Key Points
A nationwide epidemiologic study for acquired PRCA identified 1055 patients, an incidence rate of 1.06 patients per million per year.
The median age was 73 years old with female predominance (1.5:1), and 69% of the PRCA was idiopathic.
Abstract
Acquired pure red cell aplasia (PRCA) is a rare syndrome characterized by anemia with reticulocytopenia and a marked reduction in erythroid precursors. Given its rarity, the true incidence is largely unknown, and epidemiological data representing the general population, with a description of the full spectrum of etiologies, are scarce. An epidemiological study on PRCA in Japan conducted 30 years ago estimated the annual incidence as 0.3 per million. To update the data and investigate the incidence and demographics of PRCA, we conducted a nationwide epidemiological study using the Japanese Society of Hematology (JSH) Hematologic Disease Registry, a hematologic disease registration database managed by the JSH and the Diagnosis Procedure Combination (DPC) study data available at a website of the Ministry of Health, Labor, and Welfare (MHLW) of Japan. A total of 1055 patients with newly diagnosed acquired PRCA were identified between 2012 and 2019, and the average annual incidence was calculated at 1.06 (95% confidence interval [CI], 0.83-1.28) per million. The median age was 73 (range, 18-99) years. The female-to-male ratio was 1.5:1, and the female predominance was most prominent in the child-bearing age group. Sixty-nine percent of acquired PRCA was idiopathic. The incidence of PRCA was approximately 20% of that of aplastic anemia (AA) during the same period. Approximately 0.98 patients per million per year (95% CI, 0.89-1.07) required hospitalization for the treatment of PRCA. These results are expected to contribute to the discussion of resource allocation for PRCA in the aging population in many countries, including Japan.
Introduction
Acquired pure red cell aplasia (PRCA) has been defined as a bone marrow disorder characterized by anemia, reticulocytopenia, and erythroid hypoplasia.1,2 It presents as cytopenia with a single lineage but shares some clinical features with other bone marrow failure syndromes and autoimmune cytopenias. Nosologically, PRCA is classified either into a congenital entity, such as Diamond-Blackfan anemia, or acquired PRCA. The latter may be associated with various underlying medical conditions and diseases, that may include thymoma, lymphoid, and myeloid malignancies, solid malignancies, infections, drugs and chemicals, collagenous diseases, pregnancy, and ABO-mismatched allogeneic transplantation.3 A further understanding of the pathogenesis may provide clues to aid in the development of effective therapies.4
Acquired PRCA is considered a rare disease. Relevant epidemiologic literature is sparse, and most previous studies were based on small cohorts or focused on a subtype of PRCA. Clark and colleagues, for example, described 37 patients with PRCA from 3 medical institutions in the United States between 1966 and 1982; the median age was 45 (range, 17-79) years, with no marked difference in frequency between sexes.5 Another study from the United States was conducted at a single institution by Balasubramanian and colleagues, identifying 62 individuals with PRCA between 2000 and 2016 with a median age of 62 (range, 25-87) years.6 Among these patients, 52% had idiopathic PRCA; this was followed in frequency by thymoma-associated PRCA, large granular lymphocytic leukemia (LGLL)-associated PRCA, and other PRCAs. Additionally, Malhotra and colleagues described 9 patients in India.7 These studies, which covered the full spectrum of PRCA subtypes, may be informative with regard to the epidemiological diversity of the disease, although they are not always representative of the general population.
However, a number of case series have focused on certain underlying diseases or pathological conditions that may give rise to secondary PRCA as a complication. For example, PRCA occurred as a complication in 1.8% to 14.3% of patients with thymoma,8-11 28.8% to 68.2% of patients with LGLL,12-14 0.5% to 1.5% of patients with chronic lymphocytic leukemia (CLL),2,15 and 7.5% to 10.5% of patients with major ABO-mismatched hematopoietic transplantation.16,17 The causative association of exogenous erythropoietin-associated autoantibody with PRCA was attributed to specific drug formulations produced between 1989 and 2004, and such antibody-medicated PRCA has been extremely rare since modifications to those drug formulations.18,19 Therefore, epidemiological descriptions of PRCA with a full spectrum of etiologies are still limited, and data on the incidence of PRCA are largely unavailable.
