Key Points
iTTP survivors have a mortality rate nearly twofold higher than expected based on the US reference population.
Cardiovascular disease and iTTP recurrence are the leading causes of mortality in iTTP survivors.
Abstract
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) survivors experience high rates of adverse health sequelae and increased mortality over long-term follow-up. We conducted this multicenter cohort study to evaluate long-term mortality and causes of death in iTTP survivors. Between 2003 and 2020, 222 patients were enrolled in the Ohio State University and Johns Hopkins TTP registries and followed for a median of 4.5 (interquartile range [IQR], 75 0.4-11.5) years. Nine patients died during their first iTTP episode, and 29 patients died during follow-up. Mortality rate was 1.8 times higher than expected from an age-, sex-, and race-adjusted reference population. Cardiovascular disease was a leading primary cause of death (27.6%) tied with relapsed iTTP (27.6%), followed by malignancy (20.7%), infection (13.8%), and other causes (10.3%). Male sex (hazard ratio [HR], 3.74; 95% confidence interval [CI], 1.65-8.48), increasing age (HR, 1.04; 95% CI, 1.01-1.07), and number of iTTP episodes (HR, 1.10; 95% CI, 1.01-1.20) were associated with mortality in a model adjusted for African American race (HR, 0.70; 95% CI, 0.30-1.65), hypertension (HR, 0.47; 95% CI, 0.20-1.08), chronic kidney disease (HR 1.46; 95% CI, 0.65-3.30), and site (HR, 1.46; 95% CI, 0.64–3.30). There was a trend toward shorter survival in patients with lower ADAMTS13 activity during remission (P = .078). Our study highlights the need for survivorship care and investigation focused on cardiovascular disease and early mortality in TTP survivors.
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare, potentially fatal hematological disorder caused by an antibody-mediated deficiency of ADAMTS13 (a disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13), a von Willebrand factor (VWF)–cleaving protease.1 Ultra-large VWF multimers in iTTP lead to microvascular platelet aggregation, manifesting as thrombocytopenia and microangiopathic hemolytic anemia with variable end-organ dysfunction.1 Prior to the advent of plasma exchange, iTTP was nearly uniformly fatal, with a mortality rate of >90%.2 Currently, prompt therapy with plasma exchange and immunosuppression leads to survival in >90% of patients.3,4 iTTP survivors were previously thought to return to their baseline premorbid level of health, with the exception of the risk for iTTP recurrence.5 However, recent studies indicate a high rate of adverse health sequelae in iTTP survivors, including hypertension, obesity, stroke, cognitive impairment, mood disorders, and poor quality of life.6-9 iTTP survivors also appear to have higher all-cause mortality than expected from an age-, race-, and sex-matched control population.9,10 A recent study found that iTTP survivors have a nearly fivefold increased risk of incident stroke, which was associated with ADAMTS13 activity ≤70% during remission.11 Lower ADAMTS13 activity has been identified as a risk factor for coronary artery disease,12,13 stroke,14 and all-cause and cardiovascular mortality in the general population.15 Based on this, we hypothesized that cardiovascular disease contributes to early mortality in iTTP survivors. We conducted this multicenter cohort study to evaluate cause of death in patients who survived their first iTTP episode and risk factors for earlier mortality in this population.
Methods
Study cohort
The study included patients from 2 iTTP cohorts: (1) The Ohio State University (OSU) TTP/aHUS Registry, established in 2003, is a prospective cohort of consecutive patients who are referred or present to the institution with a thrombotic microangiopathy (TMA); and (2) The Johns Hopkins University (JHU) TTP cohort includes patients enrolled in the prospective Hopkins Thrombotic Microangiopathy (and Complement Associated Disorders Registry) established in 2014 as well as a retrospective cohort of additional patients with iTTP treated at Johns Hopkins Hospital prior to 2014 who were identified from plasma exchange records from the therapeutic apheresis service. Many of these patients have subsequently enrolled in the registry at follow-up visits.
