Key Points
Caplacizumab reduces exacerbation and refractoriness in iTTP.
As initial therapy, caplacizumab accelerates response and reduces the need for PEX and hospital stay.
Abstract
Immune thrombotic thrombocytopenic purpura (iTTP) is a thrombotic microangiopathy caused by anti-ADAMTS13 antibodies. Caplacizumab is approved for adults with an acute episode of iTTP in conjunction with plasma exchange (PEX) and immunosuppression. The objective of this study was to analyze and compare the safety and efficacy of caplacizumab vs the standard of care and assess the effect of the concomitant use of rituximab. A retrospective study from the Spanish TTP Registry of patients treated with caplacizumab vs those who did not receive it was conducted. A total of 155 patients with iTTP (77 caplacizumab, 78 no caplacizumab) were included. Patients initially treated with caplacizumab had fewer exacerbations (4.5% vs 20.5%; P < .05) and less refractoriness (4.5% vs 14.1%; P < .05) than those who were not treated. Time to clinical response was shorter when caplacizumab was used as initial treatment vs caplacizumab used after refractoriness or exacerbation. The multivariate analysis showed that its use in the first 3 days after PEX was associated with a lower number of PEX (odds ratio, 7.5; CI, 2.3-12.7; P < .05) and days of hospitalization (odds ratio, 11.2; CI, 5.6-16.9; P < .001) compared with standard therapy. There was no difference in time to clinical remission in patients treated with caplacizumab compared with the use of rituximab. No severe adverse event was described in the caplacizumab group. In summary, caplacizumab reduced exacerbations and refractoriness compared with standard of care regimens. When administered within the first 3 days after PEX, it also provided a faster clinical response, reducing hospitalization time and the need for PEX.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is a rare disease characterized by microangiopathic hemolytic anemia, severe thrombocytopenia (<30 × 109/L), and a variable degree of ischemic organ damage.1 Organ ischemia includes basically the cerebral, myocardial, renal, and gastrointestinal systems.2 iTTP is characterized by severely deficient activity (<10% levels) of ADAMTS13, a cleaving protease of von Willebrand factor (vWF). In iTTP, ADAMTS13 deficiency is acquired via the production of autoantibodies against ADAMTS13, which inhibit ADAMTS13 proteolytic activity toward vWF or increase its clearance.3,4 The lack of ADAMTS13 activity leads to platelet/vWF multimer aggregation, causing microthrombi within small arterioles.
In Spain, the estimated annual incidence of iTTP is 2.67 individuals per million,5 in line with data reported in other western countries.6 Given the rarity of the disease, iTTP registries are essential if a deeper knowledge of the disease and its management is to be obtained.
iTTP is associated with high morbidity and mortality. If it is left untreated, the mortality rate rises to 90% but falls to 5% to 20% when appropriately treated.7,8 Death usually occurs within the initial days of management. Therefore, early diagnosis and treatment are of paramount importance. Until recently, the standard first-line therapy for iTTP has been empirical and includes plasma exchange (PEX) and concomitant immunosuppressive therapy (corticosteroids with or without rituximab).2,9 PEX and immunosuppressant treatment may take several days or weeks, however, to produce a response. Consequently, some patients, mostly those with risk factors for death, die of the disease.8,10
Caplacizumab, a novel humanized nanobody targeting vWF that blocks platelet–vWF aggregation, was the first drug approved in the European Union for iTTP (2018). It has shown a fast effect within hours, preventing thrombi formation. Early caplacizumab has been shown to reduce the time to clinical response, refractoriness, mortality rate, and recurrence rate, and is efficacious both in the initial episode and in exacerbation or relapse.11-13
Several European registries have already reported their experience outside clinical trial populations.14-16 To provide real-world evidence in our country, the objective of this study was to retrospectively analyze the efficacy and safety of caplacizumab in patients with iTTP included in the Spanish TTP Registry (Registro Español de PTT [REPTT]) and to compare these data vs a cohort of patients not treated with this drug. Finally, we sought to describe the role of rituximab associated with caplacizumab.
Methods
In this retrospective study, subjects were identified from the REPTT. During the study period, caplacizumab was only available in Spain to treat patients with iTTP through the Sanofi MAP (Medical Access Program). The MAP allowed for the extended use of caplacizumab for 30 days after PEX, although it could be used even longer if ADAMTS13 was abnormal. Patients with iTTP who received caplacizumab between June 2018 and November 2020 were included. The decision to treat with caplacizumab depended on timely access to MAP, clinical criteria, and the physicians’ knowledge of drug availability. This group was compared with all the REPTT patients treated during the same period who did not receive caplacizumab.
Demographic, clinical, and analytical data were collected retrospectively from medical records. The expression “initial treatment” is henceforth used to indicate the intention to use a drug or therapy from the beginning of treatment, although due to the procedures required to obtain the medication it could be delayed several days. The caplacizumab treatment initiation day was defined as the day PEX began. We considered caplacizumab to be a second-line treatment if it was used due to exacerbation or refractoriness.