In Japan, the Japanese National Research Group on Idiopathic Bone Marrow Failure Syndromes of the MHLW conducted questionnaire surveys in 1993, 2004, and 2006, which recruited 185 patients with PRCA.20,21 The mean age was 55 (range, 18-89) years, with a female predominance (1:1.7).21 However, it has been almost 30 years since the first epidemiological survey was conducted, and the age structure of society at present differs from that in the past. Medical advances have helped foster a deeper understanding of PRCA and its etiologies. To reflect the changing age structure of the population in past decades, the incidence and demographic characteristics of acquired PRCA need to be updated. We, therefore, conducted a nationwide epidemiological study using 2 independent databases: the JSH Hematologic Disease Registry and the DPC study data (Table 1).
A comparison of characteristics of JSH Hematologic Disease Registry and DPC study databases
| Database . | JSH Hematologic Disease Registry . | DPC study . |
|---|---|---|
| Who organizes the registration database? | JSH | Ministry of Health, Labor, and Welfare |
| Which patients are registered? | All patients with newly diagnosed hematological diseases at the JSH member hospitals | All patients discharged from the participating hospitals |
| When are the patients registered? | At diagnosis | At reimbursement for an inpatient care |
| What is available from the database? | ||
| Diagnosis of the patients | With ICD10 code | With ICD10 code |
| Number of subtypes | 5 | 3 |
| Gender of the patients | Yes | No |
| Age of the patients | Yes | No |
| Treatment information | No | No |
| Outcome and survival | Limited | No |
| Incentives to increase registration | JSH certification as an education center | Medical fee reimbursement |
| Who participates in the registration? | The JSH member hospitals | Any hospitals participating in the survey |
| What size does the database have? | 42 000 new cases in 2020 394 000 cases as of 2020 | 1.0 million beds (64.8% of total hospital beds in Japan) as of 2020 |
| Accessibility to database | Partly accessible as an open document | Partly accessible as an open document |
| Database . | JSH Hematologic Disease Registry . | DPC study . |
|---|---|---|
| Who organizes the registration database? | JSH | Ministry of Health, Labor, and Welfare |
| Which patients are registered? | All patients with newly diagnosed hematological diseases at the JSH member hospitals | All patients discharged from the participating hospitals |
| When are the patients registered? | At diagnosis | At reimbursement for an inpatient care |
| What is available from the database? | ||
| Diagnosis of the patients | With ICD10 code | With ICD10 code |
| Number of subtypes | 5 | 3 |
| Gender of the patients | Yes | No |
| Age of the patients | Yes | No |
| Treatment information | No | No |
| Outcome and survival | Limited | No |
| Incentives to increase registration | JSH certification as an education center | Medical fee reimbursement |
| Who participates in the registration? | The JSH member hospitals | Any hospitals participating in the survey |
| What size does the database have? | 42 000 new cases in 2020 394 000 cases as of 2020 | 1.0 million beds (64.8% of total hospital beds in Japan) as of 2020 |
| Accessibility to database | Partly accessible as an open document | Partly accessible as an open document |
The JSH Hematologic Disease Registry is a registration database in which all instances of patients with newly diagnosed hematological disorders except iron deficiency anemia are registered in Japan. The incidence data obtained from the JSH Registry may be approximated to the true incidence of a hematological disease in Japan. The DPC study database is an annual summary of reimbursement claims for medical fee points of hospitalized patients in Japan. The frequency data of PRCA obtained from the DPC study database may thus reflect the number of patients with PRCA requiring hospitalized care for any medical or surgical indications, including PRCA itself.
The JSH Hematologic Disease Registry is a registration database managed by the Japanese Society of Hematology (JSH), in which member hematologists register all patients with a newly diagnosed hematological disorder, except for iron deficiency anemia.22 All patients are registered consecutively without duplication. This registration is a prerequisite for a medical institution in Japan to be certified by the JSH as an education center for hematology, which is an academic incentive to minimize underestimation. The annual frequency of PRCA derived from the JSH registry data may therefore closely reflect the true incidence of newly diagnosed PRCA in Japan.
In contrast, the DPC database, managed by the MHLW, is a collection of reimbursement claims for medical fee points of hospitalized patients.23 An annual summary of the survey, the DPC study database, which lists the number of hospitalized patients whose care was reimbursed each year, is readily accessible on a government website. The database covers 1.0 million hospital beds, accounting for approximately 65% of the total hospital beds and 90% of the hospital beds available for acute care in Japan.23 It is widely accepted that inpatient treatment for PRCA usually involves hematological expertise, and 81% of JSH hematologists are registered at a hospital listed in the DPC study24; thus, it is likely that the majority of patients with PRCA who require hospital care are included in the DPC study database. This database includes patients who are hospitalized for the treatment of PRCA itself, as well as those hospitalized with any medical or surgical indications other than PRCA but who require treatment for PRCA during their hospital stay. The frequency data derived from the study should therefore reflect the number of patients with PRCA in Japan who require hospital care for any medical or surgical indications. Of note, the DPC study data do not include the reimbursement data for outpatient care and do not reflect the true prevalence of the disease; however, this database may still be useful for discussing the allocation of medical resources. These 2 nationwide databases were therefore searched to obtain an up-to-date overview of the epidemiology of PRCA in Japan.