The diagnosis of iTTP was based on the presence of thrombocytopenia (platelet count, <100 × 109/L), microangiopathic hemolytic anemia (hemoglobin level, <10 g/dL, along with schistocytes on the peripheral blood smear), a clinical course consistent with iTTP (response to plasma exchange therapy), and the absence of alternative thrombotic microangiopathies, such as atypical hemolytic uremic syndrome or transplant-associated microangiopathy. After 2005 (OSU) and 2006 (JHU), when ADAMTS13 activity measurement was available at the participating institutions, documented ADAMTS13 activity <10% was included in the diagnostic criteria. At OSU, ADAMTS13 activity is measured by surface-enhanced laser desorption/ionizationtime of flight mass spectrometry, which has been previously described.16 At JHU, ADAMTS13 is measured by the standard Fluorescence Resonance Energy Transfer (FRETS)-vWF73 assay. Only ADAMTS13 activity drawn prior to initiating plasma exchange was considered. A subset of patients who had their initial episode before 2006 had ADAMTS13 deficiency documented at time of relapse. For the analysis of mortality after recovery from acute iTTP, we excluded patients who died during the index iTTP episode and those who had <1 month of follow-up after recovery from iTTP. Patients were observed from iTTP diagnosis (or first clinical contact after iTTP diagnosis for patients who were transferred to our centers after being diagnosed with iTTP at other hospitals) until death or last clinical contact. Between both registries, 124 patients had their last clinical contact >1 year before date of last data collection. To overcome the issue of loss to follow-up leading to possibly erroneous death rates, we have expressed mortality rate as deaths/100 000 patient years of follow-up.
During follow-up, current practice at both institutions is to follow patients and obtain laboratory studies (including ADAMTS13 activity) every 3 months during remission. However, testing is sometimes more frequent immediately following an acute episode, which may reflect individual practitioner preference and the use of ADAMTS13 activity to tailor immunosuppression, especially since the assay is readily available with same-day results at both institutions. Additionally, some patients opt to follow-up every 6 to 12 months if they have not had a relapse in several years. The institutional review boards at the respective institutions (OSU and JHU) approved the study. The study was conducted in accordance with the Declaration of Helsinki.
Data management and study outcomes
All available patients in the OSU and JHU TMA registries were reviewed. Patients with confirmed iTTP based on the diagnostic criteria described above were included in the analysis. We extracted data from the registries and the electronic medical record, including patient demographics, details of iTTP presentation, diagnosis and treatment, and the presence of comorbidities, including hypertension, diabetes mellitus, obesity (defined as body mass index >30 kg/m2), hyperlipidemia, atrial fibrillation, congestive heart failure, smoking, chronic kidney disease (CKD; defined as a glomerular filtration rate <60 mL/min per 1.73 m2 persisting over at least 3 months), systemic lupus erythematosus, and other autoimmune diseases. Comorbidities (period prevalence at end of follow-up) were determined by ICD-9/ICD-10 codes, documentation in health care provider notes or documented requirement of regularly scheduled medications for these disorders (along with documentation of the diagnosis). Mortality during follow-up after acute iTTP was recorded as the primary outcome. In these cases, primary and secondary cause of death (where applicable) were determined by autopsy report (if performed), electronic medical records, and/or the death certificate.
Statistical analysis
Data were summarized as counts and percentages and medians and interquartile range (IQR; representing the 25th to 75th percentiles) for categorical and continuous variables, respectively. Death rate per 100 000 patient years was calculated, and cause of death is reported in counts (percentage). We compared the observed mortality rate with age- and sex-adjusted expected rates for a reference population,17 available from the National Center for Health Statistics (1999-2018), using indirect standardization methods.18 To identify risk factors for mortality, we initially tested differences by mortality status using the χ2 test for categorical covariates and Wilcoxon Mann-Whitney U test for continuous variables. We then used Cox proportional hazards regression to evaluate risk factors for mortality, including age, sex, and the presence of comorbidities. Date of iTTP diagnosis was set as time 0, and covariates were selected based on plausible association with the outcome and results of univariate analysis.