The definitions of clinical response, clinical remission, exacerbation, and relapse considered were as previously defined by the International Working Group for Thrombotic Thrombocytopenic Purpura.17,18 Refractoriness was defined as platelet counts <50 × 109/L and persistently increased lactate dehydrogenase (LDH) levels (>1.5 upper limit of normal) despite five PEX. Time to normalization of platelet count was defined as the time from the first PEX to the first day with a count ≥150 × 109/L. Exacerbation was defined as an unjustified decrease in platelet counts and elevation of LDH within 30 days as of the suspension of treatment for clinical remission and requiring the re-initiation of PEX. The survival analysis was measured in days or months and was defined as the period between the PEX and the occurrence of the event of interest. ADAMTS13 levels during follow-up were measured almost only in patients treated with caplacizumab according to MAP requirements. Adverse events were also recorded and graded according to the Common Toxicity Criteria for Adverse Events. Refractoriness was defined as platelet counts <50 × 109/L and a persistently raised LDH level (>1.5 upper limit of normal) despite five PEX. First PEX to exacerbation was defined as an unjustified decrease in platelet counts and raise of LDH before 30 days from treatment suspension due to clinical remission and that requires re-start PEX. PEX ADAMTS13 levels during follow-up were measured almost only in patients treated with caplacizumab according to MAP requirements.
The primary end point was the time to clinical response since the first PEX. The secondary end points were the total number of PEX procedures, hospital stay, and percentage of clinical remission.
Statistical analysis
A descriptive analysis of the demographic and clinical data was performed. For continuous variables, the mean and standard deviation or median with interquartile range (IQR) were calculated. Categorical variables are presented as number (n) and frequencies (percentages). The Shapiro-Wilks normality test was used to decide whether t test/analysis of variance or Mann-Whitney/Kruskal-Wallis tests should be used and whether the normality assumption was fulfilled to compare groups for quantitative outcomes. Pearson’s χ2 test and Fisher’s exact test were used for bivariate comparisons with nominal variables. Kaplan-Meier survival estimates were used to describe “time to” variables. Cox regression was used to evaluate factors related to “time to” variables. Multivariate regression analysis was used to evaluate factors related to length of hospitalization, intensive care unit stay, and number of PEX procedures.
No formal sample size calculation was performed. All tests were two-sided, and P < .05 was considered statistically significant. The statistical analysis was conducted by using SPSS version 25.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY). All patients gave their informed consent for their data to be recorded in the REPTT to be used for research purposes. The Ethics Committee of the Hospital Clínic de Barcelona evaluated and approved the study protocol. The study was conducted according to the Declaration of Helsinki.
Results
Patient characteristics
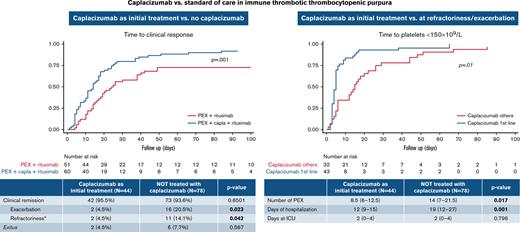
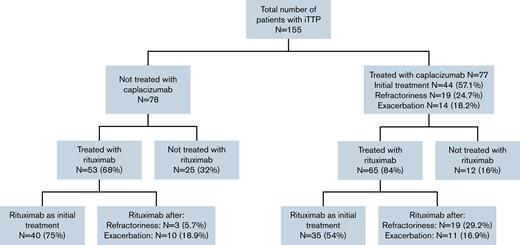
In this retrospective study, 155 patients with iTTP from 35 centers were included: 77 received caplacizumab and 78 did not (control group). All the patients were also treated with PEX and prednisone 1 mg/kg per day or the equivalent dose of corticosteroids (standard therapy). Eighty-four percent (65 of 77) of the patients treated with caplacizumab also received rituximab, and almost 68% (53 of 78) of the patients treated with standard therapy alone also received rituximab. The reasons for starting treatment with caplacizumab and rituximab are described in Figure 1.
The starting day of caplacizumab was variable (median time from PEX was 5 days; IQR, 2-11 days), with a median duration of 35 days (IQR, 31-40 days). Overall, 44 (57%) patients received caplacizumab as initial treatment, and the other 33 patients received caplacizumab as second line: 19 (24.7%) at refractoriness and 14 (18.2%) after exacerbation. The median time to start caplacizumab treatment in each of these subgroups is shown in Table 1. The median follow-up of the caplacizumab group was 216 days (IQR: 141-417 days) vs 214 days (IQR: 138-467 days) in the non-caplacizumab group.
