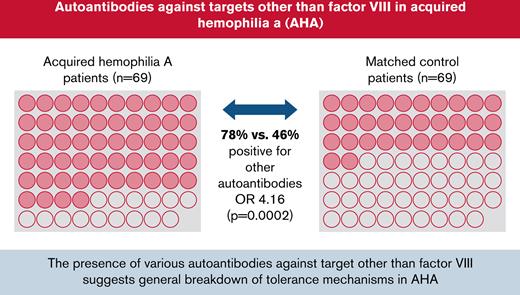
Key Points
Seventy-eight percent of patients with AHA were found to have autoantibodies against targets other than factor VIII.
The data suggest that a generalized breakdown of self-tolerance mechanisms is involved in the pathology of AHA.
Abstract
The root cause of autoantibody formation against factor VIII (FVIII) in acquired hemophilia A (AHA) remains unclear. We aimed to assess whether AHA is exclusively associated with autoantibodies toward FVIII or whether patients also produce increased levels of autoantibodies against other targets. A case-control study was performed enrolling patients with AHA and age-matched controls. Human epithelial cell (HEp-2) immunofluorescence was applied to screen for antinuclear (ANA) and anticytoplasmic autoantibodies. Screening for autoantibodies against extractable nuclear antigens was performed by enzyme immunoassay detecting SS-A/Ro, SS-B/La, U1RNP, Scl-70, Jo-1, centromere B, Sm, double-stranded DNA, and α-fodrin (AF). Patients with AHA were more often positive for ANA than control patients (64% vs 30%; odds ratio [OR] 4.02, 1.98-8.18) and had higher ANA titers detected than controls. Cytoplasmic autoantibodies and anti-AF immunoglobulin A autoantibodies were also more frequent in patients with AHA compared with controls. Autoantibodies against any target other than FVIII were found in 78% of patients with AHA compared with 46% of controls (OR 4.16, 1.98-8.39). Results were similar preforming sensitivity analyses (excluding either subjects with autoimmune disorders, cancer, pregnancy, or immunosuppressive medication at baseline) and in multivariable binary logistic regression. To exclude that autoantibody staining was merely a result of cross-reactivity of anti-FVIII autoantibodies, we tested a mix of 7 well-characterized monoclonal anti-FVIII antibodies. These antibodies did not stain HEp-2 cells used for ANA detection. In conclusion, a diverse pattern of autoantibodies is associated with AHA, suggesting that a more general breakdown of immune tolerance might be involved in its pathology.
Introduction
Neutralizing autoantibodies against coagulation factor VIII (FVIII inhibitors) cause acquired hemophilia A (AHA). The disorder affects women and men of all ages but mainly the elderly.1 Progress has been achieved in managing acute hemorrhage in AHA as documented by declining rates of bleed-related mortality.2 Nowadays, the leading cause of mortality remains infection, often related to the immunosuppressive therapy (IST) used to suppress autoantibody formation and to induce long-term remission of the disease.3,4
IST in AHA consists of corticosteroids, rituximab, and cytotoxic drugs like cyclophosphamide.5 More targeted, biological therapies that revolutionized the treatment of rheumatoid and other autoimmune disorders have not been introduced in AHA. This can be attributed in part to a lack of understanding how the autoimmune process against FVIII arises, how it is sustained, and how it is suppressed in healthy individuals or in patients achieving remission.
FVIII is the coagulation factor most commonly targeted by autoimmune inhibitors,6 but it is also considered highly immunogenic when given as replacement therapy to patients suffering from congenital hemophilia A, who express no or dysfunctional FVIII protein due to mutations in the F8 gene.7,8
Nonneutralizing anti-FVIII autoantibodies have been observed in healthy individuals.9 We previously reported that such autoantibodies are of low affinity and belong to the immunoglobulin G1 (IgG1) or IgG3 subclasses but never to the IgG4 subclass.10,11 In contrast, 98% of patients with AHA show high-affinity anti-FVIII autoantibodies of the IgG1 and IgG4 subclasses.11,12 Differentiation of B cells into long-lived plasma cells, secreting high-affinity antibodies, typically requires cognate interactions with antigen-specific follicular helper CD4 T cells in specific structures of secondary lymphoid organs, called germinal centers.13,14 Most likely, the pathogenesis of AHA also involves CD4 T-cell–dependent differentiation, enabling class switch and affinity maturation of the B-cell receptor.13-15 Such a process would require the activation of autoreactive FVIII-specific follicular CD4 T helper cells able to provide costimulatory signals to FVIII-reactive B cells.