Methods
Databases and obtaining relevant data
The JSH Hematologic Disease Registry, launched in 2008, collects clinical information on 20 000 to 40 000 patients a year with newly diagnosed hematological diseases, having accumulated approximately 394 000 cases of hematological disease as of 2020. This database includes patient age, sex, the diagnosis of hematological disease with the International Classification of Diseases version 10 (ICD-10) code, the date and place of the diagnosis, and the last date of confirmed survival. Regarding the etiological classifications of acquired PRCA, cases are registered as either idiopathic PRCA or secondary PRCA. The underlying causes of secondary PRCA are selected from among thymoma, CLL, LGLL, or “others” at registration. Physicians are also encouraged to update patient survival data in the JSH Hematologic Disease Registry database at the time of data locking each year. In April 2021, with the approval of the JSH committee, we obtained an anonymized list of patients with acquired PRCA and patients with aplastic anemia (AA) (as reference cases), along with their demographic features. These cases were all registered in the JSH Hematologic Disease Registry database between 2012 and 2019. The data of the patients <18 years of age were excluded from our subsequent analysis to minimize the possibility of unintentional inclusion of congenital subtypes.
For the DPC survey data, we obtained annual reports of the survey of inpatient care for PRCA and AA between 2015 and 2019 from a website maintained by the MHLW.23 While the report provides the number of hospitalized patients with PRCA or AA separately, the demographic characteristics of the patients (eg, age and sex), are summarized under the same DPC category in the report, and thus they were not available for this study. The etiological classification described in the DPC study database included chronic acquired PRCA, transient acquired PRCA, and acquired PRCA, unspecified.
To calculate the annual incidence of PRCA, vital statistics were obtained from a government database available online (e-Stat), a portal site of official statistics of Japan managed by the Statistics Bureau.25 The population of each prefecture as of 2015 was also obtained from the e-Stat, and the number of JSH-certifying hematologists registered in each prefecture as of 2021 was obtained from the JSH website.24
Statistical analyses
For the survival analysis, we included only the patients whose subtypes, survival information, and date of the outcome were available. Overall survival (OS) was defined as the period from the date of the diagnosis to the date of the outcome. A statistical software EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 2.13.0), was used to perform the statistical analyses.26 Demographic figures were created using the Prism v9.0 software program (GraphPad Software, San Diego, CA).
Ethical approval
This study was approved by the institutional review board of Shinshu University School of Medicine (Approval number: 5090, 29 March 2021) and conducted in accordance with the Declaration of Helsinki.
Results
Age and sex
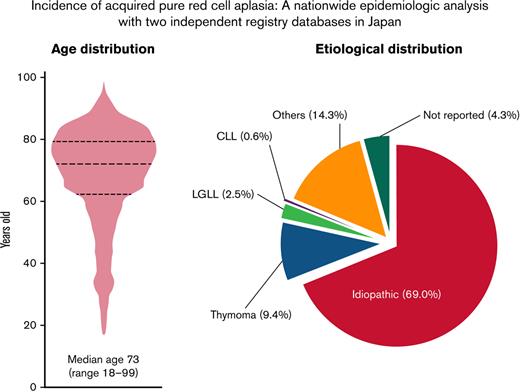
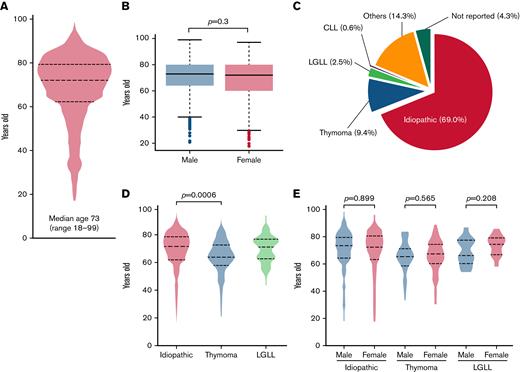
A total of 1055 patients who were newly diagnosed with acquired PRCA between 2012 and 2019 were identified in the JSH Hematologic Disease Registry database; thus, there was an average of 132 patients per year (Table 2). Given the national population of approximately 120 million, the average annual incidence of PRCA was estimated to be 1.06 (95% confidence interval [CI], 0.83-1.28) per million. The median age of the patients with PRCA was 73 (range, 18-99) years, and approximately 77% were >60 years of age (Figure 1A). The age distributions did not differ to a statistically significant extent between the sexes (Figure 1B).