Time to start caplacizumab treatment as initial treatment or after refractoriness or exacerbation in days
| . | N . | Median . | IQR . | P . |
|---|---|---|---|---|
| Initial treatment | 44 | 2 | 1-4 | <.0001 |
| Exacerbation | 19 | 14 | 10-16 | |
| Refractoriness | 14 | 10 | 8-13 |
| . | N . | Median . | IQR . | P . |
|---|---|---|---|---|
| Initial treatment | 44 | 2 | 1-4 | <.0001 |
| Exacerbation | 19 | 14 | 10-16 | |
| Refractoriness | 14 | 10 | 8-13 |
The demographic and initial disease characteristics of the patients included in the study are described in Table 2. No differences were observed between treatment groups regarding gender or age. In the caplacizumab cohort, 65% of the episodes were disease onset compared with 92% in the nontreated group (P < .001). Before treatment, signs of bleeding diathesis were more frequent in the caplacizumab group (58.4% vs 29.5%; P < .001). The patients enrolled had a median platelet count of 12 × 109/L, and more than one-half of them presented neurologic involvement. There were no differences in the laboratory parameters at diagnosis between groups.
Baseline patient characteristics
| Baseline characteristic . | Caplacizumab cohort (n = 77) . | Non-caplacizumab cohort (n = 78) . | P . |
|---|---|---|---|
| Female, N (%) | 58/77 (75%) | 61/78 (78%) | .6711 |
| Age, mean ± SD, y | 47.1 ± 14 | 46.5 ± 14 | .833 |
| First episode iTTP, N (%) | 50/77 (65%) | 72/78 (92%) | <.001 |
| Symptoms/signs, N (%) | |||
| Anemic syndrome∗ | 41/77 (53.2%) | 36/78 (46.2%) | .3772 |
| Neurologic involvement | 43/77 (55.8%) | 47/78 (60.3%) | .4510 |
| Neurologic focal signs† | 22/43 (51.2%) | 16/47 (34.0%) | |
| Headache | 11/43 (25.6%) | 15/47 (31.9%) | |
| Others | 11/43 (25.6%) | 10/47 (21.3%) | |
| Elevated troponin | 22/44 (50%) | NA | |
| Hemorrhagic diathesis‡ | 45/77 (58.4%) | 23/78 (29.5%) | <.001 |
| Mucocutaneous hemorrhage | 39/45 (86.7%) | 18/23(78.3%) | .010 |
| Menorrhagia | 5/46 (10.9%) | 0/23 (0%) | |
| Others | 1/45 (2.2%) | 5/23 (21.7%) | |
| Renal insufficiency/failure§ | 19/77 (24.7%) | 19/76 (25.0%) | .9629 |
| Laboratory parameters at diagnosis, median (IQR) [range] | |||
| Hemoglobin, g/dL | 8.7 (7.2-11.1) [5.7-16.6] | 8.7 (6.85-10.3) [5.7-14.6] | .219 |
| Platelets, ×109/L | 12 (8-20) [3-94] | 12 (8-17.5) [3-57] | .802 |
| WBC, ×109/L | 8.19 (6.7-11.4) [3.2-23] | 9.1 (7.13-12.09) [3.2-23.3] | .226 |
| Indirect bilirubin, mg/dL | 1.61 (0.995-2.35) [ 0.2-8] | NA | |
| LDH, U/L | 1040 (607-1546) [169-7334] | 1086 (715.5-1550) [291-7334] | .497 |
| Creatinine, mg/dL | 0.95 (0.73-1.2) [0.45-2.78] | 0.92 (0.72-1.3) [0.54-5.49] | .803 |
| ADAMTS13, % | 0 (0-0.5) [0-13] | 0 (0-0.5) [0-19] | .954 |
| ADAMTS13 inhibitor, BU | 3.3 (7-20.93) [0-112.2] | NA | |
| Baseline characteristic . | Caplacizumab cohort (n = 77) . | Non-caplacizumab cohort (n = 78) . | P . |
|---|---|---|---|
| Female, N (%) | 58/77 (75%) | 61/78 (78%) | .6711 |
| Age, mean ± SD, y | 47.1 ± 14 | 46.5 ± 14 | .833 |
| First episode iTTP, N (%) | 50/77 (65%) | 72/78 (92%) | <.001 |
| Symptoms/signs, N (%) | |||
| Anemic syndrome∗ | 41/77 (53.2%) | 36/78 (46.2%) | .3772 |
| Neurologic involvement | 43/77 (55.8%) | 47/78 (60.3%) | .4510 |
| Neurologic focal signs† | 22/43 (51.2%) | 16/47 (34.0%) | |
| Headache | 11/43 (25.6%) | 15/47 (31.9%) | |
| Others | 11/43 (25.6%) | 10/47 (21.3%) | |
| Elevated troponin | 22/44 (50%) | NA | |
| Hemorrhagic diathesis‡ | 45/77 (58.4%) | 23/78 (29.5%) | <.001 |
| Mucocutaneous hemorrhage | 39/45 (86.7%) | 18/23(78.3%) | .010 |
| Menorrhagia | 5/46 (10.9%) | 0/23 (0%) | |
| Others | 1/45 (2.2%) | 5/23 (21.7%) | |
| Renal insufficiency/failure§ | 19/77 (24.7%) | 19/76 (25.0%) | .9629 |
| Laboratory parameters at diagnosis, median (IQR) [range] | |||
| Hemoglobin, g/dL | 8.7 (7.2-11.1) [5.7-16.6] | 8.7 (6.85-10.3) [5.7-14.6] | .219 |
| Platelets, ×109/L | 12 (8-20) [3-94] | 12 (8-17.5) [3-57] | .802 |
| WBC, ×109/L | 8.19 (6.7-11.4) [3.2-23] | 9.1 (7.13-12.09) [3.2-23.3] | .226 |
| Indirect bilirubin, mg/dL | 1.61 (0.995-2.35) [ 0.2-8] | NA | |
| LDH, U/L | 1040 (607-1546) [169-7334] | 1086 (715.5-1550) [291-7334] | .497 |
| Creatinine, mg/dL | 0.95 (0.73-1.2) [0.45-2.78] | 0.92 (0.72-1.3) [0.54-5.49] | .803 |
| ADAMTS13, % | 0 (0-0.5) [0-13] | 0 (0-0.5) [0-19] | .954 |
| ADAMTS13 inhibitor, BU | 3.3 (7-20.93) [0-112.2] | NA | |
BU, Bethesda unit; LDH, lactate dehydrogenase; NA, not available; SD, standard deviation; WBC, white blood cells.
