Abstract
Patients with multiple myeloma (MM) have a diminished immune response to coronavirus disease 2019 (COVID-19) vaccines. Risk factors for an impaired immune response are yet to be determined. We aimed to summarize the COVID-19 vaccine immunogenicity and to identify factors that influence the humoral immune response in patients with MM. Two reviewers independently conducted a literature search in MEDLINE, Embase, ISI Web of Science, Cochrane library, and Clinicaltrials.gov from existence until 24 May 24 2022. (PROSPERO: CRD42021277005). A total of 15 studies were included in the systematic review and 5 were included in the meta-analysis. The average rate (range) of positive functional T-lymphocyte response was 44.2% (34.2%-48.5%) after 2 doses of messenger RNA (mRNA) COVID-19 vaccines. The average antispike antibody response rates (range) were 42.7% (20.8%-88.5%) and 78.2% (55.8%-94.2%) after 1 and 2 doses of mRNA COVID-19 vaccines, respectively. The average neutralizing antibody response rates (range) were 25% (1 study) and 62.7% (53.3%-68.6%) after 1 and 2 doses of mRNA COVID-19 vaccines, respectively. Patients with high-risk cytogenetics or receiving anti-CD38 therapy were less likely to have a humoral immune response with pooled odds ratios of 0.36 (95% confidence interval [95% CI], 0.18, 0.69), I2 = 0% and 0.42 (95% CI, 0.22, 0.79), I2 = 14%, respectively. Patients who were not on active MM treatment were more likely to respond with pooled odds ratio of 2.42 (95% CI, 1.10, 5.33), I2 = 7%. Patients with MM had low rates of humoral and cellular immune responses to the mRNA COVID-19 vaccines. Further studies are needed to determine the optimal doses of vaccines and evaluate the use of monoclonal antibodies for pre-exposure prophylaxis in this population.
Introduction
Patients with multiple myeloma (MM) have an increased risk of severe coronavirus disease 2019 (COVID-19), with a mortality rate of 34%-37%.1-3 Several vaccine platforms have been shown to reduce disease transmission, severity, and mortality in the general population.4-6 However, many immunocompromised patient populations, including people with MM, were not included in clinical trials of COVID-19 vaccines.5,7,8 MM, caused by the abnormal proliferation of clonal plasma cells producing monoclonal immunoglobulin, is the second most common hematologic malignancy in the United States and accounts for 10% of total hematologic malignancies.9,10 Patients with MM are known to have diminished humoral and cellular immune response to influenza, pneumococcal, and Haemophilus influenzae type B vaccines.11 Unfortunately, recent studies have also shown that patients with MM had an inferior immune response to COVID-19 vaccines compared with the general population.12-14 The impaired immune response of patients with MM has raised concerns about breakthrough infections and the ineffective protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).15 This systematic review and meta-analysis were conducted to summarize the current information regarding the immunogenicity of COVID-19 vaccines and identify the factors that contribute to low rates of humoral response to COVID-19 vaccines in patients with MM.
Methods
Data sources and searches
Two authors (N.C. and K.M.) independently conducted the systematic search in MEDLINE, Embase, ISI Web of Science, Cochrane library, and ClinicalTrials.gov databases from the beginning of the pandemic until May 24th, 2022. SARS-CoV-2 vaccine, COVID-19 vaccine, BNT162b2, Pfizer, mRNA-1273, AZD1222, Janssen, CoronaVac, and were used as search terms. Full search terms are available in the supplemental material (Method S). Duplicate studies were excluded. We did not limit our search by language. Google Translate was used to translate non-English studies during title and abstract screening. We conducted the study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.16 The International Prospective Register of Systematic Reviews (PROSPERO) registration number is CRD42021277005.
Study selection
All studies were reviewed independently by 2 authors (N.C. and K.M.). We included clinical trials and observational studies consisting of prospective cohort, retrospective cohort, and case-control studies. Studies were selected if they reported the immune response to COVID-19 vaccines in patients with MM. Studies of subjects with prior COVID-19 were excluded to prevent the confounding effects of immune responses from natural infection of SARS-CoV-2. If needed, we contacted the corresponding authors for additional information regarding antibody testing. Conflicts were resolved by mutual consensus among the reviewers.
