 2021.
Contribution: F.P. conceived the study; C.V., R.G., and F.P. wrote the manuscript; R.G., S.A., A.C., and S.M.S. managed patients; C.V., L.S., and C.N. supervised blood samples and data collection and performed the analysis; P.M.M. critically revised the manuscript; and all authors reviewed the data and revised the manuscript.
Conflict-of-interest disclosure: F.P. is a member of advisory boards of Sanofi, Sobi, Takeda, Roche, Biomarin and a speaker at educational meetings of Grifols and Roche. R.G. is a member of advisory boards of Roche, Takeda, Bayer, Pfizer, and Novo Nordisk. C.N. is consultant for Werfen and Takeda. A.C. is a consultant for Bayer. P.M.M. is a speaker for Bayer, Kedrion, and Roche and a member of the advisory board for Bayer Award. The remaining authors declare no competing financial interests.
Correspondence: Flora Peyvandi, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Via Pace 9, 20122 Milan, Italy; e-mail: flora.peyvandi@unimi.it.
References
Author notes
Data are available on request from the corresponding author, Flora Peyvandi (flora.peyvandi@unimi.it).