Boldface means statistical significant.
Includes fatigue, weakness, and dizziness symptoms.
Includes stroke, cardiovascular events, and transient ischemic attacks.
Required only 1 red blood cell transfusion and stopping caplacizumab treatment for 48 hours in 1 patient with rectal bleeding due to gut polyposis. The event remitted and did not require vWF or other treatments.
Defined as creatinine level >1.5 mg/dL.
Description of caplacizumab use and disease course
Global clinical remission was high and similar between groups: 95.7% of patients treated with caplacizumab and 94.8% in patients not treated with caplacizumab. Four (5.2%) of 77 patients treated with caplacizumab presented exacerbations, and 5 (6.5%) of 77 had refractoriness, compared with 16 (20.5%) of 78 and 11 (14%) of 78 in the nontreated group, respectively. When the patients treated with caplacizumab as initial treatment were analyzed, only 4.5% of them experienced exacerbations, compared with 20.5% of those not treated with caplacizumab (P < .05). In addition, fewer patients treated initially with caplacizumab were refractory compared with those not treated with caplacizumab (4.5% vs 14.1%; P < .05) (Table 3). Overall, caplacizumab as initial treatment rescued 95% of refractory patients (18 of 19) and 93% of patients with exacerbations (13 of 14).
Outcomes of patients treated with caplacizumab as initial treatment vs patients not treated with caplacizumab
| . | patients with iTTP treated with caplacizumab as initial treatment (n = 44) . | patients with iTTP not treated with caplacizumab (n = 78) . | P . |
|---|---|---|---|
| Clinical remission | 42/44 (95.5%) | 73/78 (93.6%) | .6501 |
| Exacerbation | 2/44 (4.5%) | 16/78 (20.5%) | .023 |
| Refractoriness∗ | 2/44 (4.5%) | 11/78 (14.1%) | .042 |
| Death | 2/44 (4.5%) | 6/78 (7.7%) | .567 |
| . | patients with iTTP treated with caplacizumab as initial treatment (n = 44) . | patients with iTTP not treated with caplacizumab (n = 78) . | P . |
|---|---|---|---|
| Clinical remission | 42/44 (95.5%) | 73/78 (93.6%) | .6501 |
| Exacerbation | 2/44 (4.5%) | 16/78 (20.5%) | .023 |
| Refractoriness∗ | 2/44 (4.5%) | 11/78 (14.1%) | .042 |
| Death | 2/44 (4.5%) | 6/78 (7.7%) | .567 |
The 2 patients who were refractory to treatment were those who died, although they were different from the other 2 who had an exacerbation.
Fewer of the patients treated with caplacizumab (3 of 77 [3.8%]) died than those who were not treated with it (6 of 78 [7.7%]), albeit without statistically significant differences. Two of 3 patients in the caplacizumab group died during an acute episode (supplemental Table 1). The third patient was a 48-year-old patient who died 4 months after achieving clinical remission and normalization of ADAMTS13 in the context of cytomegalovirus and Aspergillus infection. This patient was treated with steroids (156 days from start to suspension), 21 daily PEX, rituximab 375 mg/m2 per week for 44 total doses, and N-acetyl cysteine. Caplacizumab was started because of exacerbation. The patient achieved clinical response after 13 PEX after diagnosis, but she experienced an exacerbation 9 days later, whereupon PEX and caplacizumab were reinitiated, and a clinical response was obtained in 8 days. A total of 9 PEX and a further 30 doses of caplacizumab were used. The patient had no other infectious risk factors or immunodeficiency other than immunosuppressive treatment for an acute iTTP episode. Supplemental Table 1 summarizes the clinical characteristics and evolving features of the 8 patients (2 with and 6 without caplacizumab) who died during an acute episode.