Data extraction and quality assessment
The checklist for critical appraisal and data extraction for systematic reviews of prediction modeling studies (CHARMS) was employed to guide comprehensive data extraction from included studies.17 We extracted study design, country, center, study year, study period, type of SARS-CoV-2 vaccine, immunogenicity tests, study limitations, and other important comments. Our primary outcomes were the humoral and cellular immune response rates to the COVID-19 vaccines. The seroconversion rates were calculated from the number of responders and total participants. We defined responders as participants who tested positive for humoral or cellular response according to the study’s cutoffs and definitions.
Our secondary outcomes were factors that affected the humoral immune response to COVID-19 vaccines. We collected the number of responders, total participants, and odds ratios (ORs) with a 95% confidence interval (95% CI) of the factors that were tested for an association with vaccine response. If ORs were not available, we used crude number of responders, nonresponders, and total participants for the OR calculation. We used the Newcastle-Ottawa scale for assessing the risk of bias in the studies (supplemental Table 1).18
Data synthesis and analysis
We used descriptive statistics to summarize the humoral and cellular immune response data from each COVID-19 platform and dosage. We used the weighted means for the positive humoral and cellular immune response rates. Comprehensive Meta-Analysis 3.3 software from Biostat, Inc (Englewood, NJ) was used to perform a meta-analysis and Egger’s regression to identify the risk factors associated with poor humoral immune response. We performed the meta-analysis with the random-effects model to obtain the pooled ORs (pOR) with 95% CI, for binary or categorical variables, of factors that affected the immunogenicity. Adjusted ORs were used if the study provided both adjusted and unadjusted ORs. We used raw data to calculate unadjusted ORs if the study did not provide ORs. We performed sensitivity analyses using a leave-one-out method.19 Publication bias was assessed by Funnel plot and Egger’s regression.20 If the P value of Egger’s regression was below .05, the publication bias was considered significant.21 If there were concerns for publication bias, data were further adjusted by the Duval and Tweedie trim-and-fill method.22 The I2 statistic was used to assess the heterogeneity of effect size estimates of each study. The I2 statistic value ranged from 0% to 100% (I2 < 25%, low heterogeneity; I2 = 25%-60%, moderate heterogeneity; and I2 > 60%, substantial heterogeneity).
Results
Study and patient characteristics
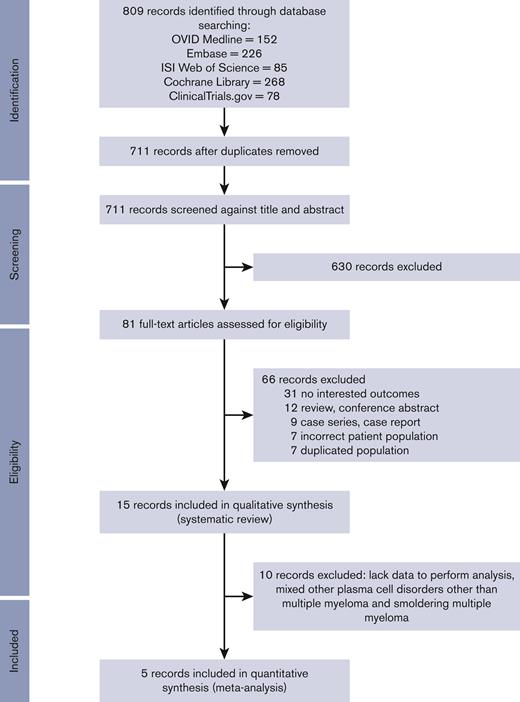
