Key Points
CD70 is highly expressed in mature TCL, especially in CTCL, and an ideal therapeutic target for ADC.
SGN-CD70A, a novel ADC, induces complete eradication of established tumors assessed by cell-free DNA and prolongs survival in CTCL PDXs.
Abstract
CD70 is a member of the tumor necrosis factor receptor superfamily. Emerging data indicate that CD70 may be a suitable target for various malignancies. We investigated the expression of CD70 in cutaneous and systemic T-cell lymphomas and conducted preclinical studies of SGN-CD70A, a CD70-directed antibody-drug conjugate (ADC), using patient-derived xenograft cutaneous T-cell lymphoma (CTCL PDX) models. CD70 expression was examined by immunohistochemical (IHC) stains in 49 diagnostic specimens of T-cell lymphomas. The activities of SGN-CD70A in growth inhibition and apoptosis induction were examined in CTCL cell lines and primary CTCL tumor cells. Using previously established CTCL PDXs, we conducted a dose-finding trial followed by a phase 2-like trial to evaluate the optimal dosing and the efficacy of SGN-CD70A in tumor-bearing PDX animals. The therapeutic efficacy of SGN-CD70A was measured by tumor-associated cell-free DNA (cfDNA) and survival of treated PDXs. We found that CD70 is highly expressed in T-cell lymphomas, especially in CTCL. SGN-CD70A inhibited cell growth and induced apoptosis in CD70-expressing CTCL cell lines and primary tumors cells. Additionally, SGN-CD70A at 100 μg/kg and 300 μg/kg prolonged the survival of PDXs in a dose-dependent manner. Finally, treatment with 3 doses of SGN-CD70A at 300 μg/kg was superior to a single-dose treatment in survival prolongation (median survival: 111 days vs 39 days; P = .017). Most importantly, multiple dosing of SGN-CD70A induced complete eradication of established tumors in PDXs measured by cfDNA. Our results demonstrated marked antitumor activity of SGN-CD70A in CTCL PDXs, providing compelling support for its clinical investigation.
Introduction
Cutaneous T-cell lymphoma (CTCL) is a subtype of non-Hodgkin’s lymphoma and is a malignancy of skin-homing T cells. Mycosis fungoides (MF) and Sézary syndrome are the most common subtypes of CTCL. Early-stage CTCL is generally treated with skin-directed therapy and has a favorable prognosis. In contrast, advanced disease has an overall survival of 3.5 to 5.6 years, a result that has not improved for decades.1-4 This highlights unmet needs for targeted and effective therapy for the treatment of CTCL.
CD70 is a member of the tumor necrosis factor receptor superfamily and interacts with a ligand, CD27. CD70 has several unique properties that make it an ideal therapeutic target in cancer. First, CD70 is only transiently expressed on activated T- and B-lymphocytes, mature killer cells, and mature dendritic cells,5-9 and has limited expression on normal, nonimmune cells. However, it is more widely expressed in various solid tumors and hematologic malignancies, including various subtypes of B-cell and systemic T-cell lymphomas. Second, interactions between CD70 and CD27 serve as a costimulatory signal in T and B lymphocyte activation and induce lymphocytic proliferation.10 Thus, blocking CD70-CD27 interaction may exert antiproliferative activity in lymphomas. Finally, CD70-CD27 interaction has been implicated as one of the mechanisms of immune escape through the promotion of T regulatory cells in the tumor microenvironment.11,12 Indeed, CD70 has emerged as a promising therapeutic target in recent years. A phase 1 clinical trial with ARGX-110, a defucosylated anti-CD70 monoclonal antibody conducted in patients with CD70-expressing advanced malignancies, showed preliminary evidence of clinical activity.13 Additionally, a phase 1/2 trial with ARGX-110 in advanced CTCL patients demonstrated modest clinical activity with an overall response rate (ORR) of 23%.14 Recently, clinical activities of antibody-drug conjugates (ADCs) targeting CD70 have also been explored.15,16 SGN-CD70A is a potent ADC, linking an anti-CD70 monoclonal antibody with a cytotoxic DNA-crosslinking agent, pyrrolobenzodiazepine (PBD) dimer.17 Recent trials of SGN-CD70A in metastatic renal cell carcinoma and diffuse large B-cell lymphoma showed modest activity.16,18 However, the clinical activity of SGN-CD70A in CTCL has not been explored. Herein, we examined the frequency of CD70 expression in systemic and primary cutaneous T-cell lymphomas and investigated ex vivo and in vivo activities of SGN-CD70A in preclinical models using patient-derived xenograft (PDX) models for CTCL.
Materials and methods
Patient selection and specimen preparation
Patient data and archived slides were obtained from the University of California, San Francisco (UCSF) Medical Center. The study was performed according to a protocol approved by the UCSF Medical Center Institutional Review Board. The surgical and dermatopathology databases were searched for cases with the diagnosis of T-cell lymphomas between 2002 and 2014. A total of 49 cases of T-cell lymphomas were selected, including MFs (n = 13), primary cutaneous anaplastic large cell lymphoma (pcALCL) (n = 7), systemic anaplastic large cell lymphoma (ALCL), anaplastic lymphoma kinase (ALK)+ (n = 9), systemic ALCL, ALK− (n = 6), and peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) (n = 14). For MF specimens, 10 of 13 were from tumor lesions, and the remaining 3 samples were from lesions with large cell transformations. Twelve of 13 MF specimens were skin biopsies, and 1 was a lymph node biopsy. For systemic ALCL and PTCL NOS specimens, 16 from lymph nodes, 3 from lungs, 3 from tonsils, and 1 sample from each of the following sites: nasal mass, mediastinal mass, orbital mass, bone marrow, GI, skin, and liver.
Cell lines and reagents
Human CTCL cell lines (HH, H9, MJ, Hut 78) and T-cell acute lymphoblastic leukemia (T-ALL) cell lines (HPB-ALL, PF-382, CCRF-CEM, and Jurkat) were acquired from American Type Culture Collection (ATCC) and cultured in complete media recommended by ATCC. All cell lines were passaged 3 times per week and maintained at a cell density below 106 cells/mL, and logarithmically growing cells were used for all experiments.