Time to platelet count normalization, PEX, and length of hospital stay
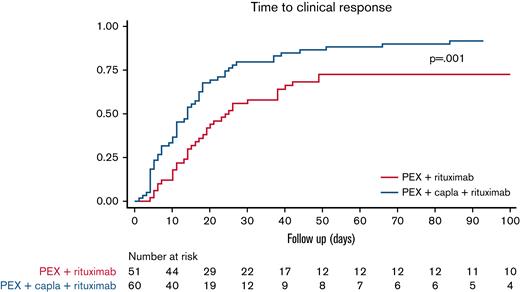
The median time to clinical response was shorter in patients treated with caplacizumab as initial treatment compared with patients not treated with caplacizumab: 8.5 (IQR, 6-12.5) days vs 14 (IQR, 7-21) days (P = .009). To assess the real impact of caplacizumab treatment on the course of the disease and to avoid potential biases, we compared the subgroup of patients treated with caplacizumab as initial treatment (n = 44) vs patients treated with caplacizumab only after an exacerbation or refractoriness. The median time to clinical response was shorter when caplacizumab was used initially than when it was used after refractoriness or an exacerbation (median, 5 days vs 15 days; P < .01; hazard ratio, 2.30; 95% CI, 1.41-3.76) (Figure 2).
Survival analysis. Time to clinical response in patients treated with caplacizumab as initial treatment (n = 43∗) vs caplacizumab used in refractoriness or exacerbation (n = 32∗). (It should be noted that all patients also received PEX and corticosteroids). ∗Missing values.
Survival analysis. Time to clinical response in patients treated with caplacizumab as initial treatment (n = 43∗) vs caplacizumab used in refractoriness or exacerbation (n = 32∗). (It should be noted that all patients also received PEX and corticosteroids). ∗Missing values.
Moreover, among patients who received caplacizumab as initial treatment, the time to clinical response was significantly shorter in patients treated within the first 3 days compared with those who started later. When caplacizumab was started within the first 3 days, the median time to platelet count normalization was 4 days (IQR, 3-5 days), compared with 5 days (IQR, 5-9 days) if it was started between 4 and 6 days, and 14.5 days (IQR, 10-26.5 days) if it was delayed for ≥7 days (P = .003) (supplemental Table 2). In other words, the earlier the caplacizumab treatment was started, the faster a clinical response was achieved.
Efficacy outcomes linked to health care resource utilization were compared between patients treated initially with caplacizumab and patients not treated with caplacizumab. Caplacizumab reduced the number of PEX (P < .05) and length of hospitalization (P < .001). No difference in the length of intensive care unit stay between treatments was observed (P = .796) (Table 4). Subsequently, a multivariate analysis was performed to identify factors linked to length of hospitalization and number of PEX, and it transpired that patients treated with caplacizumab within 3 days from the first PEX had shorter hospitalizations (11.2 days fewer on average). Moreover, a hemoglobin level <8 g/dL was related to longer hospitalizations (7.5 more days on average) (supplemental Table 3). Furthermore, treatment with caplacizumab within the first 3 days from the first PEX was related to less need for PEX. In contrast, older patients and treatment with rituximab were associated with a higher number of PEX. Neurologic manifestations were associated with both shorter hospitalizations and fewer PEX. We were unable to identify any variable associated with the use of rituximab or the presence of neurologic alterations that might have skewed these findings.
Response of patients treated with caplacizumab as initial treatment vs patients not treated with caplacizumab in terms of number of PEX, days of hospitalization, and days in the intensive care unit
| . | patients with iTTP treated with caplacizumab as initial treatment . | patients with iTTP not treated with caplacizumab . | P . |
|---|---|---|---|
| No. of PEX | |||
| N | 44 | 76 | .017 |
| Mean ± SD | 12.39 ± 11.8 | 18.51 ± 19.05 | |
| Median (IQR) | 8.5 (6-12.5) | 14 (7-21.5) | |
| Days of hospitalization | |||
| N | 44 | 51 | .001 |
| Mean ± SD | 14.68 ± 12.36 | 21.94 ± 14.42 | |
| Median (IQR) | 12 (9-15) | 19 (12-27) | |
| Days in ICU | |||
| N | 42 | 71 | .796 |
| Mean ± SD | 2.31 ± 2.62 | 2.79 ± 3.51 | |
| Median (IQR) | 2 (0-4) | 2 (0-4) | |
| . | patients with iTTP treated with caplacizumab as initial treatment . | patients with iTTP not treated with caplacizumab . | P . |
|---|---|---|---|
| No. of PEX | |||
| N | 44 | 76 | .017 |
| Mean ± SD | 12.39 ± 11.8 | 18.51 ± 19.05 | |
| Median (IQR) | 8.5 (6-12.5) | 14 (7-21.5) | |
| Days of hospitalization | |||
| N | 44 | 51 | .001 |
| Mean ± SD | 14.68 ± 12.36 | 21.94 ± 14.42 | |
| Median (IQR) | 12 (9-15) | 19 (12-27) | |
| Days in ICU | |||
| N | 42 | 71 | .796 |
| Mean ± SD | 2.31 ± 2.62 | 2.79 ± 3.51 | |
| Median (IQR) | 2 (0-4) | 2 (0-4) | |
ICU, intensive care unit; SD, standard deviation.