Based on reports that lower ADAMTS13 activity is associated with higher all-cause mortality and cardiovascular mortality in the general population,15 we also evaluated the association of mortality with remission ADAMTS13 activity in a subset of patients for whom remission ADAMTS13 data were available. Since ADAMTS13 activity may fluctuate during remission, we treated this as a time-dependent covariate in Cox regression models evaluating the association with mortality. In addition, we also compared average remission ADAMTS13 activity between patients who died vs survivors. Average remission ADAMTS13 was calculated as the average of all multiple available measures; we excluded any ADAMTS13 measurement taken within 3 months after an acute episode and also limited the analysis to only 1 value within any 3-month period to avoid oversampling of data from a period immediately following an acute episode. Finally, we evaluated the association of the most recent ADAMTS13 measurement (closest to death or last follow-up) with survival in a Kaplan-Meier analysis. We first evaluated remission ADAMTS13 activity as a continuous variable and also evaluated associations with mortality when ADAMTS13 was categorized using an ADAMTS13 activity threshold of ≤70%, which has been associated with stroke during remission.11 This cutoff also correlates with the median of the lowest quartile ADAMTS13 activity (71%) in the population-based Rotterdam cohort, which was associated with an increased risk of ischemic stroke, all-cause, and cardiovascular mortality.15
Results
Cohort demographics
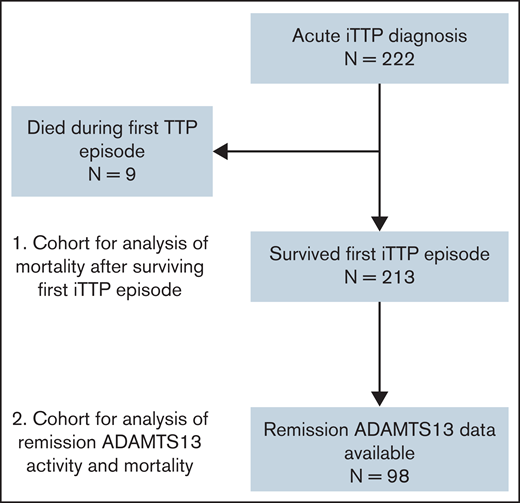
Between 2003 and 2020, 222 patients diagnosed with iTTP were enrolled in the OSU and JHU TMA registries (62 at OSU and 160 at JHU; supplemental Table 2). Median age at entry into the cohort was 42 (IQR, 29-55) years, and 70.3% were female. Patients were followed for a median (IQR) duration of 4.5 (0.4-11.5) years, with a total of 1318 patient years of observation. Characteristics of the study cohort are summarized in Table 1. Thirty-eight patients died during the follow-up period, of whom 9 died during their first episode of iTTP and 29 died after surviving the first iTTP episode (Figure 1).
Flowchart of individuals included in the analysis. A total of 222 individual patients were treated for acute iTTP at the Johns Hopkins Hospital or OSU, of whom 9 died during the index iTTP episode. The remaining 213 patients were including in the analysis of mortality after an episode of acute iTTP. A subset of 98 patients had available measurements of ADAMTS13 activity in remission and were included the analysis evaluating the association of reduced ADAMTS13 activity in remission with mortality.
Flowchart of individuals included in the analysis. A total of 222 individual patients were treated for acute iTTP at the Johns Hopkins Hospital or OSU, of whom 9 died during the index iTTP episode. The remaining 213 patients were including in the analysis of mortality after an episode of acute iTTP. A subset of 98 patients had available measurements of ADAMTS13 activity in remission and were included the analysis evaluating the association of reduced ADAMTS13 activity in remission with mortality.