SGN-CD70A (h1F6239C-PBD) and ADC-IgG control (hIgG239C-PBD) were provided by Seagen Inc. (Seattle, WA).17 T-cell activation/expansion kit and recombinant cytokines, human IL-2 (hIL-2) and hIL-7, were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
Immunohistochemical (IHC) staining and interpretation
Polyclonal antibody derived against 17 amino acids and synthetic peptide derived from the internal region of human CD70 (Abcam, Cambridge, MA) was optimized for IHC staining of lymphoid tissue in the Pathology Core Facility at UCSF. The slides with paraffin-embedded specimen sections were stained with hematoxylin and eosin or optimal concentrations of polyclonal antibodies against human CD70 as described previously. Positive controls were included in each staining run and consisted of tonsil and spleen tissue. Negative controls using isotype control antibodies were also included in each staining run. CD70 immunostaining of skin biopsies was performed by a contract laboratory through Seagen Inc. Scoring of CD70 expression in the systemic and cutaneous T-cell lymphoma specimens was performed by 2 independent hematopathologists (L.W. and K.W.W.) and dermatopathologists (L.P. and B.H.), respectively. CD70 expression on the lesional lymphocytes was scored in 4 categories: <5%, 5% to 25%, 25% to 50%, and >50%.
Flow cytometry analysis
Cells harvested from the spleens of PDX mice or various cell lines were washed with phosphate-buffered saline (PBS) and resuspended in cell staining buffer (Biolegend, San Diego, CA) at a cell density of 1 to 2 × 105 cells/mL. For surface antigen staining, the cells were incubated with Fc receptor blocking solution (Biolegend) for 15 minutes at 4°C and then stained with cocktails containing combinations of fluorochrome-conjugated monoclonal antibodies against human CD3, CD4, and CD70 (Biolegend) in the dark for 30 minutes at 4°C. After staining, the cells were washed with PBS containing 2% fetal bovine serum and resuspended in cell staining buffer. For the apoptosis assay, treated cells were stained with annexin V-FITC/PI reagents (BD Bioscience, San Jose, CA). Data were acquired by a BD FACS-Aria III (BD Bioscience) and analyzed by FlowJo 10.6.1 software (TreeStar Inc., Ashland, OR).
In vitro and ex vivo assays
CTCL and T-ALL cell lines were treated with various concentrations of SGN-CD70A or PBS for 72 hours. The cell proliferation was measured by CellTiter-Glo (Promega, Leiden, Netherlands), and apoptosis was analyzed by the annexin-V/PI assay.
Primary tumor cells from PDX mice were cultured in complete media, RPMI1640 with 20% human AB serum (MP, Solon, OH). For the proliferation assays, primary tumor cells from PDXs were stimulated to grow with CD2/CD3/CD28 activation beads and incubated with hIL-2 (500 U/mL) and hIL-7 (1000 U/mL) (Miltenyi Biotec) at an optimal cell density of 5 to 10 × 105 cells/mL. After 24 or 48 hours of activation, the cells were treated with various concentrations of SGN-CD70A as indicated. The proliferation of primary tumor cells was determined by Real-Time Glo (Promega) at 24, 48, and 72 hours after drug treatment. Apoptosis assays using primary tumor cells were performed as described previously.20 Briefly, primary tumor cells were harvested from the spleens of PDX mice and cultured in a complete medium with 250 U/mL of hIL-2 without activation beads. At 24 hours, cells were treated with various concentrations of SGN-CD70A or ADC-IgG control. After 72 hours of treatment, cells were analyzed by the annexin-V/PI assay.
In vivo activity of SGN-CD70A in patient-derived CTCL xenograft models
Assessment of the antitumor activity of SGN-CD70A in CTCL PDX models was performed as published previously.20 Briefly, on day 0, 8-week-old female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were implanted in a flank with 4 × 106 cells from a PDX mouse, PRS-1. On day 7, when the tumor volumes reached 60 to 80 mm3, mice were divided into 3 or 4 groups as indicated. In the dose-finding trial, mice were intraperitoneally administrated with 1 dose of PBS (control) or SGN-CD70A at 100 or 300 μg/kg on day 7 (denoted as SGN-CD70A100 and SGN-CD70A300, respectively). In the phase 2-like mouse trial, mice were intraperitoneally injected on day 7 with 1 dose of PBS (control), isotype IgG control conjugated with the PBD dimer (300 μg/kg, IgG control), SGN-CD70A at 300 μg/kg (SGN-CD70A300), or 3 weekly doses of 300 μg/kg (days 7, 14, and 21). The size of the tumor lesion was measured twice weekly with calibers. Plasma samples were collected from mice twice per week starting from day 6 and stored at -80°C for cfDNA.
cfDNA quantification
Serial blood samples (250 μL) were collected by retroorbital bleeding from PDX mice using microtainer tubes with dipotassium EDTA (BD Biosciences, San Jose, CA). cfDNA was isolated from 100 µL of the plasma samples using the NucleoSpin Plasma kit (Macherey-Nagel, Düren, Germany). cfDNA concentration in the plasma was subsequently determined by quantitative polymerase chain reaction using the Custom TaqMan assay with the 7900HT Fast Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA). Human β-actin primer pair and probe set are as follows: forward primer, 5′-ATCCTAAAAGCCACCCCACT-3′; reverse primer, 5′-CTCAAGTTGGGGGACAAAAA-3′; and probe, 5′-FAM-CACAGGGGAGGTGATAGCAT-MGB-3′. Serial dilutions of genomic DNA extracted from the Hut78 CTCL cell lines were used to calibrate for cfDNA quantification. Sample cfDNA concentrations were extrapolated from the standard curve using Prism software v.6.0 (GraphPad Software, La Jolla, CA).
Statistical analysis
The results of tumor volume and plasma tumor cfDNA for each treatment group were expressed as mean ± SEM. Statistical differences between 2 groups of tumor volume and plasma tumor cfDNA were analyzed by an unpaired, 1-tailed t test. P < .05 was considered statistically significant. The survival curves were generated by the Kaplan-Meier method and analyzed by Mann-Whitney U test performed using Prism software v6.0, and P < .05 was considered statistically significant.