Previous studies established that central mechanisms of immune tolerance do not completely delete autoreactive B and T cells. These are found in the periphery, exhibit low to medium affinity for their target antigen, and need to be controlled by peripheral mechanisms of self-tolerance.16-19 In the spleen, transitional B cells that strongly bind self-antigen are removed by mechanisms of clonal deletion or anergy. The same happens to naïve B cells not receiving costimulatory signals from T helper cells during antigen encounter in the lymph node, which further reduces the frequency of autoreactive B cells. Autoreactive B cells can be generated de novo through somatic hypermutation of the B-cell receptor during CD4 T-cell–dependent affinity maturation in germinal centers.20 Mechanisms exist against self-reactive B-cell receptor affinity maturation, but the development of autoimmunity as a consequence of somatic hypermutation has been described in human studies relating to autoantibody-induced diseases such as pemphigus vulgaris or pulmonary alveolar proteinosis.20
AHA is primarily a disease of the elderly, and its pathogenesis might include an age-related deterioration of peripheral mechanisms of self-tolerance, caused by immunosenescence and inflammaging.21,22
Therefore, we were interested to investigate whether AHA is exclusively associated with autoantibodies toward FVIII or whether patients with AHA also more frequently generate autoantibodies against other targets. If the latter was true, it could be a valuable hint toward a more general breakdown of peripheral control mechanisms of self-tolerance rather than a specific pathogenic autoantibody response against FVIII (eg, caused by molecular mimicry or abnormal proliferation of FVIII-specific germline B cells).
To this end, we employed an autoantibody screening strategy in a consecutive cohort of patients with AHA and age-matched controls. To exclude that autoantibody staining was merely a result of crossreactivity of the anti-FVIII autoantibodies contained in patient plasma, we also tested well-characterized monoclonal anti-FVIII antibodies in a control experiment. Here, we report both the results of the clinical study and our additional experiments that collectively demonstrate an association of AHA with more general markers of autoantibody formation and suggest that a generalized breakdown of peripheral mechanisms of self-tolerance might be involved in the pathogenesis of the disease.
Methods
Study design
The current study was a case-control study enrolling patients with AHA from the GTH-AH 01/2010 multicenter prospective observational study and age-matched control patients.
Patients with AHA were enrolled from German and Austrian hemophilia centers that participate in the GTH-AH 01/2010 registry. A diagnosis of AHA was made based on a neutralizing FVIII inhibitor ≥ 0.6 Bethesda units (BU)/mL (lower limit of detection) and a factor VIII activity < 50 IU/dL (lower limit of normal).3 Information on underlying and concomitant disorders was collected by participating physicians from medical records and reported using standardized case report forms. Autoimmune and malignant disorders were classified according to international classification of diseases version 10. The ethics committees of all participating centers approved the study protocol, and patients gave informed consent before enrolment.
Adult control patients were recruited from Hannover Medical School Emergency and Admission Unit if a sample was routinely drawn for coagulation testing. These patients had been admitted for various acute or chronic medical disorders. Recruitment into the control group was performed in age tiers of 5 years according to the age distribution of patients with AHA; recruitment was consecutive over a period of 3 weeks until an age tier was complete. Control patients with active bleeding or known hemostasis disorders were excluded. Otherwise, no matching was actively attempted. In addition to age and gender, we collected concomitant disorders and medication from the control patients’ medical records. Hannover Medical School ethics committee approved the recruitment of the control group; informed consent was not required because only residual material was used, and data were completely anonymized.
Citrated plasma from AHA and control patients was centrifuged according to local standards and stored at −80°C until analysis.
Anti-FVIII antibody detection
HEp-2 indirect immunofluorescence of clinical samples
Geometric dilution of plasma samples was performed in phosphate-buffered saline, starting at 1:80. Dilutions were incubated on human epithelial cells (HEp-2) fixed on glass slides (Aesku Diagnostics GmbH & Co. KG, Wendelsheim, Germany) in a moisture chamber for 30 minutes at room temperature. After washing, bound antibodies were detected by incubation with fluorescein isothiocyanate–conjugated anti-human immunoglobulin for 30 minutes at room temperature. Subsequently, slides were washed and assessed visually with a fluorescence microscope (Olympus IX81). The highest dilution resulting in visible staining was reported, with 1:160 or higher considered positive. The staining pattern was reported according to the International Consensus on ANA Patterns (https://www.anapatterns.org).26
Enzyme immunoassays detection of autoantibodies
Screening for autoantibodies against extractable nuclear antigens (ENA) was performed using the automated EliA Symphony S assay (Phadia AB, Uppsala, Sweden). The following autoantibodies are detected by this screening assay: SS-A/Ro, SS-B/La, U1RNP (70 kDa, A, and C protein), Scl-70, Jo-1, centromere B, and Sm. Single assays were applied for double-stranded DNA (dsDNA) and α-fodrin (AF) IgG and IgA autoantibodies. All assays were performed on a Phadia 250 system according to the manufacturer’s instructions.
HEp-2 indirect immunofluorescence with anti-FVIII monoclonal antibodies
The HEp-2 immunofluorescence assay was performed as described above, except that a mix of anti-FVIII mouse monoclonal antibodies (mAb) or isotype controls were used. FVIII mAb were a mix of equal amounts of 7 purchasable monoclonal anti-FVIII IgG antibodies directed against different FVIII epitopes (GMA-8001, GMA-8002, GMA-8011, GMA-8020, and GMA-8021, Green Mountain Antibodies, Burlington, VT; ADGESH-4 and ADGESH-8, ImmBioMed GmbH & Co. KG, Pfungstadt, Germany). All anti-FVIII mAb were of the IgG2a subclass, except for GMA-8001, which was IgG1. The isotype controls were MOPC-31C IgG1 (BD Biosciences Pharmingen) and eBM2a IgG2a (Thermo Fisher Scientific) and were mixed proportionally according to the anti-FVIII mAb subclasses. FVIII-containing immune complexes were prepared of the above anti-FVIII mAb in equal amounts (total concentration, 100 μg/mL or .667 μmol/L) and full-length recombinant FVIII (Advate, Takeda, Vienna, Austria; 100 μg/mL or .357 μmol/L), resulting in a molar ratio of mAb:FVIII of about 1.87:1). Fluorescein isothiocyanate–conjugated goat anti-mouse IgG (Thermo Fisher Scientific) was used for detection, and slides were embedded with Hoechst 33342–containing medium (Invitrogen).
