Key Points
JAM-C identifies a distinct MM cell population in bone marrow of patients and mice.
Targeting JAM-C ameliorates MM progression and offers potential therapeutic options that might complement standard treatment regimens.
Abstract
Deregulation such as overexpression of adhesion molecules influences cancer progression and survival. Metastasis of malignant cells from their primary tumor site to distant organs is the most common reason for cancer-related deaths. Junctional adhesion molecule-C (JAM-C), a member of the immunoglobulin-like JAM family, can homodimerize and aid cancer cell migration and metastasis. Here we show that this molecule is dynamically expressed on multiple myeloma (MM) cells in the bone marrow and co-localizes with blood vessels within the bone marrow of patients and mice. In addition, upregulation of JAM-C inversely correlates with the downregulation of the canonical plasma cell marker CD138 (syndecan-1), whose surface expression has recently been found to dynamically regulate a switch between MM growth in situ and MM dissemination. Moreover, targeting JAM-C in a syngeneic in vivo MM model ameliorates MM progression and improves outcome. Overall, our data demonstrate that JAM-C might serve not only as an additional novel diagnostic biomarker but also as a therapeutic target in MM disease.
Introduction
Multiple myeloma (MM) is a B-cell malignancy with clonally expanding antibody-producing plasma cells in the bone marrow (BM) that has a significant impact on morbidity and mortality. Its incidence has steadily increased in the past few decades. Although there have been therapeutic advances and major improvements in transplantation techniques and new targeted therapies, including novel agents (eg, chimeric antigen receptor T cells, bi-specific T-cell engagers, monoclonal antibodies (mAbs), and checkpoint inhibitors), MM remains incurable and has the lowest 5-year survival rate (∼40%) of the 3 blood cancers that occur most often (ie, lymphoma, leukemia, and myeloma).1 MM progression seemingly depends on interactions with the local microenvironment in the BM.2,3 The cell adhesion-mediated drug resistance of MM cells interacting with BM stromal cells and extracellular matrix components poses a major challenge in MM therapy.4,5 With its specialized endosteal and vascular niches, the BM provides a supportive microenvironment for maintaining hematopoietic and nonhematopoietic stem cells as well as long-term immune memory cells.6 Notably, disseminated tumor cells in the BM can persist in a dormant state for many years until they progress to macrometastatic lesions.7 Dissemination of malignant cells from a primary tumor site to distant tissues and organs is the most prominent cause for cancer-related deaths, which may also apply for MM, although it predominantly disseminates in the skeleton where it initiates a characteristic myeloma bone disease.8,9
To leave their site of origin, MM cells need to loosen their anchoring connection to cells of their individual primary niche within the BM and to interact with cells of the blood vessel (BV) system such as endothelial cells (ECs). Disseminating malignant cells will also need to migrate through connective tissue and enter the blood circulation using adhesion molecules such as integrins and immunoglobulin-like proteins such as members of the junctional adhesion molecule (JAM) family, as described for common leukocytes.10 JAMs have been described to support leukocyte recruitment and promote leukocyte movement through intercellular junctions.11 Because the cell adhesion and migration system in the MM microenvironment has been recognized as supporting MM cell survival, progression, and development of drug resistance, it became a key target in MM treatment.12,13 However, the specific role and function of JAMs in maintaining the MM microenvironment and its potential role in MM dissemination within and outside the BM remains largely elusive. It has recently become evident that signaling through adhesion molecules regulates MM-BM microenvironment interactions and that their overexpression and dysregulation is implicated in cancer pathogenesis.14 In a previous study, we identified a member of the JAM family (JAM-A) as a promising candidate to target MM stroma- EC interactions. JAM-A is constitutively expressed on primary patient-derived MM cells and on human MM cell lines (eg, RPMI 8226). JAM-A expression on MM cells segregated disease outcome, and higher JAM-A expression correlated with poor prognosis.15,16 Others have shown that targeting another member of the JAM family, JAM-C, prevented tumor engraftment in a xenograft model of mantle cell lymphoma and exhibited an anti-angiogenic effect in lung carcinoma tumor grafts. In addition, the dimerization of that molecule aided cancer cell migration and metastasis in a mouse lung squamous cell carcinoma model and was shown to mediate the recruitment of embryonic endothelial progenitor cells during tumor angiogenesis.17-20
The initial Relating Clinical Outcomes in Multiple Myeloma to Personal Assessment of Genetic Profile (CoMMpass) trial21 from the Multiple Myeloma Research Foundation (MMRF) revealed that patients with MM cells with high expression of cell adhesion molecule JAM-C had significantly lower progression-free survival (PFS) and overall survival (OS) compared with the patients with lower gene expression.
Here we report that JAM-C expression is upregulated in vivo in a syngeneic murine MM model, which may be crucial for distinct advanced stages of the disease such as the process of MM dissemination.22,23 Notably, we found that this induced JAM-C expression was closely associated with the previously reported dynamic regulation of CD138, which demarcates a switch between in situ growth and proliferation and a more disseminative status of MM.24 So we explored, visualized, and quantified the dynamic expression of JAM-C and CD138 on MM cells interacting with BM endothelium. We also wanted to target JAM-C and determine the subsequent effect on MM progression and dissemination in our syngeneic mouse model of MM bone disease. Subsequently, we confirmed our findings on the basis of our mouse model in the human and clinical situations and have also detected this novel CD138low/neg JAM-C+ population in primary human MM BM biopsies by flow cytometry.
Materials and methods
Ethics statement
Approval for the study was obtained from the Institutional Ethics Committee of Würzburg University Hospital and Bari University. Written informed consent was obtained from all patients with MM, and all clinical investigations were conducted in accordance with the Declaration of Helsinki. All animal experiments were performed in accordance with the German regulations for animal experimentation.
