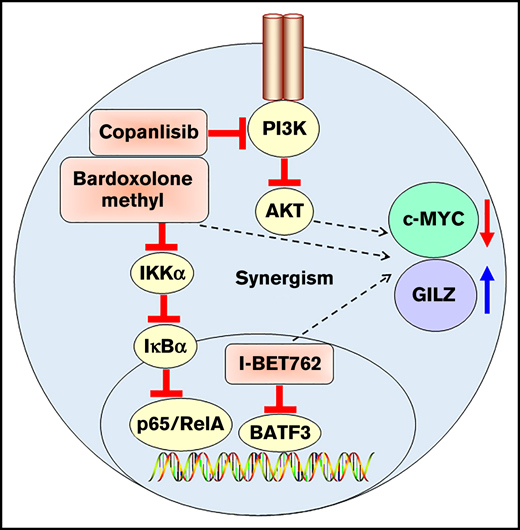
Key Points
Triple combination of I-BET762, copanlisib, and bardoxolone methyl exhibits synergistic activity against ATL in vitro and in vivo.
Triple combination synergizes to inhibit c-MYC ex vivo in PBMCs containing leukemic cells from ATL patients.
Abstract
Adult T-cell leukemia/lymphoma (ATL) is an aggressive T-cell lymphoproliferative malignancy caused by human T-cell leukemia virus type 1 (HTLV-1). ATL is an orphan disease with no curative drug treatment regimens urgently needing new combination therapy. HTLV-1-infected cells rely on viral proteins, Tax and HBZ (HTLV-1-b-ZIP factor), to activate the transcription of various host genes that are critical for promoting leukemic transformation. Inhibition of bromodomain and extraterminal motif (BET) protein was previously shown to collapse the transcriptional network directed by BATF3 super-enhancer and thereby induced ATL cell apoptosis. In the current work, by using xenograft, ex vivo, and in vitro models, we demonstrated that I-BET762 (BETi) synergized with copanlisib (PI3Ki) and bardoxolone methyl (NF-κBi) to dramatically decrease the growth of ATL cells. Mechanistically, the triple combination exhibited synergistic activity by down-regulating the expression of c-MYC while upregulating the level of the glucocorticoid-induced leucine zipper (GILZ). The triple combination also enhanced apoptosis induction by elevating the expression of active caspase-3 and cleaved PARP. Importantly, the triple combination prolonged the survival of ATL-bearing xenograft mice and inhibited the proliferation of ATL cells from peripheral blood mononuclear cells (PBMCs) of both acute and smoldering/chronic ATL patients. Therefore, our data provide the rationale for a clinical trial exploring the multiagent combination of BET, PI3K/AKT, and NF-κB inhibitors for ATL patients and expands the potential treatments for this recalcitrant malignancy.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is an aggressive T-cell lymphoproliferative malignancy that develops in individuals infected with human T-cell leukemia virus type 1 (HTLV-1). ATL cells possess a regulatory T-cell phenotype with the expression of CD3, CD4, and CD25 (IL-2Rα) on their cell surfaces.1 Based on the Shimoyama criteria,2 the acute and lymphoma subtypes are considered aggressive forms with median overall survival (OS) of only 6 to 10 months, whereas chronic and smoldering subtypes have a median OS of at least 2 years.3 The prognosis of the acute type remains poor despite chemotherapy and has not improved in the past 3 decades.4 There are modest recent advances such as treatment with a monoclonal antibody to the chemokine receptor, CCR4, that hold some promise.5
Our group has previously reported that BATF3 is one of the master regulators driving ATL cell survival and proliferation.6 HTLV-1-b-ZIP (HBZ), a transcription factor of HTLV-1, binds to an ATL-specific BATF3 super-enhancer and regulates the expression of BATF3 and its downstream targets, including c-MYC. Additionally, inhibition of bromodomain and extraterminal domain (BET) proteins with JQ1 showed efficacy in ATL models by collapsing the transcriptional network directed by BATF3, suggesting the potential for clinical applications. The BET family is composed of 4 members, BRD2, BRD3, BRD4, and BRDT. Of the 4 BET proteins, BRD4 is a key structural component of the extensive transcriptional complexes that form at genomic regions known as superenhancers, which are 15 times longer than typical enhancers.7,8 Superenhancers can act as oncogenic drivers; thus, disrupting these enhancers is recognized as a new mode of therapeutic intervention. JQ1, the first BET inhibitor, was found to bind competitively to acetyl lysine-recognizing bromodomains and shown to downregulate MYC transcription.9,10 Due to its short half-life of 1 hour, this limits the ability of JQ1 to translate into a clinical benefit.11 Similarly, I-BET762 was found to mimic acetylated histones, which disrupts chromatin complexes essential for the expression of inflammatory genes.12 I-BET762 is orally bioavailable with better pharmacodynamic/pharmacokinetic in vivo and has been used in patients with NUT carcinoma and other solid tumors.13
Recently, we demonstrated the critical role of the AKT/mTOR pathway in ATL cell survival and proliferation.14 Molecular lesions in the phosphatidylinositol-3-kinase (PI3K)/AKT pathway are very common in cancer. Class I PI3K enzymes are composed of 4 isoforms, PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ.15 Copanlisib is a pan–PI3K inhibitor with a dominant activity against PI3Kα and PI3Kδ isoforms.16 Despite the observed clinical benefit, complete remission in patients treated with copanlisib is relatively rare. Thus, copanlisib was used in combination for the treatment of relapsed or refractory hematologic malignancies.17
The HTLV-1 genome expresses 2 oncogenic proteins, the transactivator protein, Tax, and HBZ. Tax protein is critical for T-cell proliferation mainly via activation of the nuclear factor-κB (NF-κB) pathway.18 In the canonical NF-κB signaling pathway, a heterodimer of p50 and RelA (p65) is activated via IκB kinases, which leads to the translocation of NF-κB into the nucleus and the subsequent activation of genes involved in immune and inflammatory responses.19 A recent integrated molecular study also showed the alteration of the NF-κB pathway in the 426 ATL cases. Many of the somatic alterations in the malignant cells converge on TCR-NF-κB signaling genes, suggesting a critical role for the NF-κB pathway in ATL.20 Bardoxolone methyl, an NF-κB inhibitor, has been shown to inhibit constitutive and cytokine-induced activation of NF-κB, and the inhibitor was well tolerated in patients with advanced solid tumors and lymphomas.21 Despite the intensive studies of these 3 pathways in multiple cancer therapies, triple targeting has not yet been explored, particularly in the ATL model.
To understand the interactions between BET and PI3K/AKT and NF-κB pathways in ATL, we exploited the use of I-BET762, copanlisib, and bardoxolone methyl inhibitors. Triple targeting with the combination of these 3 inhibitors exhibited synergistic activity and significantly downregulated the level of c-MYC. RNA sequencing analysis also revealed a marked increase of glucocorticoid-induced leucine zipper (GILZ) expression, which was previously shown to inhibit TCR-induced IL-2/IL-2R expression and NF-κB activity. The drug synergy was consistently observed in ATL cell lines, mouse xenograft-bearing ATL tumors, and peripheral blood mononuclear cell (PBMC)-containing leukemic cells from both acute and chronic ATL patients. As shown here, the therapeutic potential of combined targeting of BET, PI3K/AKT, and NF-κB pathways in ATL clearly lends itself to further clinical development as multiagent therapies for ATL patients.