Results
Frequency of CD70 expression in cutaneous and systemic T-cell lymphomas
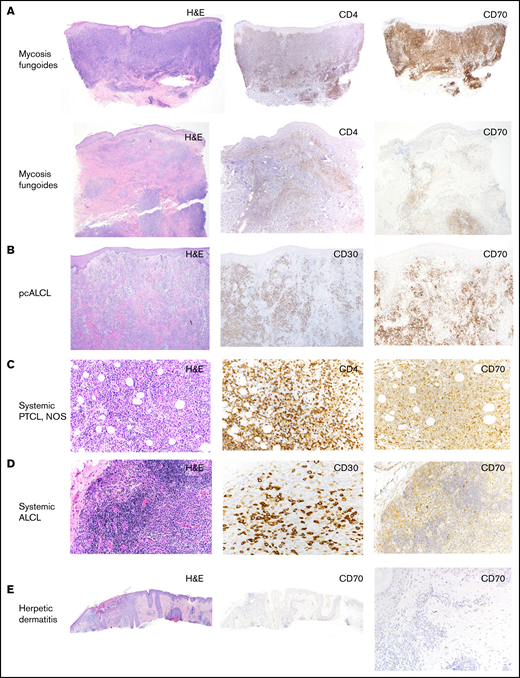
We examined the frequency and intensity of CD70 expression by IHC staining in 49 biopsy specimens of T-cell lymphomas, including MF, pcALCL, systemic ALCL, and PTCL, NOS (Figure 1). Using CD70 expression in 5% of lymphocytes as a cutoff for positive labeling, the frequencies of CD70 positivity in MF, pcALCL, PTCL, NOS, systemic ALK+, and ALK− ALCL were 95%, 100%, 64%, 78%, and 50%, respectively (Table 1). CD70 appears to be particularly highly expressed in CTCL. Among 13 cases of MF, only 1 case had an expression level <5%, and 6 cases had an expression level >50%. In pcALCL, all specimens were positive for CD70 expression, and 14% of the specimens had an expression level >50%.
CD70 expression in different subtypes of T-cell lymphomas. (A) Representative images of hematoxylin/eosin (H/E), IHC stains of CD4 and CD70 in 2 skin biopsies of MFs, showing different levels of CD70 expression (original magnification, ×40). (B) Representative images of H/E, IHC stains of CD30 and CD70 in a skin biopsy of a patient with pcALCL (original magnification, ×40). (C) Representative images of H/E, IHC stains of CD4 and CD70 in a lymph node biopsy of PTCL, NOS (original magnification, ×20). (D) Representative images of H/E, IHC stains of CD30 and CD70 in a lymph node biopsy of systemic ALCL (original magnification, ×20). (E) Images of H/E and IHC stain of CD70 in a skin biopsy of herpetic dermatitis, showing negative CD70 expression (original magnification, ×40).
CD70 expression in different subtypes of T-cell lymphomas. (A) Representative images of hematoxylin/eosin (H/E), IHC stains of CD4 and CD70 in 2 skin biopsies of MFs, showing different levels of CD70 expression (original magnification, ×40). (B) Representative images of H/E, IHC stains of CD30 and CD70 in a skin biopsy of a patient with pcALCL (original magnification, ×40). (C) Representative images of H/E, IHC stains of CD4 and CD70 in a lymph node biopsy of PTCL, NOS (original magnification, ×20). (D) Representative images of H/E, IHC stains of CD30 and CD70 in a lymph node biopsy of systemic ALCL (original magnification, ×20). (E) Images of H/E and IHC stain of CD70 in a skin biopsy of herpetic dermatitis, showing negative CD70 expression (original magnification, ×40).
Frequencies of CD70 expression in biopsies from patients with systemic or cutaneous T-cell lymphomas
| Lymphoma subtype . | n . | CD70 expression (%) . | |||
|---|---|---|---|---|---|
| <5, n (%) . | 5-25, n (%) . | 25-50, n (%) . | >50, n (%) . | ||
| Cutaneous T-cell lymphoma | 20 | ||||
| Mycosis fungoides | 13 | 1 (8) | 5 (38) | 1 (8) | 6 (46) |
| pcALCL | 7 | 0 (0) | 2 (29) | 4 (57) | 1 (14) |
| Systemic T-cell lymphoma | 29 | ||||
| PTCL | 14 | 5 (36) | 2 (14) | 3 (21) | 4 (29) |
| ALCL, ALK+ | 9 | 2 (22) | 1 (11) | 0 (0) | 6 (67) |
| ALCL, ALK− | 6 | 3 (50) | 0 (0) | 0 (0) | 3 (50) |
| Lymphoma subtype . | n . | CD70 expression (%) . | |||
|---|---|---|---|---|---|
| <5, n (%) . | 5-25, n (%) . | 25-50, n (%) . | >50, n (%) . | ||
| Cutaneous T-cell lymphoma | 20 | ||||
| Mycosis fungoides | 13 | 1 (8) | 5 (38) | 1 (8) | 6 (46) |
| pcALCL | 7 | 0 (0) | 2 (29) | 4 (57) | 1 (14) |
| Systemic T-cell lymphoma | 29 | ||||
| PTCL | 14 | 5 (36) | 2 (14) | 3 (21) | 4 (29) |
| ALCL, ALK+ | 9 | 2 (22) | 1 (11) | 0 (0) | 6 (67) |
| ALCL, ALK− | 6 | 3 (50) | 0 (0) | 0 (0) | 3 (50) |
SGN-CD70A inhibits cell growth and induces apoptosis in CD70-expressing CTCL cell lines
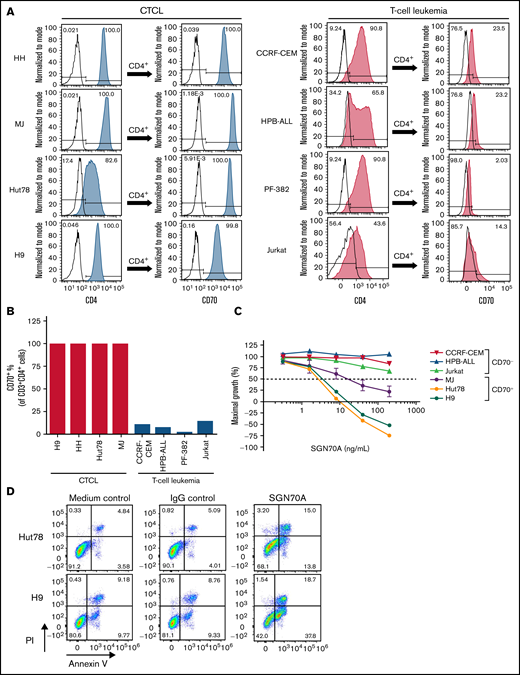
Having demonstrated the CD70 expression in T-cell lymphomas, we investigated whether CD70 is a useful therapeutic target for CTCL. We first analyzed CD70 expression by flow cytometry in a variety of T-cell lymphoma/leukemia cell lines. We found that in 4 CTCL cell lines tested (HH, MJ, Hut78, and H9), more than 99% of CD4+ cells expressed CD70, whereas, in T-cell leukemia cell lines (CCRF-CEM, HPB-ALL, PF-382, and Jurkat), only 2∼23% of cells, expressed CD70 (Figure 2A,B).