Our initial search generated 809 studies; 98 were removed owing to duplicate studies, and 630 were excluded by screening through the titles and abstracts. We performed a full-paper review with 81 articles. Sixty-six articles were subsequently excluded owing to being a review article, case report, wrong population, duplicate cohort, or different outcome of interest. A total of 15 studies were included in the systematic review and 5 studies were included in the meta-analysis (Figure 1). The characteristics of the 15 studies13,23–36 are described in Table 1. There were 1210 patients with MM and 38 patients with smoldering MM. Grading of recommendation assessment, development, and evaluation for factors influencing seroconversion is described in the supplemental material (supplemental Table 2).37
PRISMAdiagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
PRISMAdiagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics
| Study . | Vaccine . | Country . | Study period (mo/y) . | Study design . | Number of patients . | Subgroup of patients (numbers) . | Age . |
|---|---|---|---|---|---|---|---|
| Avivi 2021 | BNT162b2 | Israel | 12/20-03/21 | Prospective | 171 | MM (159); SMM (12) | Median (range) 70 (38-94) |
| Bird 2021 | BNT162b2 or AZD1222 | UK | Until 03/21 | Retrospective | 93 | MM (93) | Not reported |
| Bitoun 2021 | BNT162b2 | France | 01/21-03/21 | Prospective | 27 | MM (27) | Not reported |
| Enßle 2021 | BNT162b2 | Germany | 09/20-06/21 | Prospective | 77 | MM (73); SMM (4) | Median (IQR) 67 (60-72) |
| Gavriatopoulou 2021 | BNT162b2 | Greece | 01/21-05/21 | Prospective | 35 | MM (29); SMM (6) | Median (IQR) 66 (74) |
| Greenberg 2021 | BNT162b2 or mRNA-1273 | US | 12/20-03/21 | Prospective | 44 | MM (44) | Median (IQR) 64 (57-69) |
| Henriquez 2021 | BNT162b2 | France | 01/21-06/21 | Prospective | 60 | MM (60) | Mean (range) 70 (41-92) |
| Marasco 2021 | BNT162b2 or mRNA-1273 | Italy | 03/21-05/21 | Prospective | 263 | MM (52) | Median (range) 73 (47-78) |
| Pimpinelli 2021 | BNT162b2 | Italy | Not reported | Prospective | 42 | MM (42) | Median (range) 73 (47-78) |
| Ramasamy 2021 | BNT162b2 or AZD1222 | UK | 02/21-03/21 | Prospective | 23 | MM (23) | Mean (SD) 62.9 (9.9) |
| Rehav 2021 | BNT162b2 | Israel | Not reported | Prospective | 187 | MM (187) | Median (IQR) 66 (59-73) |
| Stampfer 2021 | BNT162b2 or mRNA-1273 | US | Not reported | Prospective | 96 | MM (96); SMM (7) | Median 68 (35-88) |
| Šušol 2022 | BNT162b2 | Czech Republic | Not reported | Prospective | 119 | MM (119) | Not reported |
| Terpos 2021 | BNT162b2 | Greece | Not reported | Prospective | 48 | MM (39); SMM (9) | Median (IQR) 74 (62-80) |
| Terpos 2022 | BNT162b2 | Greece | 09/21 – 10/21 | Prospective | 167 | MM (167) | Median (IQR) 68 (60-75) |
| Study . | Vaccine . | Country . | Study period (mo/y) . | Study design . | Number of patients . | Subgroup of patients (numbers) . | Age . |
|---|---|---|---|---|---|---|---|
| Avivi 2021 | BNT162b2 | Israel | 12/20-03/21 | Prospective | 171 | MM (159); SMM (12) | Median (range) 70 (38-94) |
| Bird 2021 | BNT162b2 or AZD1222 | UK | Until 03/21 | Retrospective | 93 | MM (93) | Not reported |
| Bitoun 2021 | BNT162b2 | France | 01/21-03/21 | Prospective | 27 | MM (27) | Not reported |