Material and methods
Reagents and cell lines
I-BET762 (GSK525762A or Molibresib, BETi), copanlisib (pan-PI3Ki), and bardoxolone methyl (NF-kBi) were purchased from Medchem Express (Monmouth Junction, NJ). CN470, a multibromodomain inhibitor that inhibits the bromodomains of CBP, p300, and BET proteins, was provided by ConverGene and NCATS. All cell lines were maintained in RPMI 1640 medium plus 10% fetal bovine serum (FBS).
Cell proliferation assay
Aliquots of 10 000 ATL cells were seeded in a 96-well plate and cultured for 72 hours in RPMI1640 plus 10% FBS with serial dilutions of drug combinations. On day 3, the cells were pulsed with 1 μCi (0.037 MBq) of 3H-thymidine and counted with a Micro-Beta2 counter (PerkinElmer, Shelton, CT).
Mouse model of ATL43Tβ (-) and therapeutic study
The xenograft tumor model was established by subcutaneous injection of 1 × 107 ATL43Tβ (-) cells into the flank of NSG mice (The Jackson Laboratory, Bar Harbor, ME). ATL43Tβ (-) was derived from the peripheral blood (PB) of adult T acute lymphoblastic leukemia patients.22 The Tax status is negative, and the mice-bearing tumor develops only a local disease. Compound administration was started when the tumor volume reached 100 mm3. The tumor volume was calculated by using the equation 1/2 (long dimension) × (short dimension).2 sIL-2Rα in the serum was measured with a human CD25/IL-2Rα Quantikine ELISA kit (R&D Systems, Minneapolis, MN).
Western blot analysis
Proteins from whole-cell lysates were detected by immunoblotting. Antibodies were from Cell Signaling Technology Inc. (Danvers, MA); p-AKTSer473 (#4060), p-4EBP1 (Thr37/46) (#2855), GAPDH (#2118), β-actin (#4970), p-NF-κB-p65 (#3033), p27Kip1 (#3686), and p-IκBα (#2859). p-IKKα (S176/S180) was from R&D Systems (Minneapolis, MN). Rabbit-polyclonal-HBZ serum was previously reported,23 and Tax antibody was kindly gifted by Cynthia Masison (NCI/NIH). Anti-c-MYC [Y69] was from Abcam (Cambridge, MA). BATF3 (3H1) was from Novus Biologicals (Centennial, CO).
Ex vivo culture and analysis of PBMCs from ATL patients
PB samples were obtained from patient volunteers with ATLs under the care of the clinical trials team, Lymphoid Malignancies Branch, NCI. Freshly isolated or frozen PBMCs from ATL patients (1 × 105 cells/well) or CD4+ T cells from healthy donors (50 000 cells/well) were cultured ex vivo for 6 days in complete medium. CD4+ T cells were stimulated with 0.5 ug/mL anti-CD3 (OKT-3) and anti-CD28 (CD28.2; BioXcell, Lebanon, NH). Frozen PBMCs were rested overnight at 37°C prior to the culture. rIL-2 (100 U/mL) was added into the culture of PBMCs from acute ATL patients. On day 6, 1 μM of I-BET762, copanlisib, bardoxolone methyl, or a combination were added for 2 hours and subjected to western blot analysis.
Cell line source, flow cytometry, real-time polymerase chain reaction (PCR), RNA sequencing, immunohistochemistry (IHC) analysis, and MTT assay are described in the supplemental Information.
Statistical analysis
For a comparison between groups, a 2-tailed unpaired Student t test or 1-way ANOVA analysis (>2 groups) was used. Kaplan-Meier of mice survival was compared by a log-rank (Mantle-Cox) test (GraphPad Prism software, version 8). P < .05 was considered statistically significant.
Study approval
This study was performed under the conditions of the World Medical Association’s Declaration of Helsinki. All patients signed written informed consent for participation in clinical studies. Clinical studies were approved by the Intramural Review Board of the National Cancer Institute (NCI). Animal studies were approved by the NCI Animal Care and Use Committee.
Results
Single BET inhibitors or copanlisib and bardoxolone methyl suppress ATL cell proliferation
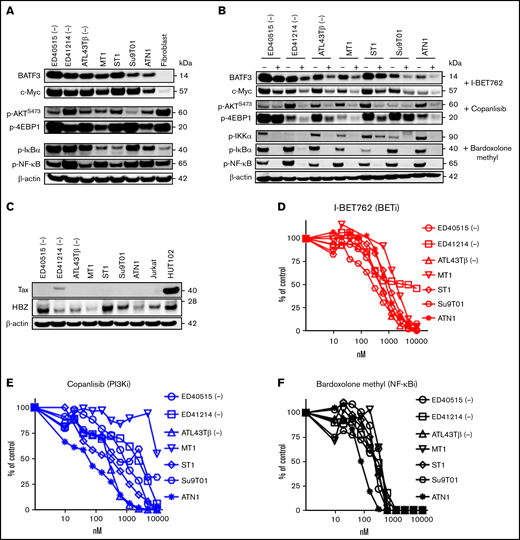
In our previous study,6 we demonstrated that the knockdown of BATF3 was highly toxic for all ATL lines, suggesting an essential role for BATF3 in ATL viability. BATF3 and c-MYC proteins were highly expressed in 7 cytokine-independent ATL cell lines, while both proteins were not expressed in fibroblast cells (Figure 1A). Additionally, p-AKTS473 and p-4EBP1 were highly expressed in most ATL cell lines and fibroblast cells. Despite the loss of Tax protein, NF-κB activation remains persistently activated in ATL associated with somatic mutations.18 We found the activation of the NF-κB pathway with the detection of p-IκBα and p-NFκB-65 expression (Figure 1A). I-BET762 significantly inhibited BATF3 and c-MYC expression in ATL cell lines (Figure 1B). We also investigated bromodomain inhibitor, CN470,24 which was recently found to inhibit the bromodomains of CBP and p300 proteins, in addition to the bromodomains of BET proteins (Imayoshi et al, submitted). Consistently, CN470 reduced c-MYC and BATF3 levels correlated with decreased cell proliferation (supplemental Figure 1A,B).
Single BET inhibitors or copanlisib or bardoxolone methyl suppress ATL cell proliferation. (A) Immunoblot analysis of BATF3, c-MYC, p-AKTS473, p-4EBP1, p-IκB-α, and p-NFκB-65 in 7 ATL cell lines and fibroblast cells. β-actin was used as a loading control. (B) Seven ATL cell lines were incubated with 1 μM of I-BET762 or copanlisib or bardoxolone methyl for 2 hours and 4 hours for p-IKKα. (C) HTLV-1 protein expression, Tax, and HBZ, in 7 ATL cell lines, Jurkat, and HuT102 cells. (D-F) Dose-response curves of I-BET762 (red), copanlisib (blue), or bardoxolone methyl (black) on ATL cell lines. Increasing concentrations (0 to 10 000 nM) of inhibitors were incubated with 10 000 ATL cells for 72 hours. The relative number of proliferating cells was analyzed by a thymidine incorporation assay. Results are representative of triplicate assays.