Cell lines and antibodies
The firefly luciferase and MOPC-315.BMP2.FUGLW23 MM cell line (which expresses enhanced green fluorescent protein [eGFP]) was cultured in complete RPMI 1640 medium: RPMI 1640 medium supplemented with 20% v/v heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 g/mL streptomycin, 1 mM sodium pyruvate, and 2 mM glutamine (all from Life Technologies, Karlsruhe, Germany). Murine EC line EOMA (ATCC CRL-2586; LGC Standards, Wesel, Germany) was maintained according to manufacturer’s instructions in EC medium (PELOBIOTECH, Planegg/Martinsried, Germany). The human MM cell line RPMI 8226 (German Collection of Microorganisms and Cell Culture, Braunschweig, Germany) was maintained in complete RPMI 1640 medium, and human umbilical vein endothelial cells (HUVECs) were cultured in EGM MV2 medium (PromoCell, Heidelberg, Germany). Details regarding antibodies used in this study are provided in the supplemental Materials and methods.
Confocal microscopy and 3D-light sheet fluorescence microscopy (LSFM) of bone
To depict the BM-infiltrating MM cells at a single-cell resolution within the intact BM compartment of MM-harboring mice, we used confocal microscopy as well as the previously described multicolor LSFM technique.25,26 Details regarding experimental procedures and image analysis are provided in supplemental Materials and methods.
Direct in vitro co-culture experiments
EOMA ECs were seeded at a density of 2.5 × 105 cells per 4 mL of EC medium in a 6-well plate and left to settle in an incubator for at least 5 hours. Afterward, 5 × 105 MM cells (MOPC-315.BMP2.FUGLW) resuspended in 4 mL MM medium were seeded directly on top of settled ECs, which resulted in a mixed cell culture in 8 mL cocktail medium that was left untouched for specific time intervals. Then, 5 × 105 MM cells were incubated without ECs in 8 mL cocktail medium for the same time intervals to serve as a control.
For experiments that included treatment, bortezomib was used at a final concentration of 2.5 ng/mL. Subsequently, MM cells were harvested by flushing them off the ECs followed by centrifugation. Pelleted cells were immediately and completely processed for further analysis. Corresponding co-culture experiments using human endothelial and MM cell lines were performed by using 4 × 104 HUVECs and 1.5 × 105 RPMI 8226 cells per 2 mL in a 24-well format. Details regarding flow cytometry and quantitative real-time polymerase chain reaction (qRT-PCR) are provided in supplemental Materials and methods.
In vivo anti–JAM-C treatment of MM-bearing mice
Female 8- to 10-week-old BALB/c mice (Charles River, Sulzfeld, Germany) were injected intratibially with 1 × 105 MOPC-315.BMP2.FUGLW cells in 15 µL of phosphate-buffered saline (PBS). One day after injection, mice were monitored with in vivo bioluminescence imaging (BLI) to confirm MM engraftment. The mice were then randomly assigned to 1 of 2 groups, each comprising mice with comparable initial tumor signal (9 treated vs 8 isotype controls). Treatment with mAbs started 1 day after MM cell injection (day 0). Mice were treated with anti–JAM-C mAb (clone #42) 100 µg/20 g body weight intraperitoneally (IP) (in 100 µL of PBS) or with a matched isotype control mAb (clone #238) 100 µg/20 g body weight IP (in 100 µL PBS). Antibodies were administered 3 times per week (9 administrations IP) on days 0, 2, 5, 7, 9, 12, 14, 16, and 19. On day 21, mice were euthanized, bones were subsequently imaged ex vivo (BLI), and BM was analyzed by flow cytometry.
Results
Patients with high expression of JAM-C have worse PFS and OS
In line with our previous study, which explored the impact of aberrant JAM-A expression on MM disease progression and outcome, we again examined the MMRF CoMMpass study21 regarding possible similar effects of divergent JAM-C expression on MM. These analyses discovered that patients with MM cells showing high JAM-C expression had a significantly shorter PFS compared with the patients with a lower JAM-C expression (median PFS, 1259 vs 773 days; hazard ratio, 1.98; 95% confidence interval, 1.44-2.72) (Figure 1A). Moreover, high JAM-C expression significantly correlated with shorter OS (median survival not reached vs 1590 days; hazard ratio, 1.66; 95% confidence interval, 1.08-2.54) (Figure 1B). JAM-C expression has already been described as relevant for hematogenous metastasis and invasion of solid cancers as well as for angiogenesis and tumor growth.19,20,27-30 Therefore, we hypothesized that JAM-C expressed on MM might be a relevant factor for vascular endothelial interactions and MM dissemination, which prompted us to investigate the mechanics of aberrant JAM-C expression in detail in our functional MM in vitro systems (murine and human), in vivo in our preclinical MM mouse model, and in primary patient-derived BM biopsies.
JAM-C high-expressing patients show worse PFS and OS. (A) PFS and (B) OS from the MMRF CoMMpass database regarding JAM-Chigh and JAM-Clow expressors. RNA sequencing (RNA-seq) expression derived from CD138-enriched cells from 572 patients with MM; JAM-Clow vs JAM-Chigh expressors are compared.
JAM-C high-expressing patients show worse PFS and OS. (A) PFS and (B) OS from the MMRF CoMMpass database regarding JAM-Chigh and JAM-Clow expressors. RNA sequencing (RNA-seq) expression derived from CD138-enriched cells from 572 patients with MM; JAM-Clow vs JAM-Chigh expressors are compared.