Characteristics of cohort, by survival status
| Characteristics . | Survivors (n = 184) . | Died during follow-up (n = 29) . | Died during first iTTP episode (n = 9) . | Total cohort (N = 222) . | P* . |
|---|---|---|---|---|---|
| Female sex | 72.8% | 55.2% | 66.7% | 70.3% | .053 |
| Race | .070 | ||||
| White | 44.6% | 65.5% | 33.3% | 46.8% | |
| African American | 53.8% | 31.0% | 44.4% | 50.5% | |
| Other/unknown | 1.6% | 3.4% | 22.2% | 2.7% | |
| Age (y), median (IQR) | 41 (28, 55) | 42 (31,50) | 44 (35, 77) | 42 (29, 55) | .451 |
| Total no. of iTTP episodes, median (IQR) | 1 (1,3) | 3 (1,5) | 1 (1,1) | 1 (1,3) | <.001 |
| Comorbidities | |||||
| Hypertension | 48.9% | 62.1% | 66.7% | 51.4% | .188 |
| Diabetes | 23.4% | 31.0% | 33.3.% | 24.8% | .372 |
| Obesity (BMI > 30) | 37.5% | 27.6% | 33.3% | 35.0% | .302 |
| Hyperlipidemia | 23.9% | 24.1% | 11.1% | 23.4% | .979 |
| Atrial fibrillation | 7.1% | 6.9% | 11.1% | 7.2% | .974 |
| Systemic lupus erythematosus | 9.8% | 6.9% | 0.0% | 9.0% | .620 |
| CKD | 19.0% | 48.3% | 11.1% | 22.5% | .001 |
| Average remission ADAMT13 activity, median (IQR) | 63.2 (42.9, 82.1) | 39.0 (23.9, 64.0) | n/a | 61.2 (37.9, 78.6) | .010 |
| Follow-up time (y), median (IQR) | 4.8 (0.7, 11.8) | 5.3 (0.8,8.0) | n/a† | 4.5 (0.4, 11.5) | .975 |
| Characteristics . | Survivors (n = 184) . | Died during follow-up (n = 29) . | Died during first iTTP episode (n = 9) . | Total cohort (N = 222) . | P* . |
|---|---|---|---|---|---|
| Female sex | 72.8% | 55.2% | 66.7% | 70.3% | .053 |
| Race | .070 | ||||
| White | 44.6% | 65.5% | 33.3% | 46.8% | |
| African American | 53.8% | 31.0% | 44.4% | 50.5% | |
| Other/unknown | 1.6% | 3.4% | 22.2% | 2.7% | |
| Age (y), median (IQR) | 41 (28, 55) | 42 (31,50) | 44 (35, 77) | 42 (29, 55) | .451 |
| Total no. of iTTP episodes, median (IQR) | 1 (1,3) | 3 (1,5) | 1 (1,1) | 1 (1,3) | <.001 |
| Comorbidities | |||||
| Hypertension | 48.9% | 62.1% | 66.7% | 51.4% | .188 |
| Diabetes | 23.4% | 31.0% | 33.3.% | 24.8% | .372 |
| Obesity (BMI > 30) | 37.5% | 27.6% | 33.3% | 35.0% | .302 |
| Hyperlipidemia | 23.9% | 24.1% | 11.1% | 23.4% | .979 |
| Atrial fibrillation | 7.1% | 6.9% | 11.1% | 7.2% | .974 |
| Systemic lupus erythematosus | 9.8% | 6.9% | 0.0% | 9.0% | .620 |
| CKD | 19.0% | 48.3% | 11.1% | 22.5% | .001 |
| Average remission ADAMT13 activity, median (IQR) | 63.2 (42.9, 82.1) | 39.0 (23.9, 64.0) | n/a | 61.2 (37.9, 78.6) | .010 |
| Follow-up time (y), median (IQR) | 4.8 (0.7, 11.8) | 5.3 (0.8,8.0) | n/a† | 4.5 (0.4, 11.5) | .975 |
BMI, body mass index.
P values are for differences between patients who died after surviving their first iTTP episode (n = 29) and those who were alive at the end of follow-up (n = 184).