Numbers of patients registered to the JSH Hematologic Disease Registry and an estimation of the annual incidence of PRCA and AA
| JSH Hematologic Disease Registry . | Year . | Estimated Incidence (95% CI) . | Total of Cohort, n (%) . | Number of Females . | Female-to-Male Ratio . | Median Age, range (yr) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | 2018 . | 2019 . | |||||||
| National population (in million) | 126.0 | 125.7 | 125.4 | 125.3 | 125.3 | 125.3 | 124.2 | 123.7 | — | — | — | — | — | — |
| PRCA | 88 | 90 | 102 | 151 | 112 | 128 | 175 | 209 | 1.06 | (0.83-1.28) | 1055 (100) | 639 | 1.54 | 73 (18-99) |
| Idiopathic | 56 | 69 | 66 | 115 | 76 | 87 | 124 | 135 | — | — | 728 (69.0) | 434 | 1.48 | 75 (20-99) |
| Thymoma | 9 | 7 | 19 | 8 | 13 | 13 | 11 | 19 | — | — | 99 (9.4) | 63 | 1.75 | 67 (33-90) |
| LGL | 3 | 3 | 4 | 5 | 1 | 1 | 3 | 6 | — | — | 26 (2.5) | 13 | 1.00 | 74.5 (56-88) |
| CLL | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 2 | — | — | 6 (0.6) | 4 | 2.00 | 64.5 (35-71) |
| Others | 19 | 10 | 12 | 23 | 21 | 7 | 12 | 47 | — | — | 151 (14.3) | 94 | 1.65 | 64 (18-89) |
| Not reported | 1 | 1 | 0 | 0 | 0 | 19 | 24 | 0 | — | — | 45 (4.3) | 31 | 2.21 | 72 (44-93) |
| AA | 514 | 499 | 564 | 634 | 566 | 752 | 828 | 881 | 5.24 | (4.46-6.02) | 5238 | 2882 | 1.22 | — |
| DPC study database | Year | Estimated Frequency (95% CI) | ||||||||||||
| — | — | — | 2015 | 2016 | 2017 | 2018 | 2019 | — | — | — | — | |||
| National population (in millions) | — | 125.3 | 125.3 | 125.3 | 124.2 | 123.7 | — | — | — | — | — | — | ||
| PRCA | — | — | — | 130 | 119 | 123 | 100 | 139 | 0.98 | (0.89-1.07) | — | — | — | |
| Chronic acquired PRCA | — | 47 | 57 | 52 | 46 | 80 | — | — | — | — | — | — | ||
| Transient acquired PRCA | — | 23 | 11 | 14 | 14 | 21 | — | — | — | — | — | — | ||
| Acquired PRCA, not specified | — | 60 | 51 | 57 | 40 | 38 | — | — | — | — | — | — | ||
| AA | — | — | — | 4257 | 4131 | 3887 | — | 4257 | — | — | — | — | ||
| JSH Hematologic Disease Registry . | Year . | Estimated Incidence (95% CI) . | Total of Cohort, n (%) . | Number of Females . | Female-to-Male Ratio . | Median Age, range (yr) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | 2018 . | 2019 . | |||||||
| National population (in million) | 126.0 | 125.7 | 125.4 | 125.3 | 125.3 | 125.3 | 124.2 | 123.7 | — | — | — | — | — | — |
| PRCA | 88 | 90 | 102 | 151 | 112 | 128 | 175 | 209 | 1.06 | (0.83-1.28) | 1055 (100) | 639 | 1.54 | 73 (18-99) |
| Idiopathic | 56 | 69 | 66 | 115 | 76 | 87 | 124 | 135 | — | — | 728 (69.0) | 434 | 1.48 | 75 (20-99) |
| Thymoma | 9 | 7 | 19 | 8 | 13 | 13 | 11 | 19 | — | — | 99 (9.4) | 63 | 1.75 | 67 (33-90) |
| LGL | 3 | 3 | 4 | 5 | 1 | 1 | 3 | 6 | — | — | 26 (2.5) | 13 | 1.00 | 74.5 (56-88) |
| CLL | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 2 | — | — | 6 (0.6) | 4 | 2.00 | 64.5 (35-71) |
| Others | 19 | 10 | 12 | 23 | 21 | 7 | 12 | 47 | — | — | 151 (14.3) | 94 | 1.65 | 64 (18-89) |
| Not reported | 1 | 1 | 0 | 0 | 0 | 19 | 24 | 0 | — | — | 45 (4.3) | 31 | 2.21 | 72 (44-93) |
| AA | 514 | 499 | 564 | 634 | 566 | 752 | 828 | 881 | 5.24 | (4.46-6.02) | 5238 | 2882 | 1.22 | — |
| DPC study database | Year | Estimated Frequency (95% CI) | ||||||||||||
| — | — | — | 2015 | 2016 | 2017 | 2018 | 2019 | — | — | — | — | |||
| National population (in millions) | — | 125.3 | 125.3 | 125.3 | 124.2 | 123.7 | — | — | — | — | — | — | ||
| PRCA | — | — | — | 130 | 119 | 123 | 100 | 139 | 0.98 | (0.89-1.07) | — | — | — | |
| Chronic acquired PRCA | — | 47 | 57 | 52 | 46 | 80 | — | — | — | — | — | — | ||
| Transient acquired PRCA | — | 23 | 11 | 14 | 14 | 21 | — | — | — | — | — | — | ||