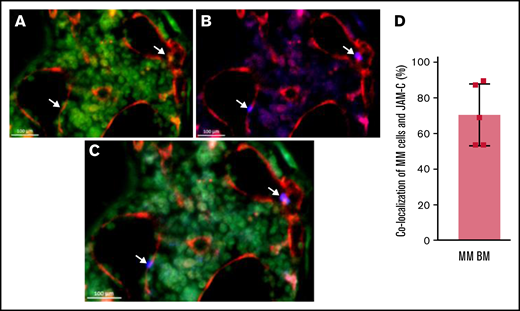
MM cells co-localize with BVs within their BM niche
To further investigate and visualize MM cells within their intact three-dimensional (3D) BM niche architecture, we first quantified MM cells co-localizing with ECs of the BV system within the intact 3D BM environment by using LSFM on cleared long bones (supplemental Figure 1A-B; supplemental video 1) of MM-harboring mice. Analysis of segmented MM and BV signals (supplemental Figure 1C-E) revealed that ∼60% of MM cells were in direct contact with vascular ECs (supplemental Figure 1F). Next, we wondered whether JAM-C could be involved in MM-EC interactions. Again using LSFM, our analysis of 3D-reconstructed multichannel stacks of segmented MM and BV signals revealed that ∼60% to 80% of MM cells were in direct contact with vascular ECs and that there is a 70% spatial co-localization of MM and JAM-C signal in the vicinity of BVs. Furthermore, JAM-Chigh cells were positioned in the immediate vicinity of BVs. Interestingly, these JAM-Chigh– expressing cells seemed to have a diminished CD138 signal (Figure 2; supplemental video 2). On the basis of these findings, we analyzed mice with established MM by using confocal microscopy on long bone sections compared with nonaffected BM compartments of the same mouse. These images showed a specific surface molecule expression signature as well as a remarkable spatial cellular distribution pattern which was not detectable in nonaffected BM compartments (Figure 3A-B).
JAM-C signal co-localizes largely with BVs and CD138. Representative LSFM of intact long bones of an MM-bearing mouse. (A) Channel signals for BVs are shown in red (CD144-AF647) and CD138 signals are shown in green (CD138-AF488). (B) Channel signals for BVs are shown in red and JAM-C signals are shown in blue (AF555). (C) Overlay of panels A and B. Arrows indicate MM cells in proximity with BVs display remarkably high JAM-C signal and low CD138 expression. (D) Graphical analysis of co-localizing MM cells with JAM-C-signal within the BM of MM-harboring mice (n = 5). Error bars indicate standard deviation (SD).
JAM-C signal co-localizes largely with BVs and CD138. Representative LSFM of intact long bones of an MM-bearing mouse. (A) Channel signals for BVs are shown in red (CD144-AF647) and CD138 signals are shown in green (CD138-AF488). (B) Channel signals for BVs are shown in red and JAM-C signals are shown in blue (AF555). (C) Overlay of panels A and B. Arrows indicate MM cells in proximity with BVs display remarkably high JAM-C signal and low CD138 expression. (D) Graphical analysis of co-localizing MM cells with JAM-C-signal within the BM of MM-harboring mice (n = 5). Error bars indicate standard deviation (SD).
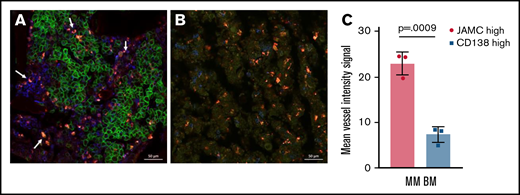
JAM-C signal is enhanced in the vicinity of BV signals but CD138 signal is diminished in these areas. Representative confocal microscopy image of intact long bones of (A) an MM-bearing mouse in comparison with (B) a nonaffected BM compartment (n = 3 mice); channel signals for BVs are shown in red (CD144-AF647), for CD138 are shown in green (CD138-AF488), and for JAM-C are shown in blue (JAM-C-AF555). Arrows indicate MM cells in proximity with BV signals that display higher JAM-C signal and lower CD138 expression. (C) Graphical analysis of mean fluorescence signal intensity of CD144 comparing CD138high and CD138low/neg JAM-C+ cells in MM-harboring BM regions. Unpaired, two-tailed Student t tests were used. Error bars indicate SD.
JAM-C signal is enhanced in the vicinity of BV signals but CD138 signal is diminished in these areas. Representative confocal microscopy image of intact long bones of (A) an MM-bearing mouse in comparison with (B) a nonaffected BM compartment (n = 3 mice); channel signals for BVs are shown in red (CD144-AF647), for CD138 are shown in green (CD138-AF488), and for JAM-C are shown in blue (JAM-C-AF555). Arrows indicate MM cells in proximity with BV signals that display higher JAM-C signal and lower CD138 expression. (C) Graphical analysis of mean fluorescence signal intensity of CD144 comparing CD138high and CD138low/neg JAM-C+ cells in MM-harboring BM regions. Unpaired, two-tailed Student t tests were used. Error bars indicate SD.
Most strikingly, the JAM-Chigh–expressing cells in close proximity to BVs apparently expressed lower CD138 levels compared with the neighboring MM cells as shown by both microscopy techniques (Figures 2A-C and 3A). Statistical analyses of mean fluorescent intensity signals comparing CD138high and CD138low/neg JAM-Chigh cell populations revealed significantly higher vessel intensities in areas where the latter population was predominantly located (Figure 3C).
However, limited penetration depth, limited availability of fluorescence channels regarding confocal images, and high autofluorescent background of cleared BM samples processed for LSFM rendered these microscopic evaluations neither adequate nor sensitive enough to discriminate small dynamic and graded differences in CD138 expression on individual MM cells, so it was not possible to determine their distinct cellular phenotype. Therefore, we used multiparameter flow cytometry to quantify subtle dynamic changes in JAM-C and CD138 expression on individual BM-infiltrating MM cells and to unequivocally determine their malignant cellular identity.