SGN-CD70A inhibits cell growth and induces apoptosis in CD70-expressing CTCL cell lines. (A-B) Histograms and bar graph of CD70 expression level in CD4+ cells of CTCL cell lines HH, MJ, Hut78, and H9, and T-cell leukemia cell lines CCRF-CEM, HPB-ALL, PF-382, and Jurkat. (C) SGN-CD70A inhibits cell proliferation in CTCL and T-cell leukemia cell lines. Cells were incubated with PBS, IgG control, or SGN-CD70A for 48 hours before the cell viability was examined. Data were presented as mean ± SEM from 3 independent experiments. (D) SGN-CD70A induces apoptosis in Hut78 and H9 cells. Cells were incubated with PBS, IgG control, or SGN-CD70A for 48 hours, and apoptosis was subsequently determined by the annexin V/PI assay.
SGN-CD70A inhibits cell growth and induces apoptosis in CD70-expressing CTCL cell lines. (A-B) Histograms and bar graph of CD70 expression level in CD4+ cells of CTCL cell lines HH, MJ, Hut78, and H9, and T-cell leukemia cell lines CCRF-CEM, HPB-ALL, PF-382, and Jurkat. (C) SGN-CD70A inhibits cell proliferation in CTCL and T-cell leukemia cell lines. Cells were incubated with PBS, IgG control, or SGN-CD70A for 48 hours before the cell viability was examined. Data were presented as mean ± SEM from 3 independent experiments. (D) SGN-CD70A induces apoptosis in Hut78 and H9 cells. Cells were incubated with PBS, IgG control, or SGN-CD70A for 48 hours, and apoptosis was subsequently determined by the annexin V/PI assay.
Next, we investigated whether SGN-CD70A has antitumor activity in CD70-expressing T-cell lymphoma/leukemia cell lines. As shown in Figure 2C, SGN-CD70A potently inhibited cell growth in CD70+ CTCL cell lines, MJ, H9, and Hut78, with a GI50 of 145.4, 3.2, and 2.4 ng/mL, respectively. In contrast, SGN-CD70A had no activity in CD70-negative (CD70−) T-cell leukemia/lymphoma lines, HPB-ALL, CCRF-CEM, and Jurkat. In addition to growth inhibition, SGN-CD70A induced apoptosis in Hut78 and H9 cells compared with medium or IgG control (Figure 2D). These results demonstrated that SGN-CD70A has antitumor activity in CTCL cell lines, and this activity is associated with CD70 expression.
Ex vivo antitumor activity of SGN-CD70A in primary CTCL tumor cells
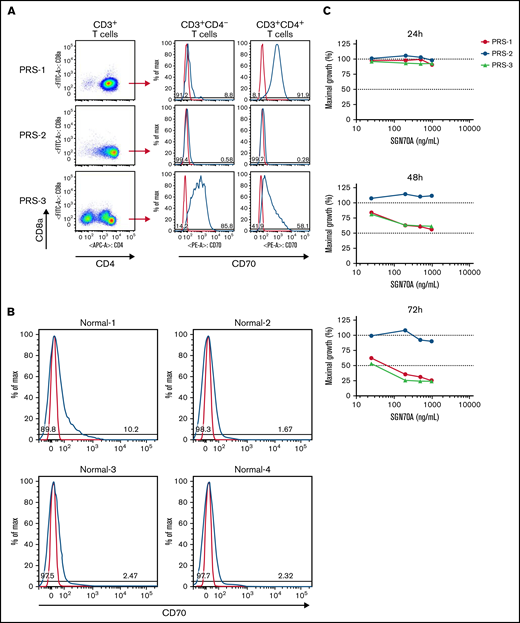
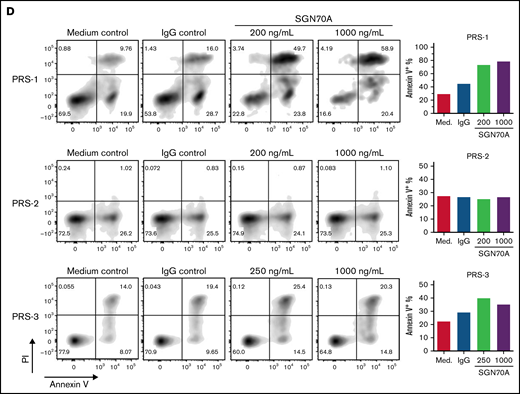
To examine the antitumor activity of SGN70A in primary CTCL tumor cells, we collected the malignant cells from the spleens of 3 CTCL PDX mice, PRS-1, PRS-2, and PRS-3, as described previously.20,21 We showed that malignant cells from PRS-1 and PRS-3 expressed CD70, whereas PRS-2 did not (Figure 3A). Of note, PRS-3 had 2 malignant cell populations, CD3+CD4+ and CD3+CD4−, which had a CD70 expression level of 58.1% and 85.8%, respectively. In contrast to primary CTCL cells, CD3+CD4+ T cells collected from the peripheral blood of healthy normal donors did not express CD70 (Figure 3B).