| Acquired PRCA, not specified | — | 60 | 51 | 57 | 40 | 38 | — | — | — | — | — | — | ||
| AA | — | — | — | 4257 | 4131 | 3887 | — | 4257 | — | — | — | — | ||
The upper table depicts the number of registered patients with PRCA and AA to the JSH Hematologic Disease Registry between 2012 and 2019. A total of 1055 cases of acquired PRCA have been documented, yielding an annual incidence rate of 1.06 patients per million. The 95% CI was 0.83 to 1.28. A total of 5238 patients with AA were identified, as a reference, during the same period of observation, yielding an annual incidence rate of 5.24 per million (95% CI, 4.46-6.02). Of note, the number of patients with PRCA was 20% of those with AA in this registration. The lower table shows the number of patients with PRCA and AA in the DPC study database between 2015 and 2019. An estimated number of patients with PRCA who require hospital care is 0.98 per million (95% CI, 0.89-1.07). A total of 4175 cases with AA were documented during the same period of registration. Of note, the total number of AA in the DPC database may have been overrepresented since the category of AA in the DPC database might include those with pancytopenia other than AA.
Age and etiological distributions of acquired PRCA in the Ptosh cohort. A total of 1055 patients with PRCA were identified in Ptosh. (A) A violin plot showing the age distribution of the patients. A total of 76% of them were >60 years old. (B) Distributions of the age of the patients with PRCA were depicted by sex, and we did not find a significant difference in age distributions (P = .3). (C) The pie chart shows an etiological distribution of PRCA in the present study. Among the 1055 patients with acquired PRCA, 69.0% had idiopathic PRCA. Other underlying causes included thymoma (9.4%), LGLL (2.5%), CLL (0.6%), and others (14.3%). (D) Age distributions in the 3 major subtypes of acquired PRCA were depicted in a violin plot, and a statistical difference was recognized between idiopathic and thymoma-associated PRCA, with median ages of 75 (range, 20-99) years old and 67 (range, 33-90) years old, respectively (P = .0006). A bimodal age distribution was observed in those with LGLL-associated PRCA. (E) The age distributions were not different between genders in respective subtypes of PRCA. The bimodal distribution in those with LGLL-associated PRCA was preserved in each gender group.
Age and etiological distributions of acquired PRCA in the Ptosh cohort. A total of 1055 patients with PRCA were identified in Ptosh. (A) A violin plot showing the age distribution of the patients. A total of 76% of them were >60 years old. (B) Distributions of the age of the patients with PRCA were depicted by sex, and we did not find a significant difference in age distributions (P = .3). (C) The pie chart shows an etiological distribution of PRCA in the present study. Among the 1055 patients with acquired PRCA, 69.0% had idiopathic PRCA. Other underlying causes included thymoma (9.4%), LGLL (2.5%), CLL (0.6%), and others (14.3%). (D) Age distributions in the 3 major subtypes of acquired PRCA were depicted in a violin plot, and a statistical difference was recognized between idiopathic and thymoma-associated PRCA, with median ages of 75 (range, 20-99) years old and 67 (range, 33-90) years old, respectively (P = .0006). A bimodal age distribution was observed in those with LGLL-associated PRCA. (E) The age distributions were not different between genders in respective subtypes of PRCA. The bimodal distribution in those with LGLL-associated PRCA was preserved in each gender group.