Statistical methods
Statistical power considerations were based on the reported frequency of ANA positivity in the elderly, ranging from 12% in a study from Finland27 to up to 22% in women > 70 years in the United States.28 We considered a twofold higher prevalence as meaningful (eg, 40% vs 20%), corresponding to an effect size of w = 0.45 in Fisher’s exact test for contingency tables. With error probabilities of α = 0.01 and β = 0.80, a total sample size of 90 (45 per each group) was estimated using G∗Power 3.1.29 Statistical testing for ENA and dsDNA was considered of interest if ANA was more frequently positive in AHA than in controls, because ENA or dsDNA positivity is usually expected in people with positive ANA.
Positivity of autoimmune markers was described in proportions using contingency tables and compared by Fisher’s exact test. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated using the Baptista-Pike method using GraphPad Prism 8. Multivariable binomial logistic regression, with group (patients with AHA or control patients) as the dependent variable and age, gender, ANA positivity, and known autoimmune disorder as covariates, was performed using SPSS version 27. Continuous data were compared by their median and using nonparametrical Mann-Whitney U tests.
Results
Demographic and baseline data for patients with AHA (n = 69) and controls (n = 69) are provided in Table 1. The 2 samples were matched for age and had a similar gender ratio. Clinically diagnosed, concomitant autoimmune disorders were more frequent in patients with AHA as compared with controls (see Table 2 for a detailed list of autoimmune disorders). Concomitant malignancy or pregnancy were present in AHA as described in previous cohort studies and were similar in frequency between AHA and control patients. Immunosuppressive therapy at the time of analysis was not different between the groups. All AHA, but no control patients, had FVIII inhibitors of at least 1 BU/mL. FVIII-binding IgG was detected in all but 1 patient with AHA but rarely and at very low concentration in the control group. The single patient with AHA without anti-FVIII IgG had anti-FVIII IgM antibodies.
Baseline characteristics
| Characteristic . | Patients with AHA (N = 69) . | Controls (N = 69) . | P value for difference . |
|---|---|---|---|
| Age in years, mean (SD, range) | 67 (15, 26-97) | 65 (18, 22-89) | .519 |
| Female, n (%) | 31 (45) | 28 (41) | .731 |
| AHA-associated clinical disorders | |||
| Autoimmune disorders | 12 (17) | 1 (1) | .002 |
| Malignancy | 8 (12) | 10 (14) | .801 |
| Pregnancy | 5 (7) | 1 (1) | .208 |
| Immunosuppressive therapy,∗n (%) | 4 (6) | 4 (6) | >.999 |
| Corticosteroids | 4 | 2 | |
| Other | 1 | 3 | |
| Inhibitor concentration in BU/mL† | |||
| Median | 15 | 0 | <.0001 |
| IQR | 7.4-71 | 0-0 | |
| Range | 1.1-1448 | 0-0 | |
| Anti-FVIII IgG in AU/mL‡ | |||
| Median | 604 | 4.6 | <.0001 |
| IQR | 237-1608 | 3.0-7.7 | |
| Range | 0.5-51500 | 0.8-19 | |
| Characteristic . | Patients with AHA (N = 69) . | Controls (N = 69) . | P value for difference . |
|---|---|---|---|
| Age in years, mean (SD, range) | 67 (15, 26-97) | 65 (18, 22-89) | .519 |
| Female, n (%) | 31 (45) | 28 (41) | .731 |
| AHA-associated clinical disorders | |||
| Autoimmune disorders | 12 (17) | 1 (1) | .002 |
| Malignancy | 8 (12) | 10 (14) | .801 |
| Pregnancy | 5 (7) | 1 (1) | .208 |
| Immunosuppressive therapy,∗n (%) | 4 (6) | 4 (6) | >.999 |
| Corticosteroids | 4 | 2 | |
| Other | 1 | 3 | |
| Inhibitor concentration in BU/mL† | |||
| Median | 15 | 0 | <.0001 |
| IQR | 7.4-71 | 0-0 | |
| Range | 1.1-1448 | 0-0 | |
| Anti-FVIII IgG in AU/mL‡ | |||
| Median | 604 | 4.6 | <.0001 |
| IQR | 237-1608 | 3.0-7.7 | |
| Range | 0.5-51500 | 0.8-19 | |
ND, not determined; SD, standard deviation.
Before diagnosis (in patients with AHA) or at the time of sampling (in controls).