| Enßle 2021 | BNT162b2 | Germany | 09/20-06/21 | Prospective | 77 | MM (73); SMM (4) | Median (IQR) 67 (60-72) |
| Gavriatopoulou 2021 | BNT162b2 | Greece | 01/21-05/21 | Prospective | 35 | MM (29); SMM (6) | Median (IQR) 66 (74) |
| Greenberg 2021 | BNT162b2 or mRNA-1273 | US | 12/20-03/21 | Prospective | 44 | MM (44) | Median (IQR) 64 (57-69) |
| Henriquez 2021 | BNT162b2 | France | 01/21-06/21 | Prospective | 60 | MM (60) | Mean (range) 70 (41-92) |
| Marasco 2021 | BNT162b2 or mRNA-1273 | Italy | 03/21-05/21 | Prospective | 263 | MM (52) | Median (range) 73 (47-78) |
| Pimpinelli 2021 | BNT162b2 | Italy | Not reported | Prospective | 42 | MM (42) | Median (range) 73 (47-78) |
| Ramasamy 2021 | BNT162b2 or AZD1222 | UK | 02/21-03/21 | Prospective | 23 | MM (23) | Mean (SD) 62.9 (9.9) |
| Rehav 2021 | BNT162b2 | Israel | Not reported | Prospective | 187 | MM (187) | Median (IQR) 66 (59-73) |
| Stampfer 2021 | BNT162b2 or mRNA-1273 | US | Not reported | Prospective | 96 | MM (96); SMM (7) | Median 68 (35-88) |
| Šušol 2022 | BNT162b2 | Czech Republic | Not reported | Prospective | 119 | MM (119) | Not reported |
| Terpos 2021 | BNT162b2 | Greece | Not reported | Prospective | 48 | MM (39); SMM (9) | Median (IQR) 74 (62-80) |
| Terpos 2022 | BNT162b2 | Greece | 09/21 – 10/21 | Prospective | 167 | MM (167) | Median (IQR) 68 (60-75) |
IQR, interquartile range; SD, standard deviation; SMM, smoldering multiple myeloma; UK, United Kingdom; US, United States.
Humoral immune responses
messenger RNA (mRNA) vaccines
A total of 15 studies of the immunogenicity of the mRNA COVID-19 vaccines were identified. There were 7 studies reporting the antibody response after 1 dose of the mRNA COVID-19 vaccines. The average positive antibody response rates after 1 dose of mRNA vaccine were 42.7% (range 20.8%-88.5%; 6 studies13,28-31,33) for antispike antibodies and 25% (1 study35) for neutralizing antibodies (Figure 2). The mean time to antibody testing was 28 (range 21-33) days after vaccination.
Twelve studies reported the antibody response rates after 2 doses of mRNA COVID-19 vaccine. The average positive antibody response rates were 78.2% (range 55.8%-94.3%; 10 studies23–25,27-30,32-34) for antispike antibodies and 62.7% (range 53.3%-68.6%; 3 studies25,26,36) for neutralizing antibodies (Figure 2; Table 2). The mean antibody testing time was 27.3 (range 14-56) days after the second dose of mRNA vaccines. We then calculated the average positive antibody response rates by vaccine type. The average rates of positive antibody response of the BNT162b2 vaccine were 77.7% (range 55.8%-87.4%; 7 studies23–25,28,30,32,34) for antispike antibodies and 62.7% (range 53.3%-68.6%; 3 studies25,26,36) for neutralizing antibodies. Terpos et al36 reported an increase of humoral response with a seropositivity of 85% for neutralizing antibodies after 3 doses of the BNT162b2 vaccine. There are no data regarding mRNA-1273 alone for analysis.