SGN-CD70A inhibits cell proliferation and induces apoptosis in CD70-expressing primary tumor cells from CTCL PDX mice. (A) CD70 expression levels by flow cytometry in primary malignant T cells harvested from spleens of CTCL PDX mice, PRS-1, PRS-2, and PRS-3. PRS-1 was generated from a patient with MF, and PRS-2 and -3 were generated from patients with Sézary syndrome, as described previously. (B) CD70 expression levels in T cells (CD3+CD4+) in the PBMC from 4 normal donors (Normal-1 through Normal-4). (C) Antiproliferative activity of SGN-CD70A in PRS-1, PRS-2, and PRS-3 primary tumor cells. The T-cell expansion was triggered by incubation with anti-CD2/CD3/CD28 beads in cell medium containing IL-2 (500 U/mL) and IL-7 (250 U/mL). Cell viability was determined at 24, 48, and 72 hours after treatment. (D) SGN-CD70A induces apoptosis in CD70-expressing primary tumor cells (PRS-1 and PRS-3) but not in tumor cells that do not express CD70 (PRS-2). The primary tumor cells harvested from spleens of PRS-1, PRS-2, and PRS-3 PDX mice were treated with various concentrations of SGN-CD70A, medium control (PBS), or IgG isotype control for 72 hours, and the apoptosis induction was analyzed by the annexin V/PI assay.
SGN-CD70A inhibits cell proliferation and induces apoptosis in CD70-expressing primary tumor cells from CTCL PDX mice. (A) CD70 expression levels by flow cytometry in primary malignant T cells harvested from spleens of CTCL PDX mice, PRS-1, PRS-2, and PRS-3. PRS-1 was generated from a patient with MF, and PRS-2 and -3 were generated from patients with Sézary syndrome, as described previously. (B) CD70 expression levels in T cells (CD3+CD4+) in the PBMC from 4 normal donors (Normal-1 through Normal-4). (C) Antiproliferative activity of SGN-CD70A in PRS-1, PRS-2, and PRS-3 primary tumor cells. The T-cell expansion was triggered by incubation with anti-CD2/CD3/CD28 beads in cell medium containing IL-2 (500 U/mL) and IL-7 (250 U/mL). Cell viability was determined at 24, 48, and 72 hours after treatment. (D) SGN-CD70A induces apoptosis in CD70-expressing primary tumor cells (PRS-1 and PRS-3) but not in tumor cells that do not express CD70 (PRS-2). The primary tumor cells harvested from spleens of PRS-1, PRS-2, and PRS-3 PDX mice were treated with various concentrations of SGN-CD70A, medium control (PBS), or IgG isotype control for 72 hours, and the apoptosis induction was analyzed by the annexin V/PI assay.
Next, we investigated the ex vivo activity of SGN-CD70A in primary tumor cells from PDXs. SGN-CD70A inhibited cell growth in a dose-dependent manner in CD70-expressing primary cells from PDXs, PRS-1, and PRS-3, but not in CD70-negative PDX cells, PRS-2 (Figure 3C). Similarly, we observed apoptosis induced by SGN-CD70A only in CD70-expressing primary tumor cells, PRS-1 and PRS-3 (Figure 3D). These results demonstrated that SGN-CD70A can inhibit cell growth and induce apoptosis in CD70-expressing primary CTCL cells.
Dose-finding trial of SGN-CD70A in CTCL PDXs
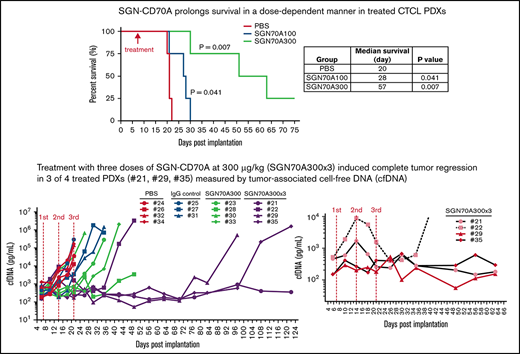
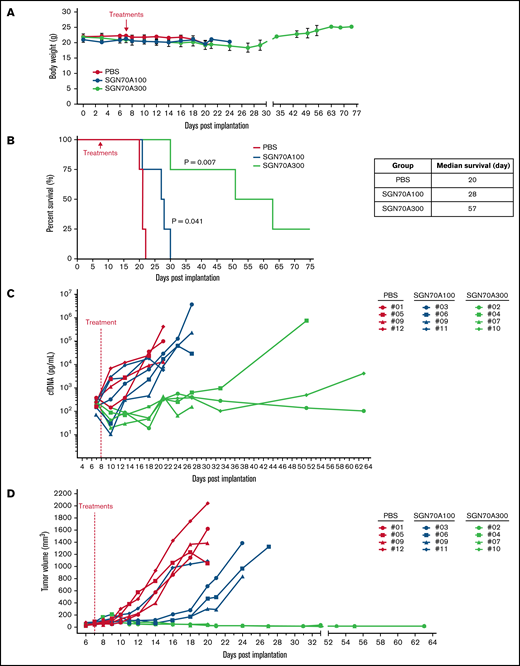
The pharmacokinetics (PKs) of SGN-CD70A in mice has been studied, showing a half-life of 12.3 days at the dose level of 300 μg/kg. In Raji lymphoma xenografts, mice treated at 100 μg/kg and 300 μg/kg had a survival advantage relative to untreated mice.18 To investigate the antitumor activity of SGN-CD70 in CTCL PDXs, we first conducted a mouse phase 1-like dose-finding trial to determine the best dose level to assess efficacy. We selected PRS-1 for this trial because, based on the above ex vivo experiments, PRS-1 has the highest level of CD70 expression and the most robust apoptosis induction when treated with SGN-CD70A ex vivo (Figure 3A,D). We treated 3 groups of PRS-1-bearing mice, with 4 mice in each group: group 1 was a control group treated with PBS, and groups 2 and 3 were treated with a single dose of 100 μg/kg and 300 μg/kg SGN-CD70A, respectively. Treatment tolerability and response were measured in each group. We found no differences in body weight between the PBS-treated and the 2 SGN70A-treated groups (Figure 4A), indicating good tolerability of SGN-CD70A at the dose levels of 100 μg/kg and 300 μg/kg. Furthermore, SGN-CD70A treatment significantly prolonged the survival of tumor-bearing animals relative to the control group. The median survivals of the control group and the 2 groups treated with 100 and 300 μg/kg of SGN CD70A were 20 days, 28 days, and 57 days, respectively; and the survival difference between the SGN-CD70A 300 and the control group was highly significant, with P = .0069 (Figure 4B).