Reference value < 0.6 BU/mL. Values below 0.6 BU/mL were reported as zero.
Reference value < 12.8 AU/mL. One out of 69 patients with AHA was negative for anti-FVIII IgG (<12.8 AU/mL) but had anti-FVIII IgM. Two out of 69 controls were positive for anti-FVIII IgG (>12.8 AU/mL) but negative for inhibitor in the NBA.
Listing of autoimmune and malignant disorders at the time of sampling
| . | Patients with AHA . | Controls . |
|---|---|---|
| Autoimmune disorders | ||
| Rheumatoid arthritis (n = 3) | Inflammatory bowel disease | |
| Systemic lupus erythematosus (n = 2) | ||
| Bullous pemphigoid (n = 2) | ||
| Hashimoto’s thyroiditis | ||
| Immune thrombocytopenia | ||
| Giant cell arteritis | ||
| Chronic graft-versus-host disease | ||
| Neurodermatitis, alopecia areata | ||
| Malignant disorders | ||
| Myeloid | Myelofibrosis | Acute myeloid leukemia |
| Lymphoid | Chronic lymphocytic leukemia | Follicular lymphoma |
| Multiple myeloma | ||
| Waldenström’s macroglobulinemia | ||
| Cancers | Lung∗ | Lung |
| Head and neck | Breast∗ | |
| Colon∗ | Head and neck | |
| Skin | Skin | |
| Esophageal | ||
| Thyroid | ||
| Renal∗ | ||
| Prostate∗ |
| . | Patients with AHA . | Controls . |
|---|---|---|
| Autoimmune disorders | ||
| Rheumatoid arthritis (n = 3) | Inflammatory bowel disease | |
| Systemic lupus erythematosus (n = 2) | ||
| Bullous pemphigoid (n = 2) | ||
| Hashimoto’s thyroiditis | ||
| Immune thrombocytopenia | ||
| Giant cell arteritis | ||
| Chronic graft-versus-host disease | ||
| Neurodermatitis, alopecia areata | ||
| Malignant disorders | ||
| Myeloid | Myelofibrosis | Acute myeloid leukemia |
| Lymphoid | Chronic lymphocytic leukemia | Follicular lymphoma |
| Multiple myeloma | ||
| Waldenström’s macroglobulinemia | ||
| Cancers | Lung∗ | Lung |
| Head and neck | Breast∗ | |
| Colon∗ | Head and neck | |
| Skin | Skin | |
| Esophageal | ||
| Thyroid | ||
| Renal∗ | ||
| Prostate∗ |
Unless otherwise indicated, every diagnosis occurred in 1 patient.
In remission at the time of sampling.
Autoimmune markers in patients with AHA and controls
ANA were detected by HEp-2 immunofluorescence in 64% and 30% of AHA and control patients, respectively (Table 3). The difference corresponded to an OR of 4.02 (95% CI, 1.98-8.18) and was highly statistically significant.
Frequency of autoantibody detection
| Characteristic . | Patients with AHA (N = 69) . | Controls (N = 69) . | OR (95% CI) . | P . |
|---|---|---|---|---|
| ANA (positive ≥ 1:160), n (%) | 44 (64) | 21 (30) | 4.02 (2.02-8.01) | .0002 |
| ANA staining pattern | ||||
| Homogenous, n/n (%) | 7/44 (16) | 7/21 (33) | ||
| Speckled, n/n (%) | 31/44 (70) | 13/21 (62) | ||
| Other, n/n (%) | 6/44 (14) | 1/21 (5) | ||
| Cytoplasmic, n (%) | 21 (30) | 13 (19) | 1.88 (0.85-4.16) | .166 |
| ENA, n (%) | 4 (6) | 2 (3) | 2.06 (0.46-11) | .680 |
| dsDNA, n (%) | 2 (3) | 3 (4) | 0.66 (0.11-3.31) | .999 |
| AF IgG, n (%) | 8 (12) | 10 (14) | 0.77 (0.28-2.00) | .801 |
| AF IgA, n (%) | 8 (12) | 0 (0) | Infinite (2.34-+∞) | .006 |
| Any of the above, n (%) | 54 (78) | 32 (46) | 4.16 (1.98-8.39) | .0002 |
| Characteristic . | Patients with AHA (N = 69) . | Controls (N = 69) . | OR (95% CI) . | P . |
|---|---|---|---|---|
| ANA (positive ≥ 1:160), n (%) | 44 (64) | 21 (30) | 4.02 (2.02-8.01) | .0002 |
| ANA staining pattern | ||||
| Homogenous, n/n (%) | 7/44 (16) | 7/21 (33) | ||
| Speckled, n/n (%) | 31/44 (70) | 13/21 (62) | ||
| Other, n/n (%) | 6/44 (14) | 1/21 (5) | ||
| Cytoplasmic, n (%) | 21 (30) | 13 (19) | 1.88 (0.85-4.16) | .166 |
| ENA, n (%) | 4 (6) | 2 (3) | 2.06 (0.46-11) | .680 |
| dsDNA, n (%) | 2 (3) | 3 (4) | 0.66 (0.11-3.31) | .999 |
| AF IgG, n (%) | 8 (12) | 10 (14) | 0.77 (0.28-2.00) | .801 |
| AF IgA, n (%) | 8 (12) | 0 (0) | Infinite (2.34-+∞) | .006 |
| Any of the above, n (%) | 54 (78) | 32 (46) | 4.16 (1.98-8.39) | .0002 |
The number of patients with a positive result according to central laboratory cutoff. ANA and cytoplasmic antibodies were detected in HEp-2 immunofluorescence; other antibodies were detected using an enzyme immunoassay.