Influence of the use of rituximab on patient response
Up to 84% (65 of 77) of the patients treated with caplacizumab also received rituximab, a higher percentage than the non-caplacizumab group (68% [53 of 78]) (P = .018) (Figure 1). In the caplacizumab group (n = 65), rituximab was used as initial treatment only in 35 (53.8%) patients, compared with 40 (75%) in the non-caplacizumab group (n = 53). Of all patients treated with rituximab (n = 111), those treated with caplacizumab achieved clinical response 1.96 times faster than those without caplacizumab: 14 days vs 24 days (hazard ratio, 1.96; 95% CI: 1.29-2.99; P < .001) (Figure 3).
Survival analysis. Time to clinical response in patients treated with rituximab + PEX, with or without the addition of caplacizumab (n = 111∗). (It should be noted that all patients also received corticosteroids.) ∗Seven patients were lost to follow-up: 2 patients receiving rituximab and 5 patients receiving rituximab and caplacizumab. Capla, caplacizumab.
Survival analysis. Time to clinical response in patients treated with rituximab + PEX, with or without the addition of caplacizumab (n = 111∗). (It should be noted that all patients also received corticosteroids.) ∗Seven patients were lost to follow-up: 2 patients receiving rituximab and 5 patients receiving rituximab and caplacizumab. Capla, caplacizumab.
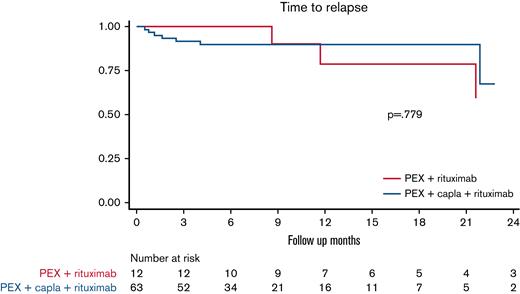
No differences were observed in the median time to relapse between patients treated with caplacizumab plus rituximab vs patients treated with caplacizumab alone (Figure 4) or in patients treated with caplacizumab and rituximab as initial treatment vs patients treated after an exacerbation or refractoriness (not shown). After the treatment response analysis, we estimated the median time to ADAMTS13 >10%, and no differences were found between patients treated with caplacizumab plus rituximab vs patients treated with caplacizumab alone (28 vs 29 days; hazard ratio, 0.93; 95% CI, 0.39-2.23; P > .05) (supplemental Figure 1).
Time to relapse in patients treated with caplacizumab + rituximab vs patients treated with caplacizumab alone (n = 75∗). (It should be noted that all patients also received PEX and corticosteroids.) ∗Two patients were lost to follow-up in the caplacizumab + rituximab group. Capla, caplacizumab.
Time to relapse in patients treated with caplacizumab + rituximab vs patients treated with caplacizumab alone (n = 75∗). (It should be noted that all patients also received PEX and corticosteroids.) ∗Two patients were lost to follow-up in the caplacizumab + rituximab group. Capla, caplacizumab.
Safety data
Overall, 36% of the patients treated with caplacizumab reported adverse events. Bleeding events were reported in 16 (20%) of 77 patients after receiving caplacizumab, although only 2 were classified as grade ≥2. Gingivorrhagia was the most common bleeding event, followed by metrorrhagia (19% and 10%, respectively). Fifty-five percent of all bleeding events resolved spontaneously; in the rest of the cases, suspending caplacizumab for <48 hours sufficed, with no need for hemostatic treatment. Thrombocytosis occurred in 20% of patients treated with caplacizumab (supplemental Table 4).
One patient died only 1 day after starting caplacizumab due to multiorgan failure with intracranial hemorrhage, confirmed in the necropsy (supplemental Table 1). Reviewing the clinical course, her doctors did not describe the adverse event as related to caplacizumab but rather to thrombocytopenia, endothelial impairment due to hypoxia, and cardiorespiratory arrest prior to multiorgan failure.
As described earlier, one of the patients treated with rituximab developed severe infections that led to death outside the acute episode 4 months later. Infections occurred as an adverse event when the patient was treated with immunosuppressants to solve TTP. The patient had also received steroids for 156 days, although the dose is unknown. No other rituximab-related adverse events were described.
Discussion
This study provides real-world data on the efficacy and safety of caplacizumab in patients with iTTP managed in Spain, confirming the therapeutic benefits of caplacizumab used as initial treatment. Our results emphasize the beneficial outcomes of caplacizumab use, especially in the first 3 days, since it is associated with a faster clinical response, less refractoriness and subsequently fewer exacerbations, and shorter length of hospitalization.