Humoral and cellular immune response after 2 doses of mRNA COVID-19 vaccines
| Study . | Vaccine (dose) . | Antibody measurement . | Methods . | Timing to Ab testing . | Responders/total (seroconversion rate) . | Cellular immune response measurement . | Timing to cellular immune response testing . | Responders/total (rate) . |
|---|---|---|---|---|---|---|---|---|
| Avivi 2021 | BNT162b2 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 14-21 d | 121/159 (76.10%) | |||
| Bird 2021 | BNT162b2 (1 dose) AZD1222 (1 dose) | Antispike Ab | Ortho Clinical Diagnostics | Median (IQR) 33 (28-38) d | 26/45 (57.78%)26/48 (54.17%) | |||
| Bitoun 2021 | BNT162b2 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 56 d | 20/27 (74.07%) | |||
| Enßle 2021 | BNT162b2 (2 doses) | Antispike Ab | ARCHITECT SARS-CoV-2 IgG II Quant assay (Abbott) | Median 21 d | 43/77 (55.84%) | IFN-γ ELISpot | 28 days | 13/38 (34.21%) |
| Gavriatopoulou 2021 | BNT162b2 (2 doses) | Neutralizing Ab | SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript) | 4 wk | 24/35 (68.57%) | |||
| Greenberg 2021 | BNT162b2 or mRNA-1273 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 1 mo | 41/44 (93.18%) | |||
| Henriquez 2021 | BNT162b2 (1 dose) BNT162b2 (2 doses) | Antispike Ab | Not reported | 30 d 1-2 mo | 26/60 (43.33%)51/60 (85.00%) | IFN-γ ELISpot | 2 months | 11/26 (42.31%) |
| Marasco 2021 | BNT162b2 or mRNA-1273 (1 dose) BNT162b2 or mRNA-1273 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 4 wk 2 wk | 46/52 (88.46%)49/52 (94.23%) | Measurement of in vitro T-helper cell type 1-associated cytokine release using ELISA | 2 weeks | 48/99 (48.48%) |
| Pimpinelli 2021 | BNT162b2 (1 dose) BNT162b2 (2 doses) | Antispike Ab | LIAISON SARS-CoV-2 S1/S2 IgG chemiluminescent assay (DiaSorin) | 21 d after first dose 35 d after first dose | 9/42 (21.43%)33/42 (78.57%) | |||
| Ramasamy 2021 | AZD1222 (1 dose) BNT162b2 (1 dose) | Antispike Ab | ARCHITECT SARS-CoV-2 IgG II Quant assay (Abbott) | >3 wk >3 wk | 7/14 (50.00%)4/9 (44.44%) | |||
| Rehav 2021 | BNT162b2 (2 doses) | Antispike Ab | In house ELISA | Median (IQR) 18 (15-23) d | 149/187 (79.68%) | |||
| Stampfer 2021 | BNT162b2 or mRNA-1273 (1 dose) BNT162b2 or mRNA-1273 (2 doses) | Antispike Ab | In house ELISA | 14-21 d 14-21 d | 20/96 (20.83%)64/96 (66.67%) | |||
| Šušol 2022 | BNT162b2 (2 doses) | Antispike Ab | EUROIMMUN SARS-CoV-2 ELISA assay | Not reported | 104/119 (87.39%) | |||
| Terpos 2021 | BNT162b2 (1 dose) | Neutralizing Ab | SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript) | 21 d | 12/48 (25.00%) | |||
| Terpos 2022 | BNT162b2 (2 doses) BNT162b2 (3 doses) | Neutralizing Ab | SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript) | 1 mo 1 mo | 110/167 (65.87%)142/167 (85.03%) |
| Study . | Vaccine (dose) . | Antibody measurement . | Methods . | Timing to Ab testing . | Responders/total (seroconversion rate) . | Cellular immune response measurement . | Timing to cellular immune response testing . | Responders/total (rate) . |
|---|---|---|---|---|---|---|---|---|
| Avivi 2021 | BNT162b2 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 14-21 d | 121/159 (76.10%) | |||
| Bird 2021 | BNT162b2 (1 dose) AZD1222 (1 dose) | Antispike Ab | Ortho Clinical Diagnostics | Median (IQR) 33 (28-38) d | 26/45 (57.78%)26/48 (54.17%) | |||
| Bitoun 2021 | BNT162b2 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 56 d | 20/27 (74.07%) | |||
| Enßle 2021 | BNT162b2 (2 doses) | Antispike Ab | ARCHITECT SARS-CoV-2 IgG II Quant assay (Abbott) | Median 21 d | 43/77 (55.84%) | IFN-γ ELISpot | 28 days | 13/38 (34.21%) |