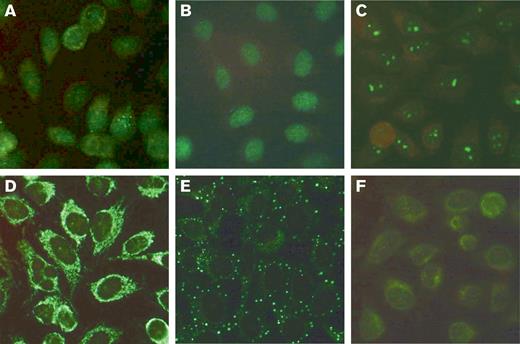
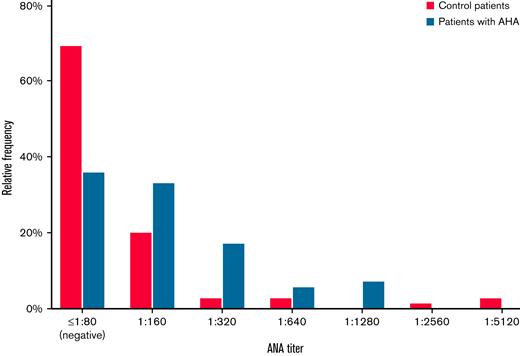
The ANA patterns in patients with AHA were diverse, including homogenous, speckled, and other patterns. Representative examples are shown in Figure 1. The distribution of ANA titers in AHA and control patients is shown in Figure 2. ANA titers were higher in patients with AHA as compared with control patients.
Representative HEp-2 immunofluorescent patterns of patients with AHA. (A) ANA (1:320) of nuclear speckled pattern with few nuclear dots in an 85-year-old male patient with FVIII:C of 2.2 IU/dL and an inhibitor of 4 BU/mL. (B) ANA (1:640) of nuclear homogeneous pattern in an 86-year-old male patient with FVIII:C of 2.4 IU/dL and an inhibitor of 9 BU/mL. (C) ANA (1:640) of nucleolar pattern in an 81-year-old female patient with FVIII:C of 2.5 IU/dL and an inhibitor of 104 BU/mL. (D) Cytoplasmic reticular, “mitochondrial” pattern (without ANA) in a 52-year-old female patient with FVIII:C of 4 IU/dL and an inhibitor of 71 BU/mL. (E) Cytoplasmic discrete dots/GW body-like pattern in a 73-year-old male patient with FVIII:C of <1 IU/dL and an inhibitor of 1.5 BU/mL. (F) Combined nuclear and cytoplasmic pattern (1:160) in a 73-year-old female patient with FVIII:C of 6 IU/dL and an inhibitor of 60 BU/mL. FVIII:C, factor VIII activity.
Representative HEp-2 immunofluorescent patterns of patients with AHA. (A) ANA (1:320) of nuclear speckled pattern with few nuclear dots in an 85-year-old male patient with FVIII:C of 2.2 IU/dL and an inhibitor of 4 BU/mL. (B) ANA (1:640) of nuclear homogeneous pattern in an 86-year-old male patient with FVIII:C of 2.4 IU/dL and an inhibitor of 9 BU/mL. (C) ANA (1:640) of nucleolar pattern in an 81-year-old female patient with FVIII:C of 2.5 IU/dL and an inhibitor of 104 BU/mL. (D) Cytoplasmic reticular, “mitochondrial” pattern (without ANA) in a 52-year-old female patient with FVIII:C of 4 IU/dL and an inhibitor of 71 BU/mL. (E) Cytoplasmic discrete dots/GW body-like pattern in a 73-year-old male patient with FVIII:C of <1 IU/dL and an inhibitor of 1.5 BU/mL. (F) Combined nuclear and cytoplasmic pattern (1:160) in a 73-year-old female patient with FVIII:C of 6 IU/dL and an inhibitor of 60 BU/mL. FVIII:C, factor VIII activity.
ANA titers in patients with AHA and controls. Histogramof HEp-2 immunofluorescent ANA titers of patients with AHA (n = 69) compared with matched control patients (n = 69). Only nuclear patterns were considered.
ANA titers in patients with AHA and controls. Histogramof HEp-2 immunofluorescent ANA titers of patients with AHA (n = 69) compared with matched control patients (n = 69). Only nuclear patterns were considered.
Cytoplasmic HEp-2 staining was detected in 30% and 19% of AHA and control patients, respectively (Table 2). ENA, anti-dsDNA, and anti-AF IgG were not different between the cohorts. Anti-AF IgA was more often detected in patients with AHA (12%) compared with control patients (0%) but mainly at low concentrations. Taken together, 78% of patients with AHA as compared with 46% of control patients had at least 1 autoimmune marker detected.