Histopathologic analysis of a primary BM biopsy from a patient with MM identified MM cells growing in a perivascular and interstitial manner (supplemental Figure 2A). Overall, JAM-C is widely expressed showing highest intensity in the perivascular compartment (supplemental Figure 2B). However, more primary BM-derived biopsies from newly diagnosed patients and at different time points during disease progression will be needed to further elucidate the exact role of JAM-C in the human setting.
Differential JAM-C and CD138 expression on murine MM cells in vivo
To investigate a possible function of JAM-C and CD138 in MM interaction with BM vascular ECs and subsequent MM dissemination, we used flow cytometry to analyze the surface expression of CD138 and JAM-C on BM cells and peripheral blood (PB) cells from MM-bearing mice. Mouse MM cells could be unequivocally identified by eGFP reporter gene expression. Notably, eGFP+ MM cells in the BM expressing high JAM-C levels displayed a CD138low/neg phenotype in contrast to CD138high MM cells (Figure 4A-B). Similarly, in the PB, eGFP+ JAM-C-expressing MM cells expressed lower or no CD138 (Figure 4B).
CD138low/neg MM cells upregulate JAM-C in murine BM. (A) Flow cytometry of living eGFP+ MM cells in murine BM defines CD138high and CD138low/neg MM cells; the latter show significantly elevated JAM-C expression. The horizontal lines represent the mean MFI. (B) Statistical analyses of MM cells in BM regarding CD138 and JAM-C expression (percentages of GFP+ cells). JAM-C expressing (mean fluorescent intensity [MFI]) BM and PB cells gated on living and GFP+ cells divided into CD138high and CD138low/neg cells of MM mice (n = 6) at day 21 after MM cell injection. Unpaired, two-tailed Student t tests were used. Error bars indicate SD.
CD138low/neg MM cells upregulate JAM-C in murine BM. (A) Flow cytometry of living eGFP+ MM cells in murine BM defines CD138high and CD138low/neg MM cells; the latter show significantly elevated JAM-C expression. The horizontal lines represent the mean MFI. (B) Statistical analyses of MM cells in BM regarding CD138 and JAM-C expression (percentages of GFP+ cells). JAM-C expressing (mean fluorescent intensity [MFI]) BM and PB cells gated on living and GFP+ cells divided into CD138high and CD138low/neg cells of MM mice (n = 6) at day 21 after MM cell injection. Unpaired, two-tailed Student t tests were used. Error bars indicate SD.
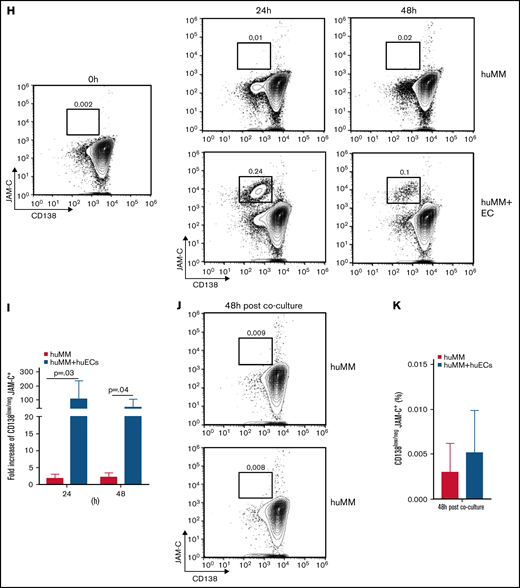
JAM-C upregulation correlates with decreased CD138 expression in vitro
To recapitulate in vivo findings (Figure 4), we performed direct co-culture assays of mouse MOPC-315.BMP2.FUGLW MM cells and mouse ECs (EOMA cells). EC-MM cell co-culture led to the emergence of an MM cell population that displayed a decreased CD138 surface expression and simultaneous upregulation of JAM-C over time (Figure 5A). The frequency of CD138low/neg/JAM-C+ MM cells significantly increased by about 3.5-fold within 48 hours in an EC-MM co-culture setting compared with MM cells without EC contact (Figure 5B). This inverse regulation of CD138 and JAM-C could be reverted when culturing MM cells from the EC-MM co-culture (after 72 hours) for another 48 hours without ECsin complete growth medium (Figure 5C-D). In line with surface receptor changes in CD138 and JAM-C on a protein level, qRT-PCR confirmed that EC-MM cell co-culture significantly decreased CD138 messenger RNA expression in MM cells within 24 hours, which inversely correlated with a marked upregulation of JAM-C messenger RNA within 48 hours (Figure 5E-F). Like the dynamic changes on a protein level, this process was completely reverted after culturing MM cells from a 72-hour EC-MM coculture again without ECs for 48 hours in complete growth medium (Figure 5G).
Direct contact of MM cells with ECs leads to a dynamic and reversible increase of CD138low/neg JAM-C+ cells in mice and humans. (A) Representative fluorescence-activated cell sorting (FACS) plots of surface CD138 and JAM-C expression on living MM cells at depicted time points of coculture without ECs (MM, upper panel) or with ECs (MM + ECs, lower panel) and (B) corresponding graphical display of increase in cell frequencies. (C) Representative FACS plots of surface CD138 and JAM-C expression on living MM cells grown without ECs for 48 hours in complete growth medium after the end of co-culture without ECs (upper panel) or with ECs (lower panel) and (D) corresponding graphical display of cell frequencies. Normalized relative transcription levels of (E) CD138 and (F) JAM-C messenger RNA (mRNA) within the MM cells cultured with ECs (MM + ECs) or without ECs (MM) determined by quantitative real-time polymerase chain reaction (qRT-PCR). (G) Normalized relative transcription levels of CD138 and JAM-C mRNA within MM cells cultured without ECS for 48 hours in complete growth medium after the end of EC co-culture compared with the expression level at 72 hours depicted in panels E and F (open columns). (H) Representative FACS plots of surface CD138 and JAM-C expression on living RPMI 8226 (MM) cells at depicted time points of co-culture without HUVECs (huMM, upper panel) or with HUVECs (huMM + ECs, lower panel) and (I) corresponding graphical display of increase in cell frequencies. (J) Representative FACS plots of surface CD138 and JAM-C expression on living huMM cells grown without ECs for 48 hours in complete growth medium after the end of co-culture without ECs (upper panel) or with ECs (lower panel) and (K) corresponding graphical display of cell frequencies. All experiments were independently repeated 3 times. Unpaired, two-tailed Student t tests were used. Error bars indicate SD for FACS analyses and standard error of the mean for qRT-PCR results.