Single BET inhibitors or copanlisib or bardoxolone methyl suppress ATL cell proliferation. (A) Immunoblot analysis of BATF3, c-MYC, p-AKTS473, p-4EBP1, p-IκB-α, and p-NFκB-65 in 7 ATL cell lines and fibroblast cells. β-actin was used as a loading control. (B) Seven ATL cell lines were incubated with 1 μM of I-BET762 or copanlisib or bardoxolone methyl for 2 hours and 4 hours for p-IKKα. (C) HTLV-1 protein expression, Tax, and HBZ, in 7 ATL cell lines, Jurkat, and HuT102 cells. (D-F) Dose-response curves of I-BET762 (red), copanlisib (blue), or bardoxolone methyl (black) on ATL cell lines. Increasing concentrations (0 to 10 000 nM) of inhibitors were incubated with 10 000 ATL cells for 72 hours. The relative number of proliferating cells was analyzed by a thymidine incorporation assay. Results are representative of triplicate assays.
Furthermore, copanlisib reduced the p-AKT and p-4EBP1, which are the downstream proteins of the PI3K pathway. As expected, bardoxolone methyl efficiently suppressed the expression of p-IKKα, p-IκBα, and p-NF-κB-p65, suggesting the persistence of the NF-κB pathway in ATL (Figure 1B). We also confirmed the expression of Tax and HBZ proteins and used Jurkat and HuT102 cell lines as negative and positive controls, respectively. Only ED41214 (-) cell line expressed Tax, whereas the other 6 cell lines lost Tax expression. In contrast, HBZ expression was observed in 7 ATL cell lines (Figure 1C; supplemental Figure 1C). In examining the dose-response curves as a single agent, I-BET762, CN470, copanlisib, and bardoxolone methyl demonstrated their potency in suppressing ATL cell proliferation (Figure 1D-F). The IC50 of these inhibitors was determined and summarized in supplemental Figure 1D.
I-BET762 shows a synergistic effect with copanlisib by suppressing ATL proliferation and downregulating the expression of c-MYC
Drug synergy is vital for cancer therapy and multiple drug combinations. An interaction between 2 or more drugs could elicit different activities such as additivity, synergism, or antagonism. A synergistic effect is more desirable than additivity as the combined effect is greater than the sum of expected additive effects.25 JQ1 has been shown in preclinical models to act synergistically with several agents, including HDACi26 and PARPi.27 Recently, copanlisib was demonstrated to elicit a synergistic activity with BCL-2 inhibitors in T-cell lymphoma models.28 We thus hypothesized that I-BET762 would exhibit synergistic activity with copanlisib in our ATL model.
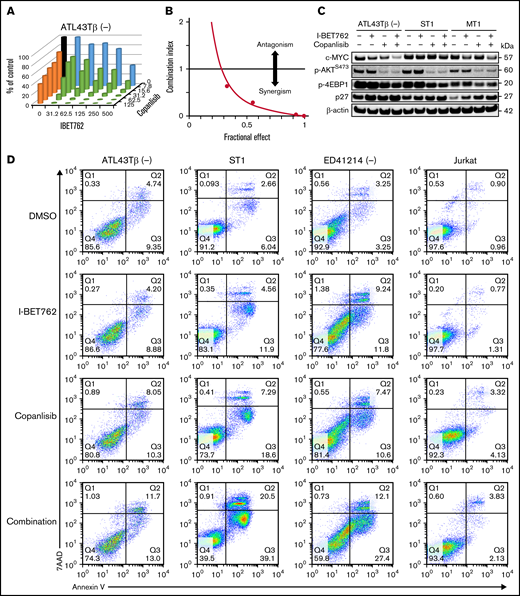
With a thymidine incorporation assay, I-BET762 used in combination with copanlisib markedly reduced the viability of the ATL43Tβ (-) cell line (Figure 2A) and the other 6 ATL cell lines (supplemental Figure 2A) after 72 hours of treatment as shown in the combination matrix graphs. To measure the synergistic effect between I-BET762 and copanlisib, the Chou-Talalay method was used.29 The synergistic effect of this drug combination with a combination index (CI) <1 was observed in 7 ATL cell lines using 5 different drug concentrations (Figure 2B; supplemental Figure 2B). We next evaluated the direct targets of the drug combination and found that I-BET762 reduced the level of c-MYC while copanlisib inhibited p-AKT and p-4EBP1. Importantly, the c-MYC level was further decreased in the combination-treated group, as demonstrated in 6 ATL cell lines (Figure 2C; supplemental Figure 2C).
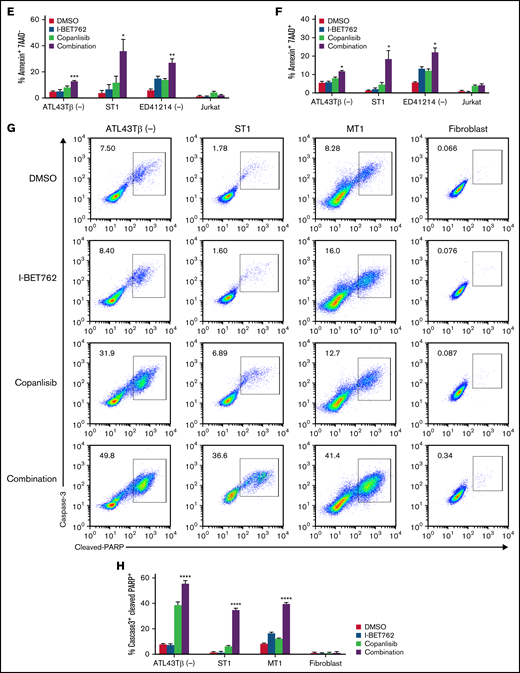
I-BET762 shows a synergistic effect with copanlisib by suppressing ATL proliferation and downregulating the expression of c-MYC. (A) The combined effects of I-BET762 with copanlisib inhibitors on ATL43Tβ (-) cell line incubated for 72 hours in the presence of 5 increasing concentrations of I-BET762 (31.2 to 500 nM, blue), copanlisib (7.8 to 125 nM, orange), or their combinations (green). A thymidine incorporation assay was performed, and the percentages of control were calculated from the counts per minute (CPM) of samples with drugs divided by the CPM of samples with no inhibitor × 100 (black bar). A representative figure of 3 independent experiments is shown. (B) CI plot of I-BET762 and copanlisib combination in ATL43Tβ (-) cell line. CI dots represent 5 combination data points, CI <1 was considered synergism. (C) Immunoblot analysis of c-MYC, p-AKTS473, p-4EBP1 after 1 hour of incubation with either 1 μM of I-BET762 or copanlisib or in combination. For p27 expression, cells were incubated for 24 hours prior to western blot analysis. (D) Induction of cell apoptosis by I-BET762 and copanlisib combination. ATL43Tβ (-), ST1, ED41214 (-), and Jurkat cells were treated with either DMSO, 1 μM of I-BET762, or copanlisib or in combination for 48 hours, and the cell apoptosis was measured with annexin V staining and analyzed by flow cytometry. (E) The bar graphs represent the percentages of early apoptotic cells (annexin V+7AAD−, lower right quadrant) and (F) late apoptosis (annexin V+7AAD+, upper right quadrant), n = 3. (G,H) Flow cytometric analysis of active caspase3 and cleaved-PARP in ATL43Tβ (-), ST1, and MT1 cell lines. The cells were treated with inhibitors for 48 hours and stained for the double-positive cells of the percentage of caspase 3+cleaved-PARP+ represented in the bar graphs (n = 3). Human fibroblast cells were used as a control. Three biological experiments were performed, and values are presented as mean ± SEM. One-way ANOVA was used to determine statistical differences. *P < .05, **P < .01, ***P < .001, ****P < .0001.