The patients included in our study were similar to those described in other iTTP registries. Our sample is fully representative of the iTTP population, although no sample size was calculated. Both groups of patients (treated and not treated with caplacizumab) were treated in the same period and had practically the same number of patients. As expected, most patients were female (women are 2-3 times likelier to develop iTTP), in their fourth to fifth decade of life, presenting neurologic symptoms at baseline, as previously reported.7 Most of the patients received caplacizumab as initial treatment (57%), and the rest started only after an exacerbation or refractoriness. It should be pointed out that all patients received caplacizumab within a MAP setting, which is therefore a different scenario from routine clinical practice in Spain. As explained in the Methods, treatment with caplacizumab or not depended on timely access to the MAP, clinical judgment and physicians' knowledge of drug availability.
Our data show that early treatment with caplacizumab permits a faster clinical response, confirming previous findings from both clinical studies and real-world data.11,12,14-16 The time to platelet count normalization and clinical remission was much shorter among patients who received caplacizumab within 3 days of the first PEX; this subsequently prevented unfavorable clinical outcomes. Patients treated with caplacizumab as initial treatment also had significantly fewer exacerbations and less refractoriness than patients receiving steroids and PEX. These data reinforce the current role of caplacizumab as initial treatment and the importance of its rapid availability in the hospital setting to avoid delays in obtaining a response.
The early prevention of microcirculation occlusion and ischemic organ damage with caplacizumab may avoid long-term complications and eventually death.19 Therefore, these data support the need for treatment with caplacizumab during the period in which a patient is at risk to avoid exacerbations. Because more than one-half of the patients already presented signs or symptoms of organ ischemia at baseline, prompt treatment response is of paramount importance. In fact, caplacizumab acts rapidly by preventing the formation of thrombi in the microcirculation and has been shown to be effective in overcoming factors associated with mortality,11,12,14-16 yielding a favorable outcome vs standard treatment.
In the current study, the patients treated with caplacizumab presented less mortality (3 patients [3.9%]) than those not treated with caplacizumab (6 patients [7.7%]). This difference was not significant, in line with data from clinical trials, in which mortality was considered a composite variable.11,12 Fortunately, deaths for iTTP are rare, and only 1 death may cause mortality percentages to vary significantly due to the small sample size of the series. It should be noted that one of the patients treated with caplacizumab died months after the acute episode and that one of the patients without caplacizumab did not receive any PEX procedure. Nevertheless, the mortality rate was higher than reported by other real-world studies (1.1% to 1.7%)14,16 and in clinical trials.11,12 However, this study included patients by intention to treat, thus accounting for deaths without initiating treatment. Furthermore, the current study had a follow-up of ∼215 days, much longer than clinical trials (28-30 days), which increases the chance of recording more death events. In contrast, another study reported a higher mortality rate with caplacizumab (5.2%) than the rate reported in this study.15 Such differences could probably be explained by patients’ baseline characteristics affecting iTTP disease severity and outcomes.
Importantly, treatment with caplacizumab also diminishes health care disease burden. The length of hospitalization was reduced by 37% and the need for PEX was reduced by 39% vs those who received other drugs, in line with previously published real-world data.14 Hospitalization data from our series were similar to other real-world data studies14,15 and even lower than a German real-world study (21.6 days of hospitalization).16 In contrast, days of hospitalization were almost twice those reported in clinical trials (mean, 14.68 vs 9.9 days of hospitalization).12 However, the data are not comparable because clinical trials operate under stricter timings, and, in our study, 43% of the patients received caplacizumab only after experiencing refractoriness or an exacerbation, thus contributing to longer hospital stays
Rituximab9 reportedly reduces relapses, and our study found analogous results. Moreover, patients treated with caplacizumab plus rituximab presented relapses similar to patients receiving caplacizumab alone and no differences in time to relapse. Nevertheless, conclusions cannot be drawn on account of the short follow-up and limited sample size. In the acute episode setting, our series found that the addition of rituximab to caplacizumab did not accelerate response. In fact, clinical remission was achieved earlier when caplacizumab was given as a single agent (12 patients) than when it was combined with rituximab (60 patients). Nevertheless, the results should be approached with caution due to the small sample size of the caplacizumab-only group.
Because these drugs have different mechanisms of action, caplacizumab and rituximab are complementary agents for disease management, with add-on benefits at different moments of the acute episode. Thus, caplacizumab acts very quickly and should be administered, if possible, within the first days after PEX. Conversely, rituximab reaches its greatest potential 2 weeks after it is started, and hence it plays a greater role in the consolidation of response and in preventing early exacerbations or relapses through its immunosuppressive effect.