Direct contact of MM cells with ECs leads to a dynamic and reversible increase of CD138low/neg JAM-C+ cells in mice and humans. (A) Representative fluorescence-activated cell sorting (FACS) plots of surface CD138 and JAM-C expression on living MM cells at depicted time points of coculture without ECs (MM, upper panel) or with ECs (MM + ECs, lower panel) and (B) corresponding graphical display of increase in cell frequencies. (C) Representative FACS plots of surface CD138 and JAM-C expression on living MM cells grown without ECs for 48 hours in complete growth medium after the end of co-culture without ECs (upper panel) or with ECs (lower panel) and (D) corresponding graphical display of cell frequencies. Normalized relative transcription levels of (E) CD138 and (F) JAM-C messenger RNA (mRNA) within the MM cells cultured with ECs (MM + ECs) or without ECs (MM) determined by quantitative real-time polymerase chain reaction (qRT-PCR). (G) Normalized relative transcription levels of CD138 and JAM-C mRNA within MM cells cultured without ECS for 48 hours in complete growth medium after the end of EC co-culture compared with the expression level at 72 hours depicted in panels E and F (open columns). (H) Representative FACS plots of surface CD138 and JAM-C expression on living RPMI 8226 (MM) cells at depicted time points of co-culture without HUVECs (huMM, upper panel) or with HUVECs (huMM + ECs, lower panel) and (I) corresponding graphical display of increase in cell frequencies. (J) Representative FACS plots of surface CD138 and JAM-C expression on living huMM cells grown without ECs for 48 hours in complete growth medium after the end of co-culture without ECs (upper panel) or with ECs (lower panel) and (K) corresponding graphical display of cell frequencies. All experiments were independently repeated 3 times. Unpaired, two-tailed Student t tests were used. Error bars indicate SD for FACS analyses and standard error of the mean for qRT-PCR results.
Similar to the observations mentioned above, co-culturing the human MM cell line RPMI 8226 with HUVECs induced significant JAM-C upregulation and CD138 downregulation on MM cells within 24 hours. Again, after co-culture, the inverse regulation of JAM-C and CD138 could be reverted within 48 hours by putting RPMI cells back into solitary culture with the corresponding growth medium (Figure 5H-K).
CD138low/neg JAM-C+ MM cells are less proliferative and more resistant to bortezomib treatment
To determine the proliferation status and the effect of JAM-C upregulation and EC interaction on drug sensitivity of this increasing CD138low/neg/JAM-C+ MM cell population, we treated cells after 48-hour murine MM-EC co-culture with bortezomib with a final concentration of 2.5 ng/mL for 24 hours. After treatment, cells were stained for surface expression of CD138 and JAM-C and intracellular levels of Ki-67. Included LIVE/DEAD Fixable Violet Dead Cell Stain was used for discriminating living cells from dead cells (supplemental Figure 3). Emerging CD138low/neg/JAM-C+ cells proliferated at a lower level than CD138high MM cells (supplemental Figure 3B-C) as shown by decreased Ki-67 levels. Furthermore, CD138low/neg/JAM-C+ cells proved to be more resistant to proteasome inhibition and significantly increased in frequency (3.3-fold), whereas treatment with bortezomib basically eradicated the highly proliferative CD138high MM population (supplemental Figure 3B-D).
Targeting JAM-C ameliorates MM progression in vivo
To assess the role of JAM-C expression on MM cells in vivo, we treated immunocompetent syngeneic mice that developed MM locally in the left tibia with our newly established anti–JAM-C–blocking (αJAM-C) mAb (supplemental Figure 4) or a matched isotype control antibody. We followed tumor progression by using sensitive in vivo BLI. MM-derived BLI signals significantly progressed more slowly in αJAM-C–treated mice than in isotype-treated mice (Figure 6A-B). Additional tumor foci in isotype-treated mice significantly appeared earlier and in larger numbers over time compared with αJAM-C–treated mice (Figure 6C). Ex vivo BLI of bones that had been removed from the mice remaining at the end of the experiment revealed an overall higher MM burden in long bones in controls than in αJAM-C–treated mice (Figure 6D). Following animal protocol guidelines, 50% of isotype-treated mice had to be euthanized prematurely because of the very high MM burden measured with BLI and/or a substantial decline in health status. In contrast, all mice in the αJAM-C–treated group could be kept until the end of the experiment without signs of impaired health status (Figure 6E). Furthermore, αJAM-C treatment markedly reduced dissemination of MM to other bones (Figure 6D,F). Fluorescence-activated cell sorting analysis of the BM of these bones confirmed our findings (Figure 6G).