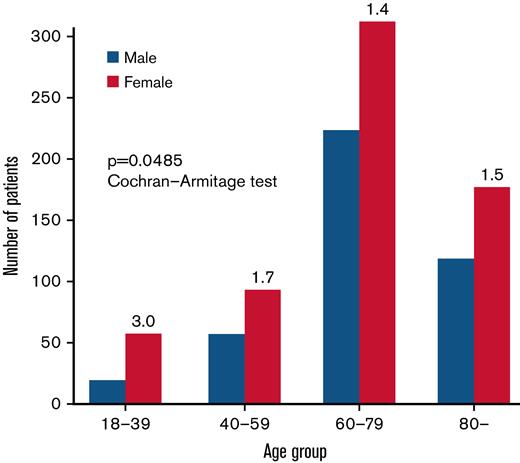
A female predominance was recognized (ratio, 1.5:1) (Table 2). Of note, a female predominance was observed in all age groups. The female-to-male ratio was 3.0 in patients <40 years of age, 1.7 in patients 40 to 59 years of age, 1.4 in patients 60 to 79 years of age, and 1.5 in patients ≥80 years of age (Figure 2). The decreasing tendency of the female-to-male ratio with aging was statistically significant (P = .0485 with Cochran-Armitage test). The female-to-male ratio did not differ to a statistically significant extent among the PRCA subgroups (P = .443 with Fisher’s exact test).
Female predominance of PRCA in different age groups. Green and red bars represent the number of female and male patients, respectively. The number on the top of the green bar indicates the female-to-male ratio of the patients in each age group. The ratio was the highest in those <40 years old or in a child-bearing age group. The decreasing trend in the female-to-male ratio along with their increased age was statistically significant (P = .0485 with Cochran-Armitage test).
Female predominance of PRCA in different age groups. Green and red bars represent the number of female and male patients, respectively. The number on the top of the green bar indicates the female-to-male ratio of the patients in each age group. The ratio was the highest in those <40 years old or in a child-bearing age group. The decreasing trend in the female-to-male ratio along with their increased age was statistically significant (P = .0485 with Cochran-Armitage test).
For reference, a total of 5238 cases with newly diagnosed AA were identified in the JSH Hematologic Disease Registry database during the same observation period (Table 2). The average annual incidence of AA was estimated to be 5.24 patients per million per year. PRCA incidence was estimated to be approximately 20% as frequent as AA. The female predominance in PRCA was statistically greater than that in AA (P = .000972) (Table 3).
Gender distribution of patients with PRCA and AA
| . | Male . | Female . | χ2 test . |
|---|---|---|---|
| PRCA | 416 | 639 | P = 0.000972 |
| AA | 2356 | 2882 |
| . | Male . | Female . | χ2 test . |
|---|---|---|---|
| PRCA | 416 | 639 | P = 0.000972 |
| AA | 2356 | 2882 |
The total number of registered patients with PRCA or AA in the JSH Hematologic Disease Registry database. A relative female predominance was observed in PRCA (P = .000972).
The DPC study data between 2015 and 2019 showed that an average of 122 patients with PRCA were hospitalized each year, amounting to approximately 0.98 patients per million per year (95% CI, 0.89-1.07) (Table 2).
Geographic distribution
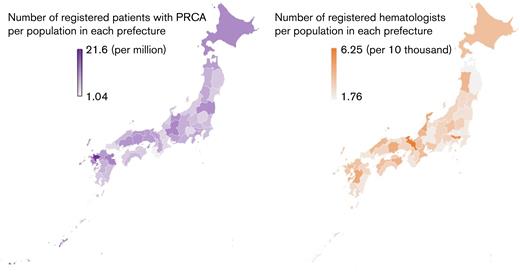
The prefectures where PRCA was diagnosed and the frequency of PRCA in the population of each prefecture are summarized in Figure 4. As a reference, the number of JSH-registered hematologists per population in each prefecture is also depicted. Slight regional differences among the prefectures were observed in the incidence of PRCA, but the density of hematologists was not significantly correlated with the density of patients with PRCA (Pearson’s product–moment correlation 0.0462; 95% CI, −0.244 to 0.329; P = .758).