I-BET762 shows a synergistic effect with copanlisib by suppressing ATL proliferation and downregulating the expression of c-MYC. (A) The combined effects of I-BET762 with copanlisib inhibitors on ATL43Tβ (-) cell line incubated for 72 hours in the presence of 5 increasing concentrations of I-BET762 (31.2 to 500 nM, blue), copanlisib (7.8 to 125 nM, orange), or their combinations (green). A thymidine incorporation assay was performed, and the percentages of control were calculated from the counts per minute (CPM) of samples with drugs divided by the CPM of samples with no inhibitor × 100 (black bar). A representative figure of 3 independent experiments is shown. (B) CI plot of I-BET762 and copanlisib combination in ATL43Tβ (-) cell line. CI dots represent 5 combination data points, CI <1 was considered synergism. (C) Immunoblot analysis of c-MYC, p-AKTS473, p-4EBP1 after 1 hour of incubation with either 1 μM of I-BET762 or copanlisib or in combination. For p27 expression, cells were incubated for 24 hours prior to western blot analysis. (D) Induction of cell apoptosis by I-BET762 and copanlisib combination. ATL43Tβ (-), ST1, ED41214 (-), and Jurkat cells were treated with either DMSO, 1 μM of I-BET762, or copanlisib or in combination for 48 hours, and the cell apoptosis was measured with annexin V staining and analyzed by flow cytometry. (E) The bar graphs represent the percentages of early apoptotic cells (annexin V+7AAD−, lower right quadrant) and (F) late apoptosis (annexin V+7AAD+, upper right quadrant), n = 3. (G,H) Flow cytometric analysis of active caspase3 and cleaved-PARP in ATL43Tβ (-), ST1, and MT1 cell lines. The cells were treated with inhibitors for 48 hours and stained for the double-positive cells of the percentage of caspase 3+cleaved-PARP+ represented in the bar graphs (n = 3). Human fibroblast cells were used as a control. Three biological experiments were performed, and values are presented as mean ± SEM. One-way ANOVA was used to determine statistical differences. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Tumor suppressors such as p16 (INK4B) and p21 (WAF1) were frequently found to be inactivated in ATL by somatic mutations or epigenetic mechanisms. Loss of p16 and p21 expression in ATL was reported in the previous studies.30 In our ATL cell lines, p16 expression was not detected, whereas p21 was partially expressed in some cell lines. We, therefore, evaluated the expression of another tumor suppressor, p27 (KIP1), which was also reported to be altered in ATL and highly correlated with patient prognosis.31 In contrast to the c-MYC level, we found an increased level of p27 in the I-BET762 and copanlisib combination-treated group (Figure 2C).
We next investigated if the drug combination induced cell apoptosis with an annexin V marker. Single I-BET762 or copanlisib slightly induced cell apoptosis after 48 hours of treatment. In contrast, the combination of these 2 agents markedly induced higher apoptosis, as shown with increased percentages of annexin V+7AAD− and annexin V+7AAD+ (Figure 2D-F). Caspase-3 mediates apoptotic cell death through the cleavage of several proteins, including poly (ADP-ribose) polymerase (PARP).32 We found a dramatic increase of caspase-3/cleaved PARP double-positive cells with the combination-treated group (Figure 2G,H).
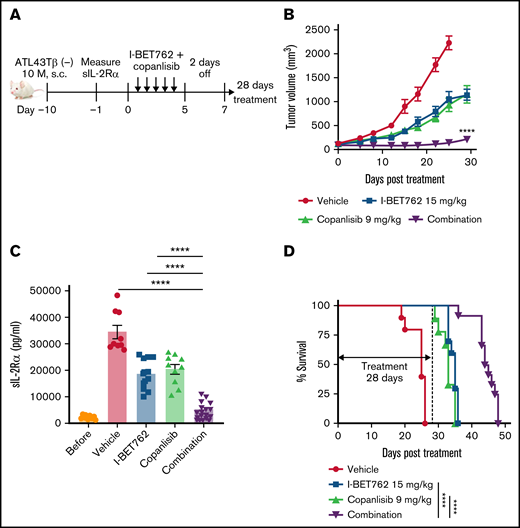
I-BET762 acts synergistically with copanlisib in vivo to inhibit ATL growth in xenograft mice-bearing ATL tumors
We next assessed the synergistic activity of I-BET762 and copanlisib in vivo using the ATL43Tβ (-) cell line. We initially defined the maximum tolerated dose (MTD) of I-BET762, which was 25 mg/kg (supplemental Figure 3A,B). In the combination study, the dose of both drugs had to be reduced by approximately half of the MTD to avoid toxicity and death of animals33 ; thus, we administered 15 mg/kg per day of I-BET762 and 9 mg/kg per day of copanlisib, 5 days on and 2 days off for 4 weeks to the xenograft mice-bearing ATL (Figure 3A). A single administration of I-BET762 or copanlisib reduced the tumor growth by approximately 50% compared with vehicle control mice. The combination therapy with these 2 inhibitors acted synergistically in vivo and dramatically reduced tumor volumes compared with single agents alone (Figure 3B). We also quantified the level of soluble interleukin-2 receptor-α (sIL-2Rα), a surrogate ATL tumor marker, in the serum of treated mice after 4 weeks. Consistent with the reduced tumor volume, the level of sIL-2Rα was greatly reduced in the serum of the combined-treated group compared with single agent-treated mice (Figure 3C). Furthermore, the single agents delayed the survival to some extent, whereas the combined treatment significantly prolonged the survival of treated mice (Figure 3D).
I-BET762 acts synergistically with copanlisib in vivo to inhibit ATL growth in xenograft mice-bearing ATL tumors. (A) Therapeutic effects of I-BET762 and copanlisib combination in xenograft mice-bearing ATL43Tβ (-) cell line. Ten million cells were subcutaneously implanted into NSG mice. The therapy was started when average tumor volumes reached approximately 100 mm3, which was 10 days after tumor inoculation. I-BET762 was given orally at a dose of 15 mg/kg, and copanlisib was administered via intraperitoneal injection at a dose of 9 mg/kg 5 days on and 2 days off for 28 days. The vehicle group received 15% solutol dissolved in water. (B) Average tumor volumes during the therapeutic time course were measured twice weekly until the tumor volume reached 2000 mm3. (C) Serum levels of human sIL-2Rα were measured on day 28 after treatment with a human CD25/IL-2Rα ELISA assay. (D) Kaplan-Meier survival curves illustrate the survival of mice that received single therapy or in combination. Data were pooled from 2 independent experiments and expressed as a mean ± SEM (n = 9 to 12). One-way ANOVA and log-rank (Mantel-Cox) test were performed to determine statistical differences. ****P < .0001.