Antitumor activity of SGN-CD70A in CTCL PDX mice. The body weight (A) and the survival (B) of mice treated with PBS, or SGN-CD70A at 100 or 300 ug/kg in the dose-finding trial. The cfDNA concentration (C) and the tumor volume (D) in mice treated with PBS, or SGN-CD70A at 100 or 300 ug/kg (n = 4 per group). NSG mice were implanted with PRS-1 primary tumor cells subcutaneously at the right flank on day 0. On day 7, mice in each group were administered intraperitoneally a single dose of PBS, 100 or 300 μg/kg of SGN-CD70A (SGN70A100 and SGN70A300). The tumor volume and cfDNA were presented as mean ± SEM and statistically analyzed by an unpaired t test with a 2-tailed P value. P < .05 represents as a significant difference. The survival curves were generated by the Kaplan-Meier method and analyzed by the Mantel-Cox test.
Antitumor activity of SGN-CD70A in CTCL PDX mice. The body weight (A) and the survival (B) of mice treated with PBS, or SGN-CD70A at 100 or 300 ug/kg in the dose-finding trial. The cfDNA concentration (C) and the tumor volume (D) in mice treated with PBS, or SGN-CD70A at 100 or 300 ug/kg (n = 4 per group). NSG mice were implanted with PRS-1 primary tumor cells subcutaneously at the right flank on day 0. On day 7, mice in each group were administered intraperitoneally a single dose of PBS, 100 or 300 μg/kg of SGN-CD70A (SGN70A100 and SGN70A300). The tumor volume and cfDNA were presented as mean ± SEM and statistically analyzed by an unpaired t test with a 2-tailed P value. P < .05 represents as a significant difference. The survival curves were generated by the Kaplan-Meier method and analyzed by the Mantel-Cox test.
Tumor-associated cfDNA in each animal was measured throughout the trial period as a surrogate for tumor burden, as reported previously.20 We found that cfDNA concentration sharply declined to a level below the baseline after SGN-CD70A dosing in 6 of 8 animals (2 of 4 in the SGN-CD70A 100 group and 4 of 4 in the SGN-CD70A 300 group). In addition, the cfDNA concentration paralleled the tumor progression during the course of treatment in each animal and peaked immediately before the animals were sacrificed per institutional guidelines, indicating that tumor progression, instead of drug toxicity, was the cause of death in these animals (Figure 4C). We previously showed that PRS-1, being an MF PDX model, reproducibly developed a cutaneous tumor lesion, as seen in MF patients, at the inoculation site.20 We found that treatment with SGN-CD70A delayed the progression of the tumor lesion in a dose-dependent manner (Figure 4D). These results demonstrated that SGN-CD70A not only delayed lymphoma progression but also had cytotoxic activity against the tumor, leading to survival prolongation in a dose-dependent manner. Since the treatment was well tolerated at all dose levels, we chose 300 μg/kg in subsequent experiments to evaluate the potential of SGN-CD70A in generating long-term survival in CTCL PDXs.
Phase 2-like trial to evaluate the efficacy of SGN-CD70A in CTCL PDXs
In the dose-finding trial, a single dose of SGN-CD70A was administered. Despite an initial reduction of tumor burden, all animals eventually had disease progression. We hypothesized that if the loss of response was not due to diminished expression of the target for SGN-CD70A, multiple dosing may result in the long-term survival of treated animals. To test this hypothesis, we first collected splenocytes from mice that died of disease progression in the mouse dose-finding trial and showed that CD70 was still highly expressed in tumor cells, which provided the rationale for multiple dosing (supplemental Figure S1).
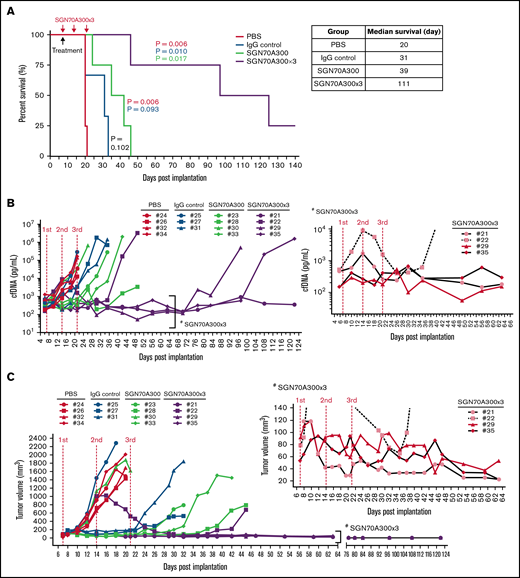
Next, we performed a therapeutic trial comparing the efficacy of treatment with a single dose (n = 4) vs triple doses of SGN-CD70A (n = 4) in CTCL PDXs. We used 2 control groups: one treated with PBS (n = 4) and the other treated with isotype IgG control conjugated with the PBD dimer (n = 3). Both SGN-CD70A-treated groups survived significantly longer as compared with either of the control groups. The median survival of the triple-dose (SGN-CD70A300x3) group was 111 days, vs 20 days in the PBS-treated group (P = .0058), and 31 days in the IgG control-treated group (P = .0101). Furthermore, the triple-dose group survived longer than the single-dose group, 111 days vs 39 days (P = .0169). In the triple-dose group, we observed a reduction of tumor burden measured by cfDNA and regression of the cutaneous tumor lesion following each dose of SGN-CD70A administration (Figure 5B,C insets). At the best response, the cfDNA level in 3 of 4 mice in the triple-dose group (#21, #29, and #35) dropped below the baseline. Additionally, we observed regression of tumor lesions in 3 of 4 mice (#21, #29, and #35) (Figure 5C, inset). These results indicated that SGN-CD70A cannot only inhibit tumor growth but also eradicate established tumors. Finally, when comparing the survival of animals receiving at least 1 dose of SGN-CD70A (n = 8) with those in the 2 control groups (n = 7), SGN-CD70A treatment reduced the risk of death by 76% (hazard ratio, 0.23; 95% confidence interval, 0.051-0.885; P = .0028). These results demonstrated that multiple dosing of SGN-CD70A was well tolerated and could eradicate established tumors, and significantly reduced the risk of death in tumor-bearing animals.