Blocking JAM-C in vivo impairs MM progression and dissemination in tumor-bearing mice. (A) Three representative mice from experimental groups that were treated with either an isotype control (n = 8) or αJAM-C antibody (n = 9) from ventral (left panel) and dorsal (right panel) views over time after treatment. (B) Absolute signal quantification by whole body BLI from ventral views over time after treatment (P = .009; 2-way analysis of variance [ANOVA]). The horizontal lines represent the mean of photons. (C) Number of additional tumor foci within the isotype control (ctrl) and αJAM-C–treated group over time after treatment (P = .031; Wilcoxon test). (D) BLI images of 4 representative bone pairs of isotype-treated (dots) and αJAM-C–treated (squares) mice at day 21 and (F) corresponding graphical and statistical signal quantification (P = .026; 2-way ANOVA). (E) Survival rate of isotype-treated and αJAM-C–treated mice over time after treatment (P = .019; Gehan-Breslow-Wilcoxon test). (G) Frequency of GFP+ cells from BM of isotype- or αJAM-C–treated mice determined by FACS analysis (P = .028; Mann-Whitney U test). Error bars indicate SD. The horizontal lines represent the % of GFP positive cells.
Blocking JAM-C in vivo impairs MM progression and dissemination in tumor-bearing mice. (A) Three representative mice from experimental groups that were treated with either an isotype control (n = 8) or αJAM-C antibody (n = 9) from ventral (left panel) and dorsal (right panel) views over time after treatment. (B) Absolute signal quantification by whole body BLI from ventral views over time after treatment (P = .009; 2-way analysis of variance [ANOVA]). The horizontal lines represent the mean of photons. (C) Number of additional tumor foci within the isotype control (ctrl) and αJAM-C–treated group over time after treatment (P = .031; Wilcoxon test). (D) BLI images of 4 representative bone pairs of isotype-treated (dots) and αJAM-C–treated (squares) mice at day 21 and (F) corresponding graphical and statistical signal quantification (P = .026; 2-way ANOVA). (E) Survival rate of isotype-treated and αJAM-C–treated mice over time after treatment (P = .019; Gehan-Breslow-Wilcoxon test). (G) Frequency of GFP+ cells from BM of isotype- or αJAM-C–treated mice determined by FACS analysis (P = .028; Mann-Whitney U test). Error bars indicate SD. The horizontal lines represent the % of GFP positive cells.
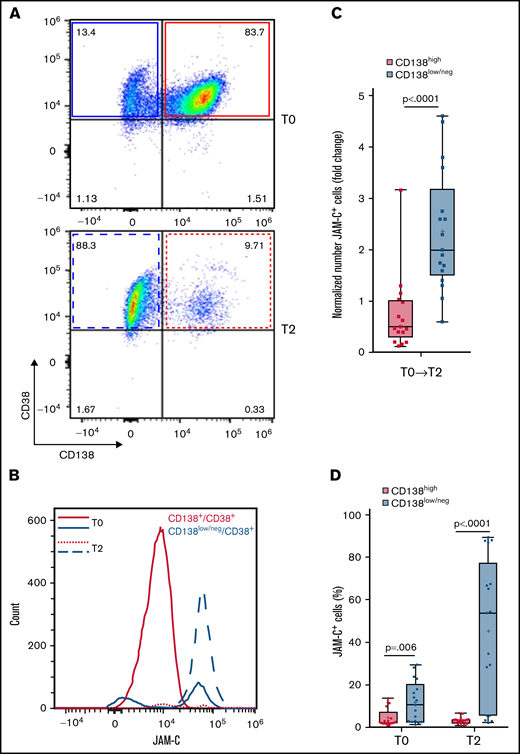
JAM-C is upregulated on CD138low/neg malignant plasma cells in BM of patients with MM
Next, we explored the regulation of JAM-C on malignant plasma cells in patients with MM. Flow cytometry of matched human BM samples from patients with MM (n = 17) at the diagnosis time point (T0) and x +1 relapse (T2) revealed that the JAM-C molecule is almost exclusively upregulated on the surface of CD138low/neg MM cells (Figure 7A-B). Notably, numbers of CD138low/neg JAM-C+ cells (gated on living CD45–CD56+CD38dim/+ cells31 ; detailed gating strategy is provided in supplemental Figure 5) significantly increased from time T0 to T2 within the majority of analyzed patient BM samples, whereas the number of CD138high JAM-C+ cells decreased on trend without being significant (Figure 7B-C). Similar observations were made when we analyzed percentages of CD138low/neg JAM-C+ events and compared them to CD138high JAM-C+ events at T0 and T2 (Figure 7D; supplemental Figure 6). Our results reveal a distinct CD138low/neg JAM-C+ MM population that could be missed by relying on CD138 expression, and they support an increase of CD138low/neg JAM-C+ malignant plasma cells with MM disease progression.
JAM-C is upregulated on CD138low/neg cells in BM of patients with MM. Representative FACS plots of surface CD138 expression on living human MM cells gated on CD45–CD56+CD38dim/+ cells in BM at (A) time of diagnosis (T0) and (B) x+1 relapse (T2). Representative graphical analysis (representative histograms) of JAM-C–expressing cells (count) in BM of patients with MM comparing CD138high (red) and CD138low/neg (blue) cells (gated on living CD45–CD56+CD38dim/+ cells) at T0 and T2. (C) Fold change in total numbers of JAM-C+ cells in BM of patients with MM (n = 17) divided into CD138high and CD138low/neg cells from T0 to T2 (Mann-Whitney U test; error bars indicate SD). The horizontal lines represent the mean. (D) Percentage of JAM-C+ cells in BM of patients with MM comparing CD138high and CD138low/neg populations at T0 and T2 (n = 17). Unpaired, two-tailed Student t tests were used. Error bars indicate SD. The horizontal lines represent the mean.