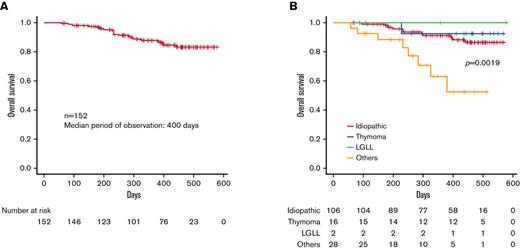
The OS of PRCA in the Ptosh database. (A) A Kaplan-Meier curve of the OS of 153 patients with PRCA in the Ptosh registration was depicted. The OS was defined as the period from the date of the diagnosis to the date of the outcome. The median period of observation was 400 days, and the presumed OS rate at 1 year was 87.6% (95% CI, 80.5-92.2%). (B) The OS curves for idiopathic and thymoma-associated PRCA were juxtaposed, while PRCA with “other” etiologies conferred an inferior survival (P = .0019). The estimated 1-year OS rates were 91.4% (95% CI, 83.4-95.6%), 92.9% (95% CI, 59.1-99.0%), and 63.1% (95% CI, 35.8-81.3%) for idiopathic, thymoma, and others, respectively.
The OS of PRCA in the Ptosh database. (A) A Kaplan-Meier curve of the OS of 153 patients with PRCA in the Ptosh registration was depicted. The OS was defined as the period from the date of the diagnosis to the date of the outcome. The median period of observation was 400 days, and the presumed OS rate at 1 year was 87.6% (95% CI, 80.5-92.2%). (B) The OS curves for idiopathic and thymoma-associated PRCA were juxtaposed, while PRCA with “other” etiologies conferred an inferior survival (P = .0019). The estimated 1-year OS rates were 91.4% (95% CI, 83.4-95.6%), 92.9% (95% CI, 59.1-99.0%), and 63.1% (95% CI, 35.8-81.3%) for idiopathic, thymoma, and others, respectively.
Geographical distribution of PRCA occurrence in Japan. The prefectural disparities in (A) the number of patients with PRCA registered in Ptosh and (B) the numbers of JSH hematologists. Although a regional PRCA incidence varies between 1.04 and 21.6 per million, it was not correlated with a hematologist density (Pearson’s product-moment correlations 0.0462; 95% CI, −0.244 to 0.329; P = .0758).
Geographical distribution of PRCA occurrence in Japan. The prefectural disparities in (A) the number of patients with PRCA registered in Ptosh and (B) the numbers of JSH hematologists. Although a regional PRCA incidence varies between 1.04 and 21.6 per million, it was not correlated with a hematologist density (Pearson’s product-moment correlations 0.0462; 95% CI, −0.244 to 0.329; P = .0758).
Etiology
Figure 1C shows the etiological breakdowns of PRCA in the JSH Hematologic Disease Registry; we identified 69.0% of the diagnoses as idiopathic PRCA, followed by thymoma-associated PRCA (9.4%), LGLL-associated PRCA (2.5%), CLL-associated PRCA (0.6%), and others (14.3%). The etiology was not reported in 4.3% of the patients. The median age (range) for the different underlying causes of acquired PRCA was 75 (20-99) years for idiopathic PRCA, 67 (33-90) years for thymoma-associated PRCA, 74.5 (56-88) years for LGLL-associated PRCA, 64.5 (35-71) years for CLL-associated PRCA, and 64 (18-89) years for others (Table 2). A significant difference in age was found between idiopathic and thymoma-associated PRCA (Steel-Dwass test: P = .0006) (Figure 1D). Of note, LGLL-associated PRCA had a bimodal age distribution pattern in both sexes.
Survival
Survival data were available for 161 patients with PRCA in the JSH Hematologic Disease Registry database (male, n = 65; female, n = 96). A Kaplan-Meier curve for the OS was drawn for 152 patients with known etiologies, as depicted in Figure 3A. The median observation period was 400 days, and the estimated 1-year OS rate was 87.6% (95% CI, 80.5-92.2%). There was no significant sex difference in OS (P = .135). The Kaplan-Meier curves for idiopathic PRCA and thymoma-associated PRCA were juxtaposed, while PRCA due to other causes conferred inferior survival (P = .0019) (Figure 3B). The estimated 1-year OS rates for idiopathic PRCA, thymoma-associated PRCA, and others were 91.4% (95% CI, 83.4-95.6%), 92.9% (95% CI, 59.1-99.0%), and 63.1% (95% CI, 35.8-81.3%), respectively.