I-BET762 acts synergistically with copanlisib in vivo to inhibit ATL growth in xenograft mice-bearing ATL tumors. (A) Therapeutic effects of I-BET762 and copanlisib combination in xenograft mice-bearing ATL43Tβ (-) cell line. Ten million cells were subcutaneously implanted into NSG mice. The therapy was started when average tumor volumes reached approximately 100 mm3, which was 10 days after tumor inoculation. I-BET762 was given orally at a dose of 15 mg/kg, and copanlisib was administered via intraperitoneal injection at a dose of 9 mg/kg 5 days on and 2 days off for 28 days. The vehicle group received 15% solutol dissolved in water. (B) Average tumor volumes during the therapeutic time course were measured twice weekly until the tumor volume reached 2000 mm3. (C) Serum levels of human sIL-2Rα were measured on day 28 after treatment with a human CD25/IL-2Rα ELISA assay. (D) Kaplan-Meier survival curves illustrate the survival of mice that received single therapy or in combination. Data were pooled from 2 independent experiments and expressed as a mean ± SEM (n = 9 to 12). One-way ANOVA and log-rank (Mantel-Cox) test were performed to determine statistical differences. ****P < .0001.
Triple combination of I-BET762 plus copanlisib and bardoxolone methyl exhibit synergistic activity and downregulate the level of c-MYC
Preclinical studies in leukemia and myeloma models indicate that BET inhibition reduced both c-MYC expression and its transcriptional downstream effects, resulting in antitumor activity.10 Direct targeting of c-MYC would be an ideal strategy for cancer therapy. Nevertheless, a therapeutic approach to target c-MYC has remained challenging due to its undruggable protein structure.34 Thus, we used bardoxolone methyl, an NF-κB inhibitor, in our triple combination study to determine if it could further enhance the c-MYC inhibition.
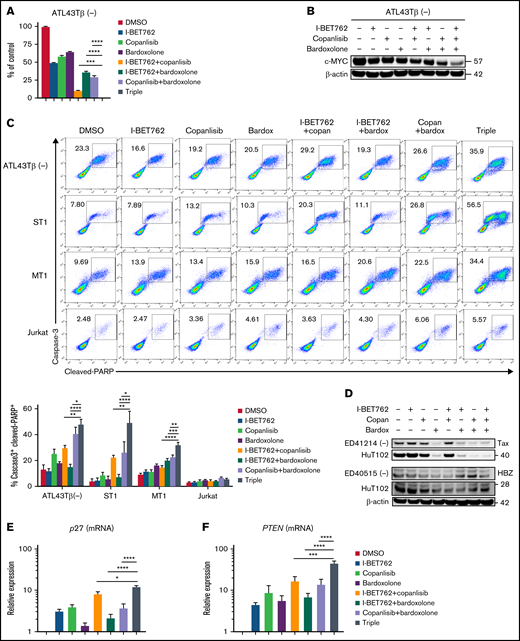
The triple combination of I-BET762 plus copanlisib and bardoxolone methyl markedly reduced the viability of the ATL43Tβ (-) cell line after 72 hours of the treatment (Figure 4A). Interestingly, the c-MYC expression was significantly reduced in the triple combination group analyzed by western blot assay (Figure 4B). In addition, the triple combination induced greater apoptosis compared with single or double agents, as shown by the percentages of double-positive caspase-3 and cleaved PARP after 48 hours of incubation (Figure 4C). Furthermore, we evaluated the effects of the triple combination on Tax protein using the ED41214 (-) cell line. Any combination with bardoxolone methyl, including the double or triple combinations, inhibited the expression of Tax, whereas I-BET762 and copanlisib showed no inhibition. Similar results were observed in the HuT102 cell line. In contrast, the triple combinations had little effect on HBZ protein in ED40515 (-) or HuT102 cell lines (Figure 4D).
Triple combination of I-BET762 plus copanlisib and bardoxolone methyl exhibit synergistic activity and downregulate the level of c-MYC. (A) Antiproliferative effects of the triple combination on the ATL43Tβ (-) cell line. Cell viability was measured with a thymidine incorporation assay after 72 hours of treatment with I-BET762 (500 nM), copanlisib (125 nM), and bardoxolone methyl (125 nM). A representative figure of 3 replicate assays is shown. (B) Immunoblot analysis of c-MYC expression of ATL43Tβ (-) cell line treated with 1 μM of a triple combination for 2 hours. (C) Increased expression of active caspase-3+ and cleaved-PARP+ in ATL43Tβ (-), ST1, and MT1 cell lines after 48 hours of treatment with triple combination measured by flow cytometry. The Jurkat cell line was used as a control. (D) Effects of the triple combination (1 µM each) on HTLV-1 proteins, Tax, and HBZ, for 4 hours. (E-F) p27 and PTEN mRNA expression in the ATL43Tβ (-) cell line after 24 hours of treatment with the triple combination measured by real time-PCR. One-way ANOVA was performed to determine statistical differences. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Triple combination of I-BET762 plus copanlisib and bardoxolone methyl exhibit synergistic activity and downregulate the level of c-MYC. (A) Antiproliferative effects of the triple combination on the ATL43Tβ (-) cell line. Cell viability was measured with a thymidine incorporation assay after 72 hours of treatment with I-BET762 (500 nM), copanlisib (125 nM), and bardoxolone methyl (125 nM). A representative figure of 3 replicate assays is shown. (B) Immunoblot analysis of c-MYC expression of ATL43Tβ (-) cell line treated with 1 μM of a triple combination for 2 hours. (C) Increased expression of active caspase-3+ and cleaved-PARP+ in ATL43Tβ (-), ST1, and MT1 cell lines after 48 hours of treatment with triple combination measured by flow cytometry. The Jurkat cell line was used as a control. (D) Effects of the triple combination (1 µM each) on HTLV-1 proteins, Tax, and HBZ, for 4 hours. (E-F) p27 and PTEN mRNA expression in the ATL43Tβ (-) cell line after 24 hours of treatment with the triple combination measured by real time-PCR. One-way ANOVA was performed to determine statistical differences. *P < .05, **P < .01, ***P < .001, ****P < .0001.
One of the negative regulators of AKT activation is the phosphatase and tensin homolog (PTEN). Loss of PTEN leads to the accumulation of PIP3 and thereby activates several proteins, including AKT family members.35 AKT is also known to downregulate p27, which mediates G1 arrest.36 In our triple combination group, we found an enhanced level of p27 and PTEN genes, suggesting that synergistic effects of the triple combination may involve the recovery of these tumor suppressors in ATL (Figure 4E,F).