ANA detection was similarly frequent in patients with AHA with or without clinically overt concomitant autoimmune disease. Excluding patients with autoimmune disease, malignancy, pregnancy, or IST at baseline did not change the results (Table 4). Likewise, multivariable binary logistic regression correcting for age, gender, and known autoimmune disorders did not change the odds for ANA positivity in binary logistic regression analysis (Table 5).
Sensitivity analysis
| Analysis . | Patients with AHA (N = 69) . | Controls (N = 69) . | OR (95% CI) . | P . |
|---|---|---|---|---|
| All patients, n (%) | 44 (64) | 21 (30) | 4.02 (2.02-8.01) | .0002 |
| Autoimmune disorders excluded, n (%) | 37 (65) | 21 (31) | 4.14 (1.93-8.99) | .0002 |
| Malignant disorders excluded, n (%) | 37 (61) | 17 (29) | 3.81 (1.73-7.79) | .0005 |
| Pregnancy excluded, n (%) | 41 (64) | 21 (31) | 3.99 (1.94-8.20) | .0002 |
| IST at baseline excluded, n (%) | 39 (60) | 18 (28) | 3.92 (1.87-8.29) | .0004 |
| Analysis . | Patients with AHA (N = 69) . | Controls (N = 69) . | OR (95% CI) . | P . |
|---|---|---|---|---|
| All patients, n (%) | 44 (64) | 21 (30) | 4.02 (2.02-8.01) | .0002 |
| Autoimmune disorders excluded, n (%) | 37 (65) | 21 (31) | 4.14 (1.93-8.99) | .0002 |
| Malignant disorders excluded, n (%) | 37 (61) | 17 (29) | 3.81 (1.73-7.79) | .0005 |
| Pregnancy excluded, n (%) | 41 (64) | 21 (31) | 3.99 (1.94-8.20) | .0002 |
| IST at baseline excluded, n (%) | 39 (60) | 18 (28) | 3.92 (1.87-8.29) | .0004 |
Numbers, proportions, and OR for ANA positivity in patients with AHA and controls (entire cohort or excluding various underlying disorders or baseline medication).
Multivariable binary logistic regression model
| Covariate . | Univariate model OR (95% CI) . | P . | Multivariate model OR (95% CI)∗ . | P . |
|---|---|---|---|---|
| ANA positive | 4.02 (1.98-8.18) | <.001 | 4.25 (2.02-8.96) | <.001 |
| Autoimmune disorders | 14.3 (1.81-113) | .012 | 17.9 (2.11-152) | .008 |
| Age (per year) | 1.01 (0.99-1.03) | .433 | 1.01 (0.99-1.04) | .330 |
| Gender (female) | 1.20 (0.61-2.35) | .606 | 0.97 (0.45-2.08) | .942 |
| Covariate . | Univariate model OR (95% CI) . | P . | Multivariate model OR (95% CI)∗ . | P . |
|---|---|---|---|---|
| ANA positive | 4.02 (1.98-8.18) | <.001 | 4.25 (2.02-8.96) | <.001 |
| Autoimmune disorders | 14.3 (1.81-113) | .012 | 17.9 (2.11-152) | .008 |
| Age (per year) | 1.01 (0.99-1.03) | .433 | 1.01 (0.99-1.04) | .330 |
| Gender (female) | 1.20 (0.61-2.35) | .606 | 0.97 (0.45-2.08) | .942 |
The dependent variable is having a diagnosis of AHA.
Containing all 4 covariates.
No difference was noted between patients with AHA positive or negative for autoimmune markers with respect to FVIII inhibitor titers, anti-FVIII IgG concentration, anti-FVIII antibody idiotype, subclass distribution, or domain epitopes (data not shown).
Monoclonal anti-FVIII antibodies and HEp-2 immunofluorescence
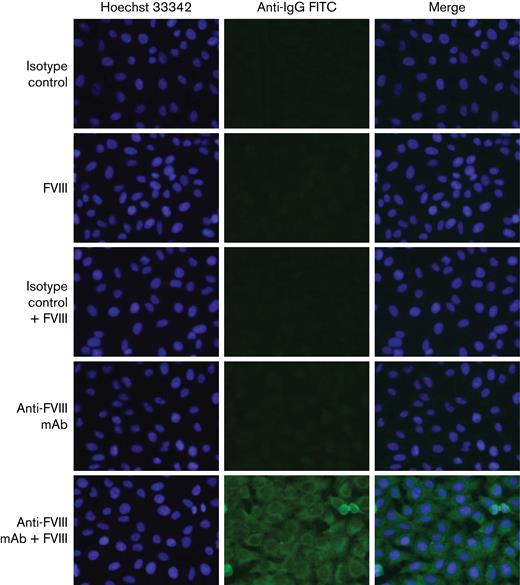
To exclude that ANA staining was merely a result of cross-reactivity of anti-FVIII autoantibodies with HEp-2 cells, we used a mix of 7 well-characterized monoclonal anti-FVIII antibodies or isotype controls in the presence or absence of human recombinant FVIII (Figure 3). No staining was observed for anti-FVIII mAb, isotype controls, or FVIII alone. Only immune complexes consisting of the combination of anti-FVIII mAb and FVIII in very high concentrations, not expected to occur in vivo, stained the cytoplasm of HEp-2 cells with a pattern similar to that observed in 6 out of 69 patients with AHA (the pattern seen in Figure 1D).