Median (IQR) follow-up time for patients who died during the first iTTP episode was 0.013 (0.002, 0.021) years.
iTTP survivors have increased mortality rates and cardiovascular disease is the leading cause of death
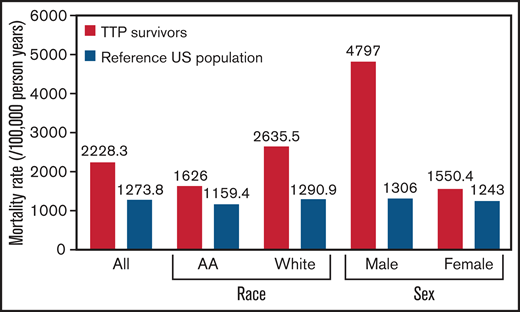
The mortality rate in individuals that survived a first episode of iTTP was higher than the expected mortality rate from an age-, sex-, and race-standardized reference US population (2228.3 per 100 000 person years vs 1273.8 per 100 000 person years; P = .007) (Figure 2). Median age at death among those that survived the first iTTP episode was 49 (IQR, 39-65) years, which was lower than the general population (78.7 years).
Mortality rate in patients surviving their first episode of iTTP was higher than the expected mortality rate from an age-, sex-, and race-standardized reference US population. Mortality rate is expressed as deaths per 100 000 person years. AA, African American.
Mortality rate in patients surviving their first episode of iTTP was higher than the expected mortality rate from an age-, sex-, and race-standardized reference US population. Mortality rate is expressed as deaths per 100 000 person years. AA, African American.
Among patients who survived their first iTTP episode, cardiovascular disease was the leading primary cause of death (27.6%), tied with relapsed iTTP (27.6%), followed by malignancy (20.7%), infection (13.8%), and other/unknown causes (10.3%) (Table 2). Cardiovascular disease was the primary or secondary cause of death in 31% of patients (9 of 29). Cardiovascular causes of death included sudden cardiac death (n = 3), decompensated heart failure/cardiogenic shock secondary to ischemic heart disease (n = 2) and stroke (n = 3). The median age of death from any cardiovascular cause of death (primary or secondary) was 49 years. Cause of death for individual patients is summarized in supplemental Table 1.
Leading causes of death in patients surviving a first episode of iTTP and the general US population
| iTTP survivors (n = 29) . | General US population (2017, CDC) . |
|---|---|
| Relapsed iTTP (27.6%) | Cardiovascular disease (29.5%) |
| Cardiovascular disease (27.6%) | Cancer (21.3%) |
| Cancer (20.7%) | Accidents (6%) |
| Infectious causes (13.8%) | Chronic lung disease (5.7%) |
| Other/unknown (10.3%) | Alzheimer disease (4.3) |
| Infectious causes (3.5%) | |
| Diabetes (3%) | |
| Renal disease (1.8%) | |
| Intentional self-harm (1.8%) | |
| Cirrhosis (1.5%) | |
| Parkinson disease (1.1%) | |
| Pneumonitis (0.7%) | |
| All other causes (20%) |
| iTTP survivors (n = 29) . | General US population (2017, CDC) . |
|---|---|
| Relapsed iTTP (27.6%) | Cardiovascular disease (29.5%) |
| Cardiovascular disease (27.6%) | Cancer (21.3%) |
| Cancer (20.7%) | Accidents (6%) |
| Infectious causes (13.8%) | Chronic lung disease (5.7%) |
| Other/unknown (10.3%) | Alzheimer disease (4.3) |
| Infectious causes (3.5%) | |
| Diabetes (3%) | |
| Renal disease (1.8%) | |
| Intentional self-harm (1.8%) | |
| Cirrhosis (1.5%) | |
| Parkinson disease (1.1%) | |
| Pneumonitis (0.7%) | |
| All other causes (20%) |
CDC, Centers for Disease Control and Prevention.
Deaths during the acute iTTP episodes (9 during first episode and 8 during subsequent episodes) were due to stroke (n = 6), myocardial infarction (n = 1), myocardial infarction and stroke (n = 1), sudden cardiac death/pulseless electrical activity (n = 6) in the setting of refractory iTTP, and other causes (n = 3; respiratory failure in 2 and infection in 1).
Risk factors for early mortality in iTTP survivors
Among patients who survived their first iTTP episode, male sex (HR, 3.74; 95% CI, 1.65-8.48; P = .002), increasing age (HR, 1.04; 95% CI, 1.01-1.07; P = .011), and number of iTTP episodes (HR, 1.10; 95% CI, 1.01-1.20; P = .022) were associated with mortality in a model adjusted for African American race (HR, 0.70; 95% CI, 0.30-1.65; P = .702), hypertension (HR, 0.47; 95% CI, 0.20-1.08; P = .076), CKD (HR, 1.46; 95% CI, 0.65-3.30; P = .358), and site (HR, 1.46; 95% CI, 0.64–3.30; P = .358).