JAM-C is upregulated on CD138low/neg cells in BM of patients with MM. Representative FACS plots of surface CD138 expression on living human MM cells gated on CD45–CD56+CD38dim/+ cells in BM at (A) time of diagnosis (T0) and (B) x+1 relapse (T2). Representative graphical analysis (representative histograms) of JAM-C–expressing cells (count) in BM of patients with MM comparing CD138high (red) and CD138low/neg (blue) cells (gated on living CD45–CD56+CD38dim/+ cells) at T0 and T2. (C) Fold change in total numbers of JAM-C+ cells in BM of patients with MM (n = 17) divided into CD138high and CD138low/neg cells from T0 to T2 (Mann-Whitney U test; error bars indicate SD). The horizontal lines represent the mean. (D) Percentage of JAM-C+ cells in BM of patients with MM comparing CD138high and CD138low/neg populations at T0 and T2 (n = 17). Unpaired, two-tailed Student t tests were used. Error bars indicate SD. The horizontal lines represent the mean.
Discussion
In this study, we demonstrate for the first time a dynamic regulation of the cell adhesion molecule JAM-C on MM cells upon MM-EC interaction, which inversely correlates with CD138 expression. Visualizing JAM-C expression in intact murine BM and human BM infiltrates revealed JAM-C at the interface of MM with BM vascular ECs, especially within the perivascular compartment that was shown to be crucial for MM cell survival.32 Importantly, JAM-C defined an MM population that was insensitive to proteasome inhibitors. The direct targeting of JAM-C with a blocking antibody in vivo inhibited progression of MM bone disease in mice and reduced overall tumor burden as well as MM dissemination. In line with those findings, JAM-C expression correlated with poor PFS and OS of 577 patients with MM, and we observed the increase of a CD138low/neg JAM-C+ malignant plasma cell population with disease progression from diagnosis to relapse in a retrospective evaluation in 17 patients with MM.
JAMs are commonly thought to have pleiotropic functions in cell physiology and development.33 JAM-C localizes at intercellular junctions, mediates tumor cell-EC interaction and helps leukocytes cross the endothelial barrier.30,34 Dissemination of malignant cells from primary tumor sites to distant organs requires migration through connective tissue and intravasation into BVs, including the process of diapedesis. These early steps are not very well understood, but they likely involve cell adhesion molecules in a way similar to that described for common leukocyte migration during inflammation.10 JAM-C plays a pivotal role in metastasis of solid cancers and acts as a counter-receptor on different cell types. Several previous studies showed JAM-C to be directly involved in cancer cell migration and metastasis.27,28,30,35,36
JAM-C expression delivers an advantage to cancer cells for crucial steps of metastasis (ie, the detachment from the primary tumor and intravasation) as described by Garrido-Urbani19 in a lung carcinoma model, and it is enriched on the surface of highly disseminating human fibrosarcoma variants.,37 Therefore, we asked whether JAM-C may also contribute to the metastatic processes of MM by promoting interaction with the vascular endothelium. The confirmation of the co-localization of MM cells with ECs in the BM niche prompted the analysis of JAM-C surface expression while using CD138 as a routine standard MM cell marker. However, we noted that in the BM of MM-bearing mice, JAM-C expression was significantly higher on the surface of CD138low/neg MM cells. Similarly, we identified this MM cell population within BM of primary patient samples especially after relapse.
In line with our results, a recent study showed that CD138 surface expression demarcates two distinct functional states of MM cells in vivo. Akhmetzyanova et al24 proposed a model in which MM cells upregulate CD138 in periods of growth and lose expression of CD138 when they sense nutrient deprivation and switch to a more motile and disseminative state. Therefore, it is tempting to speculate that when MM cells leave their increasingly inhospitable microenvironment, they need to cross the vascular barrier by using JAM-C. Previous reports have already cautioned that the evident dynamic CD138 expression in MM disease renders this surface receptor not an optimal clinical biomarker especially in late-stage disease or when monitoring imminent relapse.38,39 Therefore, new marker panels that would not exclude CD138low/neg MM cells or conversely would entirely dispense with CD138 have already been proposed.40 Our findings suggest a nearly reciprocal regulation of CD138 and JAM-C surface expression, which may add a novel molecular player to distinguish the MM cells that are switching from an intra- to an extramedullary dissemination status.
Here we show that only the direct in vitro contact with ECs leads to comparable regulation dynamics of CD138 and JAM-C as observed in vivo at the transcriptional and surface protein level. Moreover, we showed that the increasing MM cell population is basically resistant to treatment with a proteasome inhibitor, which makes those cells candidates that might withstand first-line treatment and persist within patients’ BM. Because the co-culture setting we used represents an artificial 2D system and can reflect only a minimum of the genuine MM-BM interactions, we also demonstrated that JAM-C expression is visibly enhanced at the interface of of BV system cells with MM cells within an intact BM niche in our MM mouse model. These observations are in line with the relatively recently described phenomena of overexpression of junction adhesion proteins in cancer pathogenesis.14
The development of individualized and patient-tailored therapies needs time and might strongly benefit from new initial treatments that delay disease progression. Our in vivo approach targeting JAM-C in MM mice proposes anti–JAM-C treatment as an attractive strategy for impairing MM progression and dissemination. Targeting only JAM-C ameliorated MM disease progression and significantly reduced the overall tumor burden and malignant MM dissemination. Doñate et al18 described similar effects in a xenograft model of mantle cell lymphoma. Because a high initial tumor burden is a hallmark of the intratibial model used in our study, investigation is still needed to determine whether blocking JAM-C would impair initial MM cell engraftment and homing as well as disease onset in models of MM residual disease by administering MM cells intravenously. Current experiments that combine anti–JAM-C treatment with administration of standard proteasome inhibitors will reveal whether this improves control of MM progression.
Myeloma spreading and migration are key characteristics of disease progression but may differ considerably from patient to patient. Multiple subclones that harbor different mutations might respond differently to a certain therapy. Our analysis of the MMRF CoMMpass database suggests JAM-C expression as an additional molecular biomarker for monitoring patients’ status and adapting treatment regimens, which is also shown by our evaluation of BM aspirates of patients at different time points at diagnosis and after relapse. The cell surface upregulation of JAM-C on CD138low/neg MM cells at relapse adds a novel molecule not only in terms of patient surveillance but also because it may represent a target for tailored treatment strategies in patients.