It is well known that caplacizumab does not address ADAMTS13 deficiency and that concomitant immunosuppression is required. We found no difference in time to ADAMTS13 recovery between patients treated with caplacizumab alone or caplacizumab plus rituximab. Unfortunately, we were unable to analyze the evolution of ADAMTS13 at follow-up after the acute episode in patients treated without caplacizumab because ADAMTS13 levels were not available in the majority of these patients.
The safety outcomes of the current study are consistent with those previously reported. Because caplacizumab interferes with vWF, it is associated with mucocutaneous bleeding, similar to patients with von Willebrand disease.20 In our series, the most common bleeding event was gingivorrhagia, in line with data from pivotal trials11,12 and real-world data.14-16 Although the bleeding rate was higher in patients with caplacizumab, most events were mild in severity (grade ≤2).
Although caplacizumab has shown safety and efficacy in the treatment of iTTP and the International Society on Thrombosis and Haemostasis recommends it for the treatment of TTP,9 the health authorities in Spain are still reluctant to expedite as its use as initial treatment. This is probably because the current treatment of iTTP has been shown to be effective and that caplacizumab has a high cost, which is perceived as a barrier to its widespread use. Although there are already cost analysis studies in the United States21 (which indicate it as non–cost-effective), there is a lack of analysis studies in Spain and in other European countries. However, it has been suggested that modifying the current standard of care could reduce prices.15,22-24 This change of management also implies a paradigm shift in iTTP treatment. In fact, some articles support the use of caplacizumab without PEX in selected patients,16,25,26 and treatment without PEX would be simpler and safer. Controlled trials are called for to enact progress in this regard.
As in all retrospective studies, this work has inherent limitations associated with the nature of its design. The data used were not specifically collected for the study; in this regard, certain variables with the potential to affect outcome may have been overlooked. In addition, only some patients were treated with caplacizumab as initial treatment due to some clinicians’ lack of familiarity with the drug and the paperwork required to access the MAP. However, the study focused on a by no means negligible number of patients treated early and homogeneously with caplacizumab. Therefore, the results obtained are valid for the purpose of drawing appropriate conclusions. Caplacizumab reduces exacerbations and refractoriness and disease burden. Its greatest efficacy is observed when it is used early (within 3 days) because it clearly reduces the number of days to clinical response and clinical remission, hospitalization time, and the number of PEX. Caplacizumab is well tolerated, with mild bleeding events (mostly grade ≤2) that do not require any intervention in the majority of cases. Therefore, caplacizumab is a good drug for serious clinical situations not previously covered in the treatment of iTTP and should be used early, as an initial treatment, together with PEX and immunosuppression.
Acknowledgments
The authors thank Maite Artés from Adelphi Targis S.L. and Alba Gómez for their support in the medical writing process.
Authorship
Contribution: C.P.I., M.E.M.-C., J.C., and J.d.R.-G. contributed to the design of the study, data collection, statistical analysis, and writing of the final manuscript; A.E.K.F. and J.G.-A.P. contributed to the data collection; C.P.I. and M.E.M. coordinated the team; and all authors contributed to the data collection, participated in the clinical management of these patients, contributed to the literature review on the topic, and critically reviewed the manuscript.
Conflict-of-interest disclosure: M.E.M.-C. has received honoraria for consulting and as a speaker from Sobi, Amgen, Takeda, Sanofi, Novo Nordisk, Grifols, Novartis, CSL Behring, Werfen; and grant founding from Novo Nordisk, Amgen, Takeda, Alexion. C.P.I. has served as a consultant and provided expert testimony for Sanofi and Takeda. A.E.K.F. has received honoraria for consulting and as a speaker for Sanofi and Novartis. J.G.-A.P. has received honoraria for consulting and as a speaker for Sanofi. J.C. has received research funding from Cerus, Kawasumi Laboratories, and Sanofi; and has received speaker or advisory fees from Cerus, Fresenius Kabi, Grifols, MacoPharma, Sanofi, and TerumoBCT. R.G. has received honoraria from Sanofi for consulting activities. J.d.l.R. has served as a consultant and has provided expert testimony for Sanofi. J.d.R.-G. has received honoraria for consulting from Takeda and Sanofi. D.V. has received honoraria from Sanofi as speaker and for consulting activities. I.G.-S. has received honoraria for consulting and as a speaker from Terumo BCT, Takeda and Sanofi. J.R.R.M. has received honoraria for consultancy on advisory boards and as a speaker from Janssen, AbbVie, AstraZeneca, CSL-Behring, Takeda, and GSK. The remaining authors declare no competing financial interests.
Correspondence: María Eva Mingot-Castellano, Hematology, Hospital Universitario Virgen del Rocío, Instituto de Biomedicina de Sevilla, Seville, Spain; e-mail: mariae.mingot.sspa@juntadeandalucia.es.
References
Author notes
∗C.P.I. and M.E.M.-C. contributed equally to this study.
Requests for original data may be submitted to the corresponding author, María Eva Mingot-Castellano (mariae.mingot.sspa@juntadeandalucia.es).
The full-text version of this article contains a data supplement.