ADAMTS13 activity in remission and mortality
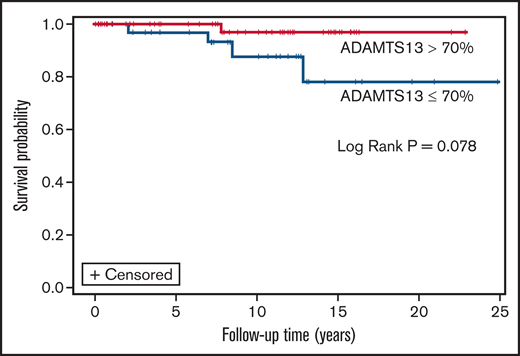
Measurements of remission ADAMTS13 activity were available for 98 of 213 patients who survived their first iTTP episode. Median average remission ADAMTS13 activity for the cohort was 61.2% (IQR, 37.9% to 78.6%). Patients who died (n = 15) had significantly lower median (IQR) ADAMTS13 activity than survivors (n = 63) (39.0% [IQR, 23.9% to 64%] vs 63.2% [IQR, 42.9% to 82.1%]; P = .010). Of the 15 patients who died during follow-up and had available remission ADAMTS13 data, median time from last measurement to death was 4.8 (0.6, 60.3) months. On evaluating the association of ADAMTS13 activity in remission (as a time varying covariate) with mortality, there was a trend toward shorter survival with lower ADAMTS13 activity (HR, 0.97; 95% CI, 0.951-1.002; P = .073 for every 1% change in ADAMTS13 activity). For every 20% change in ADAMTS13 activity, risk of death reduced by ∼38% (HR, 0.62; 95% CI, 0.36-1.04; P = .073). However, remission ADAMTS13 activity was not significantly associated with mortality (HR, 0.97; 95% CI, 0.95, 1.01; P = .127) in a multivariable Cox regression model that also included age at cohort entry (HR, 1.01; 95% CI, 0.93-1.09; P = .820), female sex (HR, 0.83; 95% CI, 0.07-9.43; P = .883), race (HR, 6.01; 95% CI, 0.44-82.38; P = .179), number of TTP episodes (HR, 1.34; 95% CI, 0.75-2.39; P = .317), hypertension (HR, 2.32; 95% CI, 0.22-23.80; P = .476), and CKD (HR, 0.97; 95% CI, 0.95-1.01). Finally, on evaluating the association of most recent ADAMTS13 activity (closest to death or last follow-up) with mortality, there was a trend toward shorter survival in patients with ADAMTS13 ≤70% vs >70% at most recent evaluation (log rank P = .078) (Figure 3).
Overall survival in patients with most recent remission ADAMTS13 >70% vs ≤70%.
We attempted to evaluate the association of remission ADAMTS13 activity with cardiovascular death; however, remission ADAMTS13 activity was available for only 5 (of 8) patients with a primary cardiovascular cause of death (supplemental Figure 1), which precluded multivariable modeling. All 5 patients who died of cardiovascular causes had average remission ADAMTS13 activity ≤70% (median [IQR] 39.3% [36.3% to 53.4%]).
Discussion
Therapeutic advances since the 1990s, including plasma exchange and effective immunosuppression, have markedly improved the outcomes of acute iTTP,4 and the majority of patients with iTTP will now survive their acute iTTP episode. Thus, iTTP survivorship and long-term sequelae have emerged as an area of active investigation.5-11,19 Best practices for screening and management of long-term sequelae in iTTP survivors are still evolving but are becoming equally important as initial management.