Triple combination manifests synergistically in xenograft mice-bearing ATL tumors
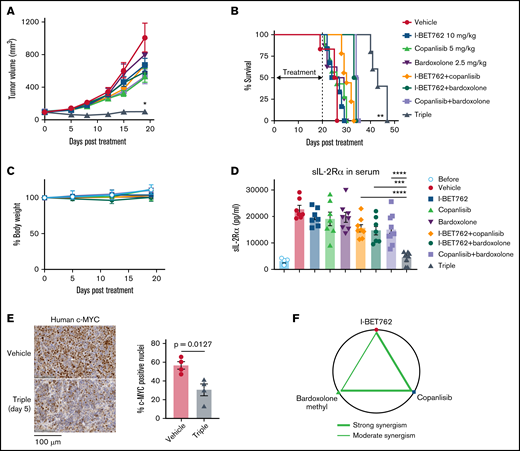
We further evaluated the efficacy of the triple combination in vivo. To avoid toxicity, the dose of I-BET762 was reduced to 10 mg/kg, and copanlisib was decreased to 5 mg/kg, while 2.5 mg/kg of bardoxolone methyl was used, either as a single agent or in double or triple combinations. The dosing schedule was optimized and adjusted to an alternate day 3 times per week to avoid toxicity. The triple combination significantly suppressed ATL growth in the xenograft-bearing ATL, whereas single or double agents had little effect (Figure 5A). When the treatment was stopped on day 20, we observed a regrowth of the tumor in the treated mice with the triple combination. Nevertheless, the reduced tumor volume resulted in prolonged survival of the triple combination group (Figure 5B). Furthermore, there were no significant changes in mouse body weight from day 0 until day 20 after treatment, indicating low toxicity of the triple combination (Figure 5C). Consistently, sIL-2Rα in the serum of triple-treated mice was markedly decreased after 20 days of treatment (Figure 5D). The triple combination also reduced c-MYC expression in the tumor tissues after 5 days of treatment analyzed by IHC staining (Figure 5E). The synergistic effects between these 3 inhibitors were determined using the Chou-Talalay method.37 As depicted in the diagram (Figure 5F), I-BET762 and copanlisib showed a strong synergism, as did copanlisib with bardoxolone methyl (shown as connected heavy lines between drugs). Whereas I-BET762 and bardoxolone methyl demonstrated a moderate synergism (shown as a thin line).
Triple combination manifests synergistically in vivo in xenograft mice-bearing ATL tumors. (A) The triple combination prolonged the survival of xenograft mice-bearing ATL43Tβ (-) tumors. Ten days after tumor inoculation, I-BET762 was given orally at a dose of 10 mg/kg, and copanlisib and bardoxolone methyl were administered via intraperitoneal injection at a dose of 5 mg/kg and 2.5 mg/kg, respectively, 3 times per week for 20 days. The vehicle group received a 15% solutol dissolved in water. Average tumor volumes during the therapeutic time course were measured twice weekly until the tumor volume reached 2000 mm3 (n = 7 to 10). (B) Kaplan-Meier curves illustrate the survival of mice that received single therapy or double or triple combinations. (C) Percentages of mouse body weight change from day 0 until day 20 after treatment. (D) Serum levels of human sIL-2Rα were measured on day 20 after treatment with an ELISA assay (n = 7 to 10 mice per group). Data were pooled from 2 independent experiments and expressed as mean ± SEM. One-way ANOVA and log-rank (Mantel-Cox) tests were performed to determine statistical differences. (E) Representative images of human-c-MYC with IHC staining of tumor isolated from the mice after treatment with vehicle or the triple combination for 5 days. Bar graphs depicted the percentages of c-MYC positive nuclei of tumor tissues (n = 4 mice per group), unpaired Student t test was used to determine statistical differences. (F) Diagram showing the interaction of the 3 drugs combination generated by the Chou-Talalay method: the heavy line is indicating a strong synergism between I-BET762 with copanlisib and copanlisib with bardoxolone methyl. The thin line indicates moderate synergism between I-BET762 and bardoxolone methyl. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Triple combination manifests synergistically in vivo in xenograft mice-bearing ATL tumors. (A) The triple combination prolonged the survival of xenograft mice-bearing ATL43Tβ (-) tumors. Ten days after tumor inoculation, I-BET762 was given orally at a dose of 10 mg/kg, and copanlisib and bardoxolone methyl were administered via intraperitoneal injection at a dose of 5 mg/kg and 2.5 mg/kg, respectively, 3 times per week for 20 days. The vehicle group received a 15% solutol dissolved in water. Average tumor volumes during the therapeutic time course were measured twice weekly until the tumor volume reached 2000 mm3 (n = 7 to 10). (B) Kaplan-Meier curves illustrate the survival of mice that received single therapy or double or triple combinations. (C) Percentages of mouse body weight change from day 0 until day 20 after treatment. (D) Serum levels of human sIL-2Rα were measured on day 20 after treatment with an ELISA assay (n = 7 to 10 mice per group). Data were pooled from 2 independent experiments and expressed as mean ± SEM. One-way ANOVA and log-rank (Mantel-Cox) tests were performed to determine statistical differences. (E) Representative images of human-c-MYC with IHC staining of tumor isolated from the mice after treatment with vehicle or the triple combination for 5 days. Bar graphs depicted the percentages of c-MYC positive nuclei of tumor tissues (n = 4 mice per group), unpaired Student t test was used to determine statistical differences. (F) Diagram showing the interaction of the 3 drugs combination generated by the Chou-Talalay method: the heavy line is indicating a strong synergism between I-BET762 with copanlisib and copanlisib with bardoxolone methyl. The thin line indicates moderate synergism between I-BET762 and bardoxolone methyl. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Triple combination downregulates c-MYC while upregulating GILZ in vivo
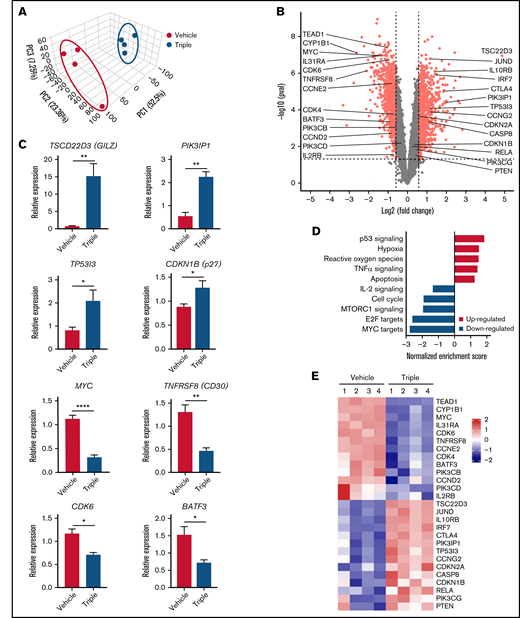
We next focused on the variability of mRNA expression to further identify novel targets and mechanisms affected by the triple combination. Thus, we performed mRNA sequencing of tumors isolated from triple-treated and vehicle mice 5 days after treatment and determined the differentially expressed genes between these groups. Principal-component analysis revealed distinct patterns between the vehicle (blue dots) and triple combination (red dots) (Figure 6A). From our sequencing results, presented as a volcano plot, 812 genes were identified as significantly upregulated (fold change >1.5 and P < .05) when the triple combination and vehicle groups were compared. These included TSC22D3 (GILZ), IL10RB, CTLA4, PI3KIP1, and TP53I3I (Figure 6B; supplemental Figure 4). Some of these genes were previously identified as negative regulators of T-cell signaling, which may be associated with the reduced tumor volume.38-41 Tsc22d3, which codes for GILZ, was the most elevated gene with 29-fold increased expression. The expressions of Tsc22d3, PI3KIP1, TP53I3I, and CDKN1B (p27) were confirmed by quantitative real-time PCR (Figure 6C). The hallmark pathway analysis revealed that the most downregulated biological pathway involved MYC-dependent targets (Figure 6D,E). E2F targets and mTORC1 signaling were also suppressed by the triple combination. Furthermore, TNFRSF8, CDK6, and BATF3 were among the 869 genes that were prominently downregulated (fold change >1.5 and P < .05). These genes were reported to be associated with lymphoma pathogenesis and subsequently identified as therapeutic targets for T-cell lymphoma.42,43
Triple combination downregulates c-MYC while upregulating GILZ in vivo. (A) Principal component analysis of gene expression between the vehicle (blue) and triple combination (red). ATL43Tβ (-) tumor isolated from NSG mice on day 5 after treatment with I-BET762 (10 mg/kg), copanlisib (5 mg/kg), and bardoxolone methyl (2.5 mg/kg) (n = 4 per group). (B) Volcano plot showing fold changes (fold change >1.5 and P < .05) of differentially expressed gene-set between triple combination vs vehicle. (C) Quantitative real-time PCR analysis of TSCD22D3 (GILZ), PIK3IP1, TP53I3, MYC, TNFRSF8 (CD30), CDK6, and BATF3 transcripts (n = 3 to 4). (D) Hallmark gene set enrichment analysis pathways in ATL43Tβ (-) tumor isolated from NSG mice. (E) Tumor-associated genes depicted on heatmap analysis. Unpaired Student t test was used for statistical analysis. *P < .05, **P < .01, ****P < .0001.