Collectively, we show that JAM-C demarks a CD138low/neg MM cell population in BM of patients and mice and that this adhesion molecule is involved in MM progression and dissemination. Because we could also detect these JAM-C+ CD138low/neg MM cells in the PB of MM-bearing mice, these circulating MM cells might also determine the prognosis of the MM patient and thus should be screened for during staging and while monitoring disease status. A recent study suggests a novel anti-CD138 antibody treatment as a promising targeting strategy in MM.41 Ultimately, therapeutic targeting of JAM-C in combination with a potential anti-CD138 treatment might impair myeloma spreading and complement standard treatment regimens to benefit patients suffering from MM.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft priority program µBONE (SPP2084) project BR 5870/1-1, the Europäische Fonds für Regionale Entwicklung, and the Interdisciplinary Center for Clinical Research (IZKF-B233) of the University of Würzburg. A.G.S. was supported by the Apulian Regional Project “Medicina di Precisione.”
Authorship
A. Brandl designed and performed the research, analyzed the data, and wrote the manuscript; A.G.S. designed and performed the research and analyzed the data; Z.M. analyzed the data; P.T., J.M., H.M., M.C.D.V., and G.A.C. performed the research and analyzed the data; M.K., S.T., and T.S. performed the research; R.E. and F.J. contributed reagents and supported writing the manuscript; and H.E. and A. Beilhack critically discussed scientific data and supported the writing process.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Brandl, Department of Internal Medicine II, Würzburg University Hospital, Zinklesweg 10, 97078 Würzburg, Germany; e-mail: brandl_a@ukw.de.
References
Author notes
Requests for data sharing may be submitted to Andreas Brandl (brandl_a@ukw.de).
The full-text version of this article contains a data supplement.



![CD138low/neg MM cells upregulate JAM-C in murine BM. (A) Flow cytometry of living eGFP+ MM cells in murine BM defines CD138high and CD138low/neg MM cells; the latter show significantly elevated JAM-C expression. The horizontal lines represent the mean MFI. (B) Statistical analyses of MM cells in BM regarding CD138 and JAM-C expression (percentages of GFP+ cells). JAM-C expressing (mean fluorescent intensity [MFI]) BM and PB cells gated on living and GFP+ cells divided into CD138high and CD138low/neg cells of MM mice (n = 6) at day 21 after MM cell injection. Unpaired, two-tailed Student t tests were used. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/7/10.1182_bloodadvances.2021004354/5/m_advancesadv2021004354f4.png?Expires=1765886796&Signature=ThLLWUMaIV8C44QrfGzef7Gj-8YThQBOLnzR07r0NNsbTEy3Hj0qfjIe8XyJ~ItO3XQQw9A0jNTAv~eI~FZDUc2Wuyg6Kcx3ONXTlvaqU7nHjO58vz9lBJ0LmlVwBzlM92qe5Kiqs9mRfkgUagdwss3uvGDWrYJmmOYun-5MAXIlq~LOefvIEyz1qIz6lpzp2QW6kP1i7lPxXzXGRNZCOZpEf082AyvDNvBDfyb~EIRSShKzT2OQ~W28UNQEMr~s5oStHxWGrY6Tsp07dg3Xb~jNF6JLsUcvZPr13snmunByALSG5zowzl0XSFfY0-b8PEpqkmZVFkFO7fxxhbzXFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Blocking JAM-C in vivo impairs MM progression and dissemination in tumor-bearing mice. (A) Three representative mice from experimental groups that were treated with either an isotype control (n = 8) or αJAM-C antibody (n = 9) from ventral (left panel) and dorsal (right panel) views over time after treatment. (B) Absolute signal quantification by whole body BLI from ventral views over time after treatment (P = .009; 2-way analysis of variance [ANOVA]). The horizontal lines represent the mean of photons. (C) Number of additional tumor foci within the isotype control (ctrl) and αJAM-C–treated group over time after treatment (P = .031; Wilcoxon test). (D) BLI images of 4 representative bone pairs of isotype-treated (dots) and αJAM-C–treated (squares) mice at day 21 and (F) corresponding graphical and statistical signal quantification (P = .026; 2-way ANOVA). (E) Survival rate of isotype-treated and αJAM-C–treated mice over time after treatment (P = .019; Gehan-Breslow-Wilcoxon test). (G) Frequency of GFP+ cells from BM of isotype- or αJAM-C–treated mice determined by FACS analysis (P = .028; Mann-Whitney U test). Error bars indicate SD. The horizontal lines represent the % of GFP positive cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/7/10.1182_bloodadvances.2021004354/5/m_advancesadv2021004354f6.png?Expires=1765886796&Signature=HoXm3tAI-tHGcdxuOTe5e3rVoCuzNmBKB~WVWVsGUdxsfi8YmcjkfECRtFZMt7qa78~-YRDj9~pBGeFeqRwePyedyWlWy~ccG5-bUhvm4AykuD4L180eDOj-hg7RmIs8lGjS~hvdw-slUJI-L4MC0vZ1rUv-K5b~IkdDIT2cgpnHnoB1hrgjT77pZb-Nnp-u0IpyR1VJ13XR9qZWuQsIcRBKD0Lg15LAv~7BUZoErAqHrbLH45U831g5uw5KJYwTqq8ALui8VhbxCSTByg3QsU7lgc2xjBdrKgFrVE5zLOYVdp4-AGt3NJaqe1~lonDL2ursBusbpKVNEh5l~bjmYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)