HEp-2 immunofluorescent staining of control antibodies. Anti-FVIII monoclonal mAb, isotype controls or human recombinant FVIII alone do not bind to HEp-2 cells, whereas immune complexes of anti-FVIII mAb plus FVIII produce a cytoplasmic reticular staining pattern like that observed in 6 patients with AHA (bottom row, compare Figure 1D).
HEp-2 immunofluorescent staining of control antibodies. Anti-FVIII monoclonal mAb, isotype controls or human recombinant FVIII alone do not bind to HEp-2 cells, whereas immune complexes of anti-FVIII mAb plus FVIII produce a cytoplasmic reticular staining pattern like that observed in 6 patients with AHA (bottom row, compare Figure 1D).
Discussion
The current case-control study found that 78% of patients with AHA had at least 1 additional marker of autoimmunity and that ANA positivity was twice as frequent in AHA patients compared with controls. These data support our initial hypothesis that autoantibody formation in AHA is not exclusive against FVIII. Of note, the pattern of ANA was highly diverse in patients with AHA, suggesting that various autoimmune targets were bound by autoantibodies rather than a single structure. ENA and dsDNA screening was mostly negative, indicating that none of the autoimmune targets typically involved in patients with connective tissue disorders or lupus erythematosus play a role.
Little is known about the root cause of autoantibody formation in AHA. Two previous studies reported association with certain HLA-DR haplotypes, alone or in combination with F8 gene polymorphisms, the latter in pregnancy- or transfusion-associated AHA.30,31 Others suggested that anti-idiotypic antibodies, present along with anti-FVIII autoantibodies in healthy human plasma, keep autoimmunity against FVIII under control.32-34 Our current study does not exclude the possible pathogenic mechanisms of AHA implied by these observations. However, the notion that autoimmune targets other than FVIII are frequent in AHA adds a new perspective to its pathogenesis (ie, a more general failure of peripheral self-tolerance control mechanisms). This failure might facilitate the proliferation and differentiation of FVIII-specific B cells to produce high-affinity, class-switched, neutralizing autoantibodies.
The failure of peripheral control mechanisms of self-tolerance that we suggest here as an explanation for our observations has been suggested or indirectly demonstrated for a number of humoral autoimmune disorders, including rheumatoid arthritis, granulomatosis with polyangiitis, pemphigus vulgaris, and systemic lupus erythematosus.18,35 In the B6 mouse model, ANA occur with aging or in association with chronic inflammatory states such as graft-versus-host disease, and, in fact, the majority of ANA clones arising was derived from germline precursors that escaped peripheral tolerance mechanisms in the context of aging or chronic inflammation.36
The loss of peripheral control mechanisms of self-tolerance in patients with AHA could be age-related in part. It is well established that aging is associated with changes in the immune system, characterized by immunosenescence and inflammaging. Immunosenescence is a dysfunction of the immune system caused by disruption of the immune organ structural architecture. In contrast, inflammaging represents a chronic, sterile, systemic inflammatory condition promoted by the accumulation of cell debris and self-antigen derived from cellular stress or infection.37 It is age-related primarily because mechanisms of clearing debris and misfolded protein become deficient with age.38 The increased prevalence of autoantibodies in elderly people, including ANA, rheumatoid factor, antineutrophil cytoplasmic antibodies, and others, was previously attributed to the phenomena of immunosenescence and inflammaging.39,40
It was also proposed that autoimmune or autoinflammatory diseases such as multiple sclerosis might be associated with premature immunosenescence.41 Novel therapeutic approaches based on rejuvenating the immune system have been proposed to reverse immunosenescence and inflammaging.41 Such approaches might also benefit patients with AHA in the future.
Our study has strengths and limitations. Reflecting on the robustness of our findings, we consider the selection of the control group as a critical element. Autoimmune disorders and the frequency of autoimmune markers are age related. AHA is a disorder of the elderly, and, therefore, it was mandatory to recruit an age-matched elderly control group rather than healthy blood donors. We decided to recruit consecutive, random medical patients admitted to a large university hospital emergency and admission unit for mainly 3 reasons: (1) There is no clear definition of “healthy” in the elderly. (2) People without known disease are difficult to find in this advanced age. (3) A random sample of medically ill people, rather than a seemingly healthy cohort, was better comparable to patients with AHA, who have multiple concomitant disorders such as infection, heart failure, diabetes, renal disorders, and others.
The selection of autoimmunity markers to test was also an important consideration. In the past few years, proteome-wide array technologies became available to screen for hundreds of targets for autoimmune disorders.42 However, the risk of chance findings increases with the number of targets, and we did not have access to a second, independent cohort of patients and controls to confirm the results of multitarget screening. Therefore, we preferred a classical autoimmunity screening strategy with a limited number of assays rather than proteome screening, being able to control the statistical power of our study and the chance of error.