Multiple dosing of SGN-CD70A eradicated established tumors, leading to long-term survival of tumor-bearing PDXs. The survival rate (A), serial measurements of cfDNA concentration (B), and tumor size in individual mice (C) throughout the drug therapy in 4 treatment groups, PBS control, IgG isotype control of SGN-CD70A, and 1 dose vs 3 weekly doses of SGN-CD70A. NSG mice were implanted with PRS-1 primary tumor cells subcutaneously at the right flank on day 0. On day 7, mice were divided into 4 groups and administrated intraperitoneally a single dose of PBS (n = 4), IgG control (n = 3), 300 μg/kg of SGN-CD70A (CD70A300, n = 4), or 3 weekly doses of SGN-CD70A (SGN70A300x3) as indicated. The tumor volume and cfDNA were represented as mean ± SEM and statistically analyzed by an unpaired t test with 2-tailed P value. P < .05 represents as a significant difference. The survival curves were generated by the Kaplan-Meier method and analyzed by the Mantel-Cox test.
Multiple dosing of SGN-CD70A eradicated established tumors, leading to long-term survival of tumor-bearing PDXs. The survival rate (A), serial measurements of cfDNA concentration (B), and tumor size in individual mice (C) throughout the drug therapy in 4 treatment groups, PBS control, IgG isotype control of SGN-CD70A, and 1 dose vs 3 weekly doses of SGN-CD70A. NSG mice were implanted with PRS-1 primary tumor cells subcutaneously at the right flank on day 0. On day 7, mice were divided into 4 groups and administrated intraperitoneally a single dose of PBS (n = 4), IgG control (n = 3), 300 μg/kg of SGN-CD70A (CD70A300, n = 4), or 3 weekly doses of SGN-CD70A (SGN70A300x3) as indicated. The tumor volume and cfDNA were represented as mean ± SEM and statistically analyzed by an unpaired t test with 2-tailed P value. P < .05 represents as a significant difference. The survival curves were generated by the Kaplan-Meier method and analyzed by the Mantel-Cox test.
Discussion
SGN-CD70A is a novel ADC consisting of a humanized anti-CD70 monoclonal IgG1 and a cytotoxic DNA-cross-linking agent, PBD dimer. It exhibits potent antitumor activity in a variety of tumor cell lines and xenograft models, including those that overexpress multidrug-resistant genes, suggesting that SGN-CD70A may overcome common chemoresistance mechanisms. Several studies have shown that CD70 is expressed in a variety of solid tumors and hematologic malignancies; however, whether CD70 is a suitable target for ADCs in T-cell lymphomas has not been established.
In this study, we showed that CD70 is particularly highly expressed in CTCL. In our cohort of 20 cases, 92% of MF and 100% of pcALCL cases were positive for CD70, which was defined as >5% lymphocytes expressing CD70. Additionally, 46% of MF and 14% of pcALCL expressed CD70 in >50% of lymphocytes. We also demonstrated that the cytotoxic activity of SGN-CD70A was only seen in CD70-expressing primary tumor cells and CTCL cell lines. Of note, we observed variable growth inhibition activities of SGN-CD70A in different CTCL cell lines. This may be due to differences in internalization rates, interacting partners, or glycosylation in different cell lines that account for varying affinities.
One of the major barriers for drug development in an orphan disease like CTCL is the lack of a suitable drug testing platform. There are few CTCL cell lines available, and xenografts established from these cell lines do not exhibit the clinical syndrome of CTCL seen in patients. In order to overcome this barrier, we developed CTCL PDX models as described previously.20 In this study, we demonstrated how drug testing for antitumor activity can be conducted systematically using the CTCL PDX platform, from ex vivo studies in primary tumor cultures to dose-finding and efficacy therapeutic trials in PDX animals, which mirror phase 1 and phase 2 studies in humans. Importantly, we were able to assess tumor response using serial measurements of tumor-associated cfDNA in living animals, which allowed us not only to accurately evaluate minimal residual tumor burden in a disease model with a disseminated tumor. Furthermore, it enalbled us to determine whether the death of an animal was due to disease progression or other causes, such as drug toxicity in the animals. Using this platform, we demonstrated that, ex vivo, SGN-CD70A can inhibit cell proliferation and induce apoptosis in CD70-expressing CTCL cells; and, in vivo, it not only inhibited tumor growth but also could completely eradicate an established tumor, leading to the long-term survival of treated animals. Of note, we found that CD27, the ligand of CD70, was not expressed on the tumor cells from any of the PDXs (supplemental Figure S2), suggesting that, at least ex vivo, interference of CD70-CD27 interaction is unlikely to be a major contributor to the antitumor activity of SGN-CD70A.
SGN-CD70A has been tested in phase 1 trials for B-cell lymphomas and metastatic renal cell carcinoma.18,22 In the B-cell lymphoma trial, 20 heavily-pretreated patients with DLBCL and mantle cell lymphoma were treated every 3 weeks (q3wk) and every 6 weeks (q6wk). ORR was 20%, with 8% in the q3wk group and 38% in the q6wk group, respectively. One of the major toxicities in this patient population is frequent high-grade thrombocytopenia, which presented a challenging barrier to the clinical development of SGN-CD70A in B-cell lymphomas. Even though SGN-CD70A only has modest clinical activity in B-cell lymphomas, given a high level of CD70 expression, it may have higher activity in CTCL, a disease with unmet needs and paucity of active drugs. In addition, our promising data may make it worthwhile investigating the mechanism of thrombocytopenia associated with SGN-CD70A.