Triple combination downregulates c-MYC while upregulating GILZ in vivo. (A) Principal component analysis of gene expression between the vehicle (blue) and triple combination (red). ATL43Tβ (-) tumor isolated from NSG mice on day 5 after treatment with I-BET762 (10 mg/kg), copanlisib (5 mg/kg), and bardoxolone methyl (2.5 mg/kg) (n = 4 per group). (B) Volcano plot showing fold changes (fold change >1.5 and P < .05) of differentially expressed gene-set between triple combination vs vehicle. (C) Quantitative real-time PCR analysis of TSCD22D3 (GILZ), PIK3IP1, TP53I3, MYC, TNFRSF8 (CD30), CDK6, and BATF3 transcripts (n = 3 to 4). (D) Hallmark gene set enrichment analysis pathways in ATL43Tβ (-) tumor isolated from NSG mice. (E) Tumor-associated genes depicted on heatmap analysis. Unpaired Student t test was used for statistical analysis. *P < .05, **P < .01, ****P < .0001.
Triple combination inhibits c-MYC ex vivo in PBMCs containing leukemic cells from ATL patients
Our group has previously demonstrated that ATL cells from PBMCs of smoldering/chronic patients possess activated JAK/STAT signaling pathways associated with spontaneous leukemic cell proliferation.44 Although PBMCs of acute ATL patients constitutively express IL-2Rα, autocrine stimulation by IL-2/IL-2R-induced proliferation was not observed in ex vivo cultures. Therefore, to induce the proliferation of acute ATL cells, exogenous rIL-2 cytokine was added to the PBMC cultures. The addition of rIL-2 significantly induced the expansion of the IL-2Rα+ leukemic cells, >50% by day 6; this was observed in 4 PBMCs of acute ATL patients (Figure 7A,B). Conversely, the leukemic cells of smoldering/chronic patients autonomously proliferated without stimuli or exogenous cytokine, indicating the presence of an IL-2/IL-2Rα autocrine loop in the leukemic cells. Strikingly, the enriched cells treated with the triple combination had markedly reduced c-MYC expression in both PBMCs from acute and smoldering/chronic ATL patients, suggesting the synergistic effect of these 3 inhibitors ex vivo (Figure 7C). The downstream proteins of the PI3K/AKT pathway were also inhibited with the reduced expression of p-AKT and p-4EBP1 (supplemental Figure 5A). Furthermore, we found the increased level of Tsc22d3 (GILZ) whereas TNFRSF8 (CD30) was reduced in PBMCs of ATL patients after being treated with the triple combination (Figure 7D). The reduced level of c-MYC expression was also correlated with the decreased proliferation of both PBMCs from acute and chronic ATL patients (Figure 7E; supplemental Figure 5B-D). In contrast, the triple combination had little effect on the proliferation of normal CD4+ T cells from healthy donors (Figure 7F; supplemental Figure 5E). In addition, we performed flow cytometry analysis on patients’ PBMCs to identify other immune cells in the culture. CD4 T cells, CD4+CD25+ (ATL), CD8 T cells, CD14 (monocytes), and CD19 (B cells) were assessed at day 0 and day 5. In acute ATL patients, their PBMCs had abnormally high white counts at day 0, and most of the cells were CD4 T cells (>80%) which accounted for 40% to 80% of CD4+CD25+. The ATL cells also remained the major population after culture (supplemental Figure 5F). On the other hand, ATL cells in the PBMCs of chronic patients were at 10% to 30% at day 0, and the percentage of ATL cells significantly increased by day 5. The remaining cells were CD8 T cells and B cells, while monocyte percentages decreased after culture.
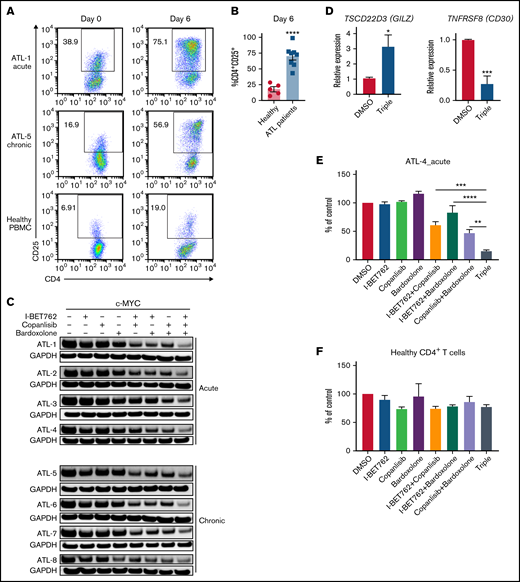
Triple combination inhibits c-MYC in PBMCs containing leukemic cells ex vivo from ATL patients. (A) Expression of CD25 on CD4+T cells of PBMCs isolated from acute ATL (n = 4), smoldering/chronic ATL patients (n = 4), and healthy donors (n = 5). The expression was measured on day 0 (before) and day 6 (after) ex vivo culture. rIL-2 cytokine (100 U/ml) was added into the culture of PBMCs from acute ATL patients and healthy donors. (B) The bar graphs represent average percentages of CD4+CD25+ on T cells of healthy donors (n = 5) and acute and chronic ATL patients (n = 8) after 6-day ex vivo culture. Error bars represent mean ± SEM. Student t test was used to determine statistical differences. ****P < .0001. (C) Western blot analysis of c-MYC after 6-day ex vivo culture treated with 1 μM of I-BET762, copanlisib, and bardoxolone methyl or combination for 2 hours (n = 8). (D) Quantitative real-time PCR analysis of TSCD22D3 (GILZ) and TNFRSF8 (CD30) transcripts in PBMC cultures of patients treated with DMSO or 1 μM of the triple combination for 4 hours (n = 5). (E) Cell viability of the PBMCs from an acute ATL patient and (F) CD4+ T cells isolated from a healthy donor, cultured with I-BET762 (250 nM), copanlisib (125 nM), and bardoxolone methyl (62.5 nM), n = 3. One-way ANOVA or Student t test was used to determine statistical differences. *P < .05, **P < .01, ****P < .0001.