The case-control design was chosen for our study because it provides the highest power for searching pathogenic factors in rare conditions. However, sources of bias exist in this type of study, in particular related to the quality of the controls.43 Our findings should ideally be verified in second, independent sets of matched patients and controls.
Finally, it should be acknowledged that our findings are in no way sufficient to explain why people develop anti-FVIII autoantibodies and AHA. Having evidence of loss of self-tolerance could be a risk factor for AHA, just as advanced age, pregnancy, or known autoimmune disorders are associated with AHA and can be considered risk factors in a classical sense. None of these risk factors, which are all quite common in the general population, would be sufficient to explain why so few people develop AHA. Clearly, there must be additional factors that finally result in the formation of clinically relevant anti-FVIII autoantibodies.
In conclusion, our study provides evidence that AHA is associated with increased frequency of autoantibodies against targets other than FVIII. Based on these results, we suggest that a more generalized failure of peripheral control mechanisms of self-tolerance might be involved in AHA pathogenesis. This failure might be related to the previously described mechanisms of immunosenescence and inflammaging. Better understanding of these mechanisms in the context of AHA will help to develop new strategies for immunomodulating therapies.
Acknowledgments
The authors acknowledge the contribution of all GTH study sites, local study coordinators, and Susanne Bartels, central study coordinator at Hannover Medical School.
This study was supported by the Thrombosis and Haemostasis Society of the German-speaking countries German, Austrian, and Swiss Society on Thrombosis and Haemostasis (GTH).
Authorship
Contribution: A.T. and B.M.R. designed the study and wrote the manuscript; O.O., S.W., A.K., and T.W. planned and performed laboratory analysis, interpreted data, and critically revised the manuscript; A.T., H.E., R.K., K.H., P.K., R.G., and P.N. enrolled patients; and all authors interpreted data and contributed critical content to the manuscript and approved its final version.
Conflict-of-interest disclosure: O.O. reports grants for research from Biotest and Octapharma outside the submitted work. S.W. reports grants for research from Biotest and Octapharma outside the submitted work. A.K. reports grants for research from Biotest and Octapharma outside the submitted work. T.W. reports fees for lectures from AbbVie, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, GSK, Lilly, Medac, Novartis, Pfizer, Roche, Sanofi, Takeda, UCB, and Viatris. H.E. reports grants for studies and research from Bayer, CSL Behring, and Pfizer and personal fees for lectures or consultancy from Bayer, BioMarin, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, and SOBI. R.K. reports grants for studies and research from Bayer, Leo, and Takeda and personal fees for lectures or consultancy from Bayer, BioMarin, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Sanofi, SOBI, and Takeda outside the submitted work. K.H. reports grants for studies and research from Bayer, CSL Behring, Pfizer, Sobi, and Roche/Chugai outside the submitted work and personal fees for lectures and consultancy from Bayer, Biotest, Chugai, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche, Sobi, and Takeda. C.H. reports travel grants and personal fees for lectures or consultancy from Bayer, BMS, CSL Behring, Daiichi Sankyo, Novo Nordisk, Octapharma, Pfizer, Roche/Chugai, Sanofi, SOBI, and Takeda outside the submitted work. C.P. reports grants for studies and research from Chugai/Roche, Leo Pharma, Zacros, and Takeda outside the submitted work and personal fees for lectures or consultancy from Bayer, BMS, Chugai/Roche, CSL Behring, Novo Nordisk, Pfizer, Sanofi, SOBI, and Takeda outside the submitted work. P.K. reports research grants, travel support, and consultancy fees from Roche, Novo Nordisk, Takeda, Octapharma, and Sanofi outside the submitted work. R.G. reports grants for research from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, Gilead, Daiichi Sankyo, and AbbVie outside the submitted work; support for travel, accommodation, and expenses from Roche, Amgen, Janssen, AstraZeneca, Novartis, MSD, Celgene, Gilead, BMS, AbbVie, and Daiichi Sankyo outside the submitted work; and honoraria or consultancy fees from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, Daiichi Sankyo, Sanofi, and Janssen outside the submitted work. P.N. reports consultancy fees from Roche, Amgen, Novo Nordisk, Bayer, Sobi, and Takeda outside the submitted work. B.M.R. reports fees for consultancy from Takeda, Biomarin, Novo Nordisk, Boehringer Ingelheim, Fresenius Kabi, and Checkimmune outside the submitted work. A.T. reports grants for studies and research from Bayer, Biotest, Chugai/Roche, Novo Nordisk, Octapharma, Pfizer, and Takeda outside the submitted work and personal fees for lectures or consultancy from Bayer, Biotest, Chugai/Roche, CSL Behring, Novo Nordisk, Octapharma, Pfizer, SOBI, and Takeda outside the submitted work.
Correspondence: Andreas Tiede, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Carl Neuberg Str. 1, 30625 Hannover, Germany; e-mail: tiede.andreas@mh-hannover.de.
References
Author notes
Requests for data sharing are to be addressed to the corresponding author, Andreas Tiede (tiede.andreas@mh-hannover.de).
B.M.R. and A.T. are joint senior authors.