ADC-induced thrombocytopenia has been observed with other ADCs, such as trastuzumab emtansine (T-DM1), an IgG1 monoclonal antibody targeting HER-2 conjugated to a cytotoxic agent DM1. Uppal and colleagues investigated the potential mechanism of T-DM1-induced thrombocytopenia.23 They demonstrated that the Fc on the IgG1 antibody, trastuzumab, binds to the FcγRIIa on the megakaryocytes (the precursor of platelets), resulting in internalization of T-DM1. Subsequently, DM1 is released intracellularly, leading to impairment of megakaryocytic maturation to platelets. To corroborate this observation, they further demonstrated that the cytotoxic effect of DM1 on megakaryocytes can be mitigated by engineering a mutation in Fc, which interferes with the Fc-FcγRIIa interaction. This example highlights the possibility of designing new CD70-directed ADCs that can circumvent undesired toxicities seen in SGN-CD70A. In fact, a new anti-CD70 ADC, SEA-CD70, is currently in phase 1 clinical trials (Clinicaltrials.gov, ID NCT04227847).
Advanced stage CTCL patients who require systemic therapy are often treated with the same agents used for systemic B- or T-cell lymphoma patients. However, these 2 patient populations seem to have differences in terms of treatment efficacy and tolerability. Most notably, it has been shown that some agents can be administered at a lower dose in CTCL patients without compromising efficacy. For instance, the standard dose of alemtuzumab is 30 mg 3 times a week. In Sézary patients, 10 mg 3 times per week was used, yielding ORR 90% and a complete response of 30%. There were no infection complications observed with this reduced dosing schedule, whereas the standard dosing of alemtuzumab is associated with a 70% to 80% infection rate.24 Similarly, pralatrexate was used at 30 mg/m2, 6 weeks on with 1 week off, for systemic T-cell lymphoma patients. Horwitz and colleagues conducted a dose deescalation trial in CTCL patients, which demonstrated that at a dose of 15 mg/m2, 3 weeks on with 1 week off, CTCL patients can achieve significant disease control with ORR 45% with reduced toxicity.25 These examples provide a rationale to explore dose level and schedule specifically in CTCL patients, which may be able to mitigate the toxicities seen in other subtypes of lymphomas without compromising the efficacy. The PKs of SGN-CD70A have been established in 2 phase 1 clinical trials showing an elimination half-life of 3 to 5 days, undetectable levels of the free cytotoxic agent, 2 partial responses observed, and recommended phase 2 dosing of 30 μg/kg on a 6-week interval to allow for recovery from thrombocytopenia.18 The phase 1 work for this agent is complete, and efficacy testing in new disease cohorts suggested by preclinical studies can be undertaken without delay.
In summary, CD70 is highly expressed in subtypes of CTCL, MF, and pcALCL. SGN-CD70A inhibits cell growth and induces apoptosis in primary CTCL tumor cells. In CTCL PDX models, SGN-CD70A can eradicate established tumors measured by tumor-associated cfDNA, which translates to long-term survival and a 76% reduction in the risk of death in treated animals. These compelling preclinical data support clinical investigation of SGN-CD70A and perhaps other CD70-directed ADCs in CTCL.
Acknowledgments
The authors thank Arthur Weiss for his advice on the project and Matthew L. Springer for his critical review of the manuscript.
This work was supported by generous gifts from the Martin and Dorothy Spatz Foundation, Summit Bank Foundation, and KL Felicitas Foundation (to W.Z.A.).
Authorship
Contribution: C.-H.W., C.-Y.Y., W.Z.A. provided conception and design and developed methodology; C.-H.W., C.-Y.Y., L.W., F.M., K.W.W., R.G., B.H., L.P., and W.Z.A. acquired data (provided animals, acquired samples from patients, provided facilities, etc.); C.-H.W., C.-Y.Y., L.W., M.M., and W.Z.A. provided analysis and interpretation of data; and C.-H.W., L.W., C.-Y.Y., K.W.W., B.H., R.G., F.M., M.M., L.P., and W.Z.A. wrote, reviewed, and revised the manuscript.
Conflict-of-interest disclosure: C.-H.W. reports research support from Nurix Therapeutics outside the submitted work. C.-Y.Y. is an employee for AbbVie and has an immediate family member who is an employee for Bayer. F.M. is a consultant for the following companies: Amgen, Daiichi Ltd., Frontiers Med, Exuma Biotech, Ideaya Biosciences, Kura Oncology, Leidos Biomedical Research Inc., PellePharm, Pfizer Inc., PMV Pharma, and Quanta Therapeutics; is a consultant and cofounder for the following companies (with ownership interest including stock options): BridgeBio, DNAtrix Inc., Olema Pharmaceuticals Inc., and Quartz; is the scientific director of the National Cancer Institute (NCI) RAS Initiative at the Frederick National Laboratory for Cancer Research/Leidos Biomedical Research Inc., and; has been the recipient of research grants from Daiichi Sankyo and Gilead Sciences and has a current grant from Boehringer-Ingelheim. W.Z.A. reports honoraria for consulting/advisory roles from Acrotech Biopharma, BeiGene, ADC Therapeutics, Kite Pharma, Kymera Therapeutics, and research support from Nurix Therapeutics outside the submitted work. All other authors declare no competing financial interests.
Correspondence: Weiyun Z. Ai, Division of Hematology and Oncology, Department of Medicine, University of California, San Francisco, 400 Parnassus Ave, 4th Floor, San Francisco, CA 94143; e-mail: Weiyun.Ai@ucsf.edu.
References
Author notes
Requests for data sharing may be submitted to Weiyun Z. Ai (weiyun.ai@ucsf.edu).
The full-text version of this article contains a data supplement.