| Gavriatopoulou 2021 | BNT162b2 (2 doses) | Neutralizing Ab | SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript) | 4 wk | 24/35 (68.57%) | |||
| Greenberg 2021 | BNT162b2 or mRNA-1273 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 1 mo | 41/44 (93.18%) | |||
| Henriquez 2021 | BNT162b2 (1 dose) BNT162b2 (2 doses) | Antispike Ab | Not reported | 30 d 1-2 mo | 26/60 (43.33%)51/60 (85.00%) | IFN-γ ELISpot | 2 months | 11/26 (42.31%) |
| Marasco 2021 | BNT162b2 or mRNA-1273 (1 dose) BNT162b2 or mRNA-1273 (2 doses) | Antispike Ab | Elecsys anti-SARS-CoV-2 (Roche) | 4 wk 2 wk | 46/52 (88.46%)49/52 (94.23%) | Measurement of in vitro T-helper cell type 1-associated cytokine release using ELISA | 2 weeks | 48/99 (48.48%) |
| Pimpinelli 2021 | BNT162b2 (1 dose) BNT162b2 (2 doses) | Antispike Ab | LIAISON SARS-CoV-2 S1/S2 IgG chemiluminescent assay (DiaSorin) | 21 d after first dose 35 d after first dose | 9/42 (21.43%)33/42 (78.57%) | |||
| Ramasamy 2021 | AZD1222 (1 dose) BNT162b2 (1 dose) | Antispike Ab | ARCHITECT SARS-CoV-2 IgG II Quant assay (Abbott) | >3 wk >3 wk | 7/14 (50.00%)4/9 (44.44%) | |||
| Rehav 2021 | BNT162b2 (2 doses) | Antispike Ab | In house ELISA | Median (IQR) 18 (15-23) d | 149/187 (79.68%) | |||
| Stampfer 2021 | BNT162b2 or mRNA-1273 (1 dose) BNT162b2 or mRNA-1273 (2 doses) | Antispike Ab | In house ELISA | 14-21 d 14-21 d | 20/96 (20.83%)64/96 (66.67%) | |||
| Šušol 2022 | BNT162b2 (2 doses) | Antispike Ab | EUROIMMUN SARS-CoV-2 ELISA assay | Not reported | 104/119 (87.39%) | |||
| Terpos 2021 | BNT162b2 (1 dose) | Neutralizing Ab | SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript) | 21 d | 12/48 (25.00%) | |||
| Terpos 2022 | BNT162b2 (2 doses) BNT162b2 (3 doses) | Neutralizing Ab | SARS-CoV-2 Surrogate Virus Neutralization Test (GenScript) | 1 mo 1 mo | 110/167 (65.87%)142/167 (85.03%) |
Ab, antibody; ELISA, enzyme-linked immunosorbent assay; ELISpot, enzyme-linked immune absorbent spot; IFN, interferon; IQR, interquartile range; SD, standard deviation.
Other vaccine platforms
Risk factors for reduced humoral immune responses after 2 doses of mRNA vaccines
We included studies reporting factors that influenced the humoral immune response after 2 doses of mRNA vaccines among patients with MM or smoldering MM to ensure an analysis of a similar disease spectrum. We reviewed host characteristics (age, sex, immunoglobulin levels, neutrophil count, lymphocyte count, previous hematopoietic cell transplantation, high-risk cytogenetics, and treatment response according to the International Myeloma Working Group definitions38) and treatment-related factors (anti- CD38 antibody–directed therapy, anti-SLAM family member 7 antibody, B-cell maturation antigen–targeted therapy, proteasome inhibitors, immunomodulatory agents, systemic corticosteroids, ≥3 lines of treatment, and no active treatment) that could potentially affect humoral immune response after 2 doses of mRNA vaccines. However, meta-analysis could only be performed with the factors below owing to a lack of data or different cutoff levels in each primary study (supplemental Table 3).
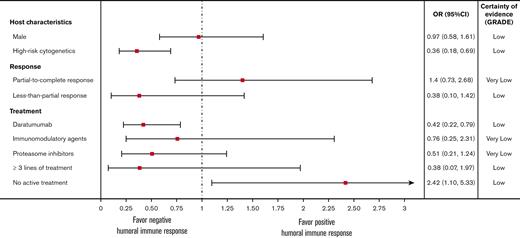
Male sex was not associated with antibody response rates. The pOR for male sex from 5 studies was 0.97 (0.58, 1.61), P = .90, I2 = 0% (Figure 3).23-25,27,30 High-risk cytogenetics, defined as having at least one of the following cytogenetic abnormalities: t(4;14), t(14;16), t(14;20), del(17p), or gain(1q) by fluorescence in situ hybridization,39 were associated with lower antibody response rates. The pOR for high-risk cytogenetics in 2 studies was 0.36 (0.18, 0.69), P = .002, I2 = 0% (