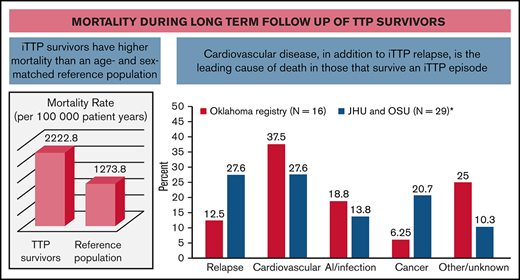
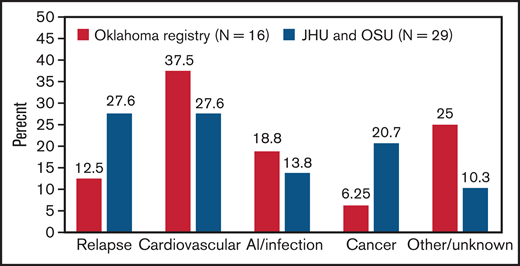
In this multicenter cohort study, we demonstrate that iTTP survivors have approximately twofold higher mortality rate than expected based on an age- and sex-adjusted reference population. Cardiovascular disease, tied with iTTP relapse, is a leading cause of death in patients who survive their first episode of iTTP. Our findings are consistent with findings from the Oklahoma TTP registry, which also noted higher than expected death rates in 77 iTTP survivors.10 Sixteen patients died during follow-up, 6 (37.5%) due to cardiovascular causes and only 2 (12.5%) due to recurrent iTTP (Figure 4).10 A recent French analysis focusing on older adults (>60 years) with iTTP reported that patients who survived their acute iTTP episode had threefold-higher long-term mortality compared with an age-matched reference population from the same geographic area.20 They hypothesized that shortened survival could be attributed to coexisting disorders, such as hypertension, depression, and cognitive decline in older patients with iTTP. In fact, iTTP has been associated with higher rates of cardiovascular risk factors such as hypertension and obesity,6,9 which may contribute to mortality and is consistent with the emergence of cardiovascular disease as a major cause of mortality in our cohort. Additionally, the magnitude of difference in mortality rates between the general population and TTP survivors was especially striking for white participants. Possible explanations include differences in the burden of comorbidities such as hypertension and renal disease between white TTP survivors and white patients in the reference population or other TTP-specific factors.
Causes of death in patients who survived their first iTTP episodes. Data from our combined cohort (29 deaths) are compared with previously published data from the Oklahoma TTP registry (16 deaths).9 Cardiovascular disease was a leading cause of death in both cohorts.
Causes of death in patients who survived their first iTTP episodes. Data from our combined cohort (29 deaths) are compared with previously published data from the Oklahoma TTP registry (16 deaths).9 Cardiovascular disease was a leading cause of death in both cohorts.
Our analysis identified increasing age and number of iTTP episodes as risk factors for shortened survival, while other known cardiovascular risk factors such as hypertension and CKD were not significantly associated with mortality. This lack of association of traditional cardiovascular risk factors with mortality is likely due to the limited sample size and few events (only 29 deaths). However, it also raises the possibility that iTTP-specific factors such as ADAMTS13 activity in remission may contribute to cardiovascular disease and death independent of traditional cardiovascular risk factors. In our analysis, there was a clear trend toward higher all-cause mortality and cardiovascular mortality in patients with lower ADAMTS13 activity in remission, though this did not reach statistical significance, likely because of the relatively small sample size (98 patients with remission ADAMTS13 activity measures) and small number of events. ADAMTS13 is a particularly interesting biomarker for long-term outcomes in patients with iTTP and has been associated with a high risk of stroke in this population. Moreover, population-based studies have established that reduced ADAMTS13 is a risk factor for all-cause mortality and cardiovascular mortality in the general population (without iTTP)15 and is also a risk factor for adverse cardiovascular outcomes such as ischemic stroke and coronary heart disease.12-14 For example, in the Rotterdam study, which followed 6130 participants aged ≥55 years for a median of 11.3 years, reduced ADAMTS13 activity was associated with a nearly 1.5-times-higher risk of all-cause or cardiovascular death.15 The association of reduced ADAMTS13 activity in iTTP remission with mortality and other adverse events needs to be evaluated in larger, multicenter cohorts. If established, this makes a case for attempting to achieve normal ADAMTS13 activity in remission rather than only targeting ADAMTS13 activity >10% to 20%, which appears to be adequate to prevent relapse.16,21,22 More systematic use of rituximab at the acute phase of disease may improve ADAMTS13 activity in remission and prevent relapses over the following 1 or 2 years.23 Our results suggest targeting higher (or normal) ADAMTS13 activity may have benefits other than relapse prevention, but additional prospective data are required before making this recommendation. Of note, the International Working Group on TTP recently published a consensus statement proposing revised definitions of remission including partial and complete ADAMTS13 remission,24 recognizing that incomplete ADAMTS13 recovery may be a risk factor for adverse outcomes such as ischemic stroke.