Triple combination inhibits c-MYC in PBMCs containing leukemic cells ex vivo from ATL patients. (A) Expression of CD25 on CD4+T cells of PBMCs isolated from acute ATL (n = 4), smoldering/chronic ATL patients (n = 4), and healthy donors (n = 5). The expression was measured on day 0 (before) and day 6 (after) ex vivo culture. rIL-2 cytokine (100 U/ml) was added into the culture of PBMCs from acute ATL patients and healthy donors. (B) The bar graphs represent average percentages of CD4+CD25+ on T cells of healthy donors (n = 5) and acute and chronic ATL patients (n = 8) after 6-day ex vivo culture. Error bars represent mean ± SEM. Student t test was used to determine statistical differences. ****P < .0001. (C) Western blot analysis of c-MYC after 6-day ex vivo culture treated with 1 μM of I-BET762, copanlisib, and bardoxolone methyl or combination for 2 hours (n = 8). (D) Quantitative real-time PCR analysis of TSCD22D3 (GILZ) and TNFRSF8 (CD30) transcripts in PBMC cultures of patients treated with DMSO or 1 μM of the triple combination for 4 hours (n = 5). (E) Cell viability of the PBMCs from an acute ATL patient and (F) CD4+ T cells isolated from a healthy donor, cultured with I-BET762 (250 nM), copanlisib (125 nM), and bardoxolone methyl (62.5 nM), n = 3. One-way ANOVA or Student t test was used to determine statistical differences. *P < .05, **P < .01, ****P < .0001.
Discussion
In the current study, we demonstrated that I-BET762 acts synergistically with copanlisib and bardoxolone methyl to inhibit the proliferation of ATL cells both in vitro and in vivo. The mechanism of synergistic activity was associated with the downregulation of c-MYC, suggesting the critical roles for BET, PI3K, and NF-κB pathways in ATL. Furthermore, RNA sequencing analysis identified GILZ as the most elevated gene with the triple combination. GILZ was previously shown to inhibit TCR-induced IL-2/IL-2R expression and NF-κB activity.40 Moreover, GILZ was demonstrated to prevent the binding of active activator protein-1 to its target DNA.45 In GILZ-transgenic mice, GILZ overexpression induced apoptosis through caspase-8 activation and decreased Bcl-xL expression46 ; an observation was consistent with our results where CASP8 transcript was increased in response to the triple combination.
Interestingly, TNFRSF8 was a transcript among the genes that substantially downregulated. TNFRSF8, also known as CD30, belongs to a member of the tumor necrosis factor receptor (TNFR) superfamily. CD30 is expressed on activated T cells and a variety of lymphoid neoplasms, including anaplastic large cell lymphoma, Hodgkin’s lymphoma, and ATL.43 A previous report demonstrated that sCD30 was elevated in the sera of ATL patients and correlated with the aggressiveness of disease,47 which suggested that the triple combination could be considered for treatment of hematologic malignancy subtypes that are CD30-positive.
Phase 1/2 clinical trials of I-BET762 showed acceptable safety and efficacy profile in patients with NUT carcinoma and advanced solid tumors.13 However, the association between the potency of BET inhibitors and the downregulation of c-MYC in a clinical setting has not yet been established.48 Our study demonstrates a correlation between the efficacy of I-BET762 and c-MYC inhibition in the PBMCs of ATL patients. Indirect targeting of c-MYC via interruption of transcription, translation, or c-MYC stability could hold a promise for future therapy. Despite the expression of IL-2Rα, the synergistic effect with the triple combination was consistently observed in PBMCs of both acute and smoldering/chronic ATL patients. The 6-day expanded cells became more responsive to the triple combination than the freshly isolated or frozen PBMCs, suggesting T cell-activation status may be critical for the outcome of the triple combination therapy.
Earlier studies by our group44,49 suggested that Tax can be induced in spontaneously proliferating chronic/smoldering ATL PBMCs ex vivo. Noting that Tax is fundamental in the progression of early HTLV-1 disease, perhaps our drug combination could be applied to a broader range of Tax-driven, HTLV-1-associated disorders.
Single-agent therapy demonstrated some partial responses with limited dose escalation.13,21,50 Thus, recent studies51-54 have been focused on using these agents in combination therapy for recurrent/relapsed diseases. The triple combination therapy is promising and recommended for acute ATL patients with advanced and aggressive disease. However, PI3K/AKT and NF-κB pathways are also present in normal T cells and other hematopoietic cells, which may lead to some adverse effects. Therefore, optimization of dosing schedules for the triple combination in patients remains challenging, and the pharmacodynamics/kinetics of the triple combination still need to be attentively evaluated to ensure successful treatment regimens. Furthermore, we observed a regrowth of ATL in xenograft-bearing tumors once the treatment was stopped, suggesting the need for inhibitors with a stronger apoptotic effect such as BCL-xL inhibitor.
A search for an alternative and novel NF-κB inhibitor is crucial to enhance the efficacy of the triple combination. Bardoxolone methyl is primarily being evaluated for patients with chronic kidney diseases (NCT04702997, NCT03918447). Bortezomib is known to inhibit NF- κB and is approved by the FDA for patients with relapsed/refractory multiple myeloma.55,56 However, the phase 2 trial of bortezomib for patients with relapsed/refractory aggressive ATL was not promising and was discontinued due to insufficient efficacy.57
In summary, the triple combination of BET, PI3K, and NF-κB inhibitors induced cytotoxic effect and mediated the downregulation of c-MYC in ATL cell lines and ATL derived from PBMCs of acute and chronic patients. Our data provide a clear rationale for a clinical trial exploring the combination of BET, PI3K, and NF-κB inhibitors for ATL patients.
Acknowledgments
The authors thank the NCI CCR genomic core, NCI sequencing facility, and CCR-SF bioinformatics group at Frederick National Laboratory for Cancer Research (FNLCR) for sequencing and analysis. They also thank Donna Butcher from Molecular Histopathology Laboratory at NCI-FNLCR for technical assistance. This paper is dedicated to Dr. Thomas A. Waldmann, who died on September 25, 2021. He was a great scientist, mentor, and friend.
This project was supported by the Intramural Research Program of the National Cancer Institute, NIH, and in part by the Division of Preclinical Innovation, National Center for Advancing Translational Sciences. P.L.G. funded by NIH/NCI P01 CA100730.
Authorship
Contribution: A.D., R.N.B., and T.A.W. designed the study; A.D. performed experiments, analyzed data, and wrote the manuscript; M.Y. and S.-M.Y. generated and provided CN470; P.H. performed RNA sequencing analysis; B.K. performed IHC analysis; B.R.B. and M.N.P. organized patient samples; C.J.T. and P.L.G. provided reagents; M.D.M. and K.C.C. performed patient care and collected patient samples; T.A.W. supervised the study and acquired funding; and all authors edited and revised the manuscript.
Conflict-of-interest disclosure: M.Y. is the vice president of research & collaborations and has ownership interest (including stock, patents, etc.) in ConverGene LLC. All other authors declare no competing financial interests.
Correspondence: Anusara Daenthanasanmak, Center for Cancer Research, National Cancer Institute/NIH, 10 Center Drive, Building 10, Room 4N112, Bethesda, MD 20892; e-mail: anusara.daen@gmail.com.
References
Author notes
Thomas A. Waldman died on 25 September 2021.
Data generated for this study are available at the Gene Expression Omnibus (GEO: GSE179307). Other data are available upon reasonable request (anusara.daen@gmail.com).
The full-text version of this article contains a data supplement.