Discussion
The present study confirmed that PRCA is a rare disease: the annual incidence is 1.06 per million, and 0.98 patients per million require hospitalization each year. Although the true prevalence of the disease is unclear from this study, our results are expected to contribute to the discussion about the allocation of resources for PRCA in the aging populations of many developed countries, including Japan. In addition, the results of the present study should reflect the growing elderly population and the rising incidence of underlying diseases, such as malignancies. Although the advent of novel therapies for CLL in the past 10 years may have altered the natural history of patients with this disease,27 CLL-associated PRCA accounted for <1% of the total cohort, and the paradigmatic impact of CLL therapies on the epidemiology of PRCA may have been quite low in Japan. Of note, this may also reflect the low incidence of CLL in the Japanese population.28
A female predominance in PRCA was previously described in some reports21,29 but denied in others.6 However, in our JSH Registry cohort, the female-to-male ratio was 1.54, and the female predominance in PRCA was significantly greater than that in AA. The fact that most autoimmune collagenous diseases show sexual predominance is often attributable to the role of sex hormones.30 The incidence of systemic lupus erythematosus (SLE), in which females outnumber males by 9:1, increases after puberty and diminishes after menopause, supporting the hypothesis that sex hormones play a role in its etiology.31,32 Likewise, in our JSH Registry cohort, the female-to-male ratio seemed most prominent in the child-bearing age group (Figure 2), and it is possible that sex hormones have etiological relevance in PRCA as well, although the sex difference was smaller than that in SLE. The female-to-male ratio varied among the subtypes of PRCA, implying that sex hormones may play different pathophysiological roles, if they play a role at all, in different subtypes.
There have been only a few reports concerning the survival of patients with PRCA. Hirokawa and colleagues showed that the estimated mean OS time of patients with idiopathic, thymoma-associated, and LGLL-associated PRCA was 212.6, 142.1, and 147.8 months, respectively.33 Our results imply that some subtypes of PRCA may confer poor survival. However, there may be a selection bias, as the outcome data were only available for 15% of the JSH Hematologic Disease Registry cohort. The median period of observation was short as well. A prospective study that includes more detailed subtype information is needed to improve the precision of the prognostication.
However, several other limitations warrant mention. First, these results apply to the Japanese population, and the epidemiology may differ among countries. Possible explanations for such differences between countries include differing age structures and incidences of underlying diseases of secondary PRCA. Given that PRCA develops among the elderly more frequently than among younger patients, a higher incidence may be expected in aging populations. The different age distribution may also result in a different incidence of PRCA and a different distribution of underlying etiologies of PRCA. Another possible reason for the disparity between countries may be differences in the accessibility of medical services. Accessibility to evidence-based medicine is an issue of global concern, especially in low-income societies. There may be a sampling bias. For example, data may not be submitted to either database when a small-scaled hospital has limited human resources for data submission or when a primary physician takes care of a patient with PRCA with a curbside consultation of a hematologist. Such underestimation cannot be ruled out; however, the academic and economic incentives for increasing submission may eventually minimize such sampling bias.
Acknowledgments
The authors would like to thank the Japanese Society of Hematology (JSH) and its committee members for providing the relevant data from the Blood Disease Registration database and all JSH members for registering their patients in the JSH Hematologic Disease Registry. They also thank Makoto Hirokawa (Akita University, Akita, Japan) for years of collaboration and advice.
This study was supported in part by a research grant from the Japanese National Research Group on Idiopathic Bone Marrow Failure Syndromes of the Ministry of Health Labor and Welfare of Japan.
Authorship
Contribution: F.I., A.O., and H.N. designed research; H.N., K.S., and F.I. performed research; H.N. and F.I. wrote the manuscript; and N.F., A.M., K.H., F.N., S.N., and K.M. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fumihiro Ishida, Department of Biomedical Laboratory Sciences, Shinshu University School of Medicine, Matsumoto, Japan, 3-1-1 Asahi, Matsumoto, Nagano 390-8621, Japan; e-mail: fumishi@shinshu-u.ac.jp; and Hideyuki Nakazawa, Department of Hematology, Shinshu University School of Medicine, 3-1-1 Asahi, Matsumoto, Nagano 390-8621, Japan; e-mail: hnaka@shinshu-u.ac.jp.
References
Author notes
∗H.N. and F.I. contributed equally to this study.
Contact the corresponding author for data sharing: fumishi@shinshu-u.ac.jp.
The full-text version of this article contains a data supplement.