A limitation of our study is that the limited sample size and small number of deaths precluded extensive multivariable analysis for risk factors for mortality. Only 15 patients in the subset of patients with remission ADAMTS13 activity died, which was a number too small to have enough power to evaluate the association. This epidemiologic study did not evaluate the mechanism by which reduced ADAMTS13 activity contributes to mortality, but we postulate 2 possible reasons for this association. First, reduced ADAMTS13 may lead to higher rates of cardiovascular disease and death, as has been noted in the general population. This is proposed to be due to reduced cleavage of ultralarge, highly active VWF multimers and may potentially lead to a state of subclinical platelet activation culminating in cumulative vascular injury.11,25 In the Rotterdam study, the combination of high VWF levels and reduced ADAMTS13 was associated with a higher risk of mortality than reduced ADAMTS13 activity alone.15 We were unable to evaluate the association of VWF levels/activity with mortality, since we did not measure these prospectively. An alternative explanation is that reduced ADAMTS13 in remission is a risk factor for iTTP recurrence,16,22,26,27 and multiple recurrences may increase risk of death either directly due to recurrent iTTP or indirectly by causing cumulative ischemic organ injury during acute episodes. Indeed, patients who died during follow-up had a higher median number of episodes than those who did not die, as seen in Table 1 (median episodes, 3 vs 1; P < .001). In either case, ADAMTS13 emerges as a potential target to reduce long-term mortality in patients with ITTP.
In conclusion, iTTP survivors at 2 tertiary care centers based in the United States have a significantly higher mortality rate compared with an age- and sex-adjusted US reference population. Cardiovascular disease is a leading cause of mortality in these patients. At our centers, iTTP survivors are monitored with ADAMTS13 activity measurements every 3 to 6 months during remission to allow consideration of preemptive therapy to prevent relapses and to monitor for long-term sequelae that may influence early mortality. Our results highlight the need to screen and aggressively manage cardiovascular risk factors in iTTP survivors, as well as the need for further investigation into the mechanisms underlying cardiovascular and other complications after iTTP. Optimizing treatment of acute iTTP, increasing recognition of long-term sequelae, and incorporating preventative strategies during follow-up are the next critical steps to improve quality of life and survival among iTTP survivors.
Acknowledgments
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (grant K99HL150594 to S.C.) and an American Society of Hematology Scholar Award (S.C.).
Authorship
Contribution: S.S. collected data and wrote, edited, and reviewed the manuscript; M.B. and S.H. collected data; R.B. and A.M. critically reviewed the manuscript; S.R.C. conceptualized the research design and critically reviewed and edited the manuscript; L.Y. performed statistical analyses; S.C. conceptualized the research design, verified all outcomes, performed and interpreted the analyses, and wrote, edited, and reviewed the manuscript; and all authors read and approved the final draft of the manuscript.
Conflict-of-interest disclosure: S.R.C. has served on advisory boards for Sanofi Genzyme, Alexion, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Shruti Chaturvedi, Division of Hematology, Johns, Hopkins University School of Medicine, 720 Rutland Ave, Ross Research Building, Room 1025, Baltimore, MD 21205; e-mail: schatur3@jhmi.edu.
References
Author notes
For original data, please contact schatur3@jhmi.edu. All internal and external requests for data will be reviewed for feasibility and priority.
Presented in oral form at the 63rd Annual Meeting of the American Society of Hematology, Atlanta, GA, 6 December 2020.
The full-text version of this article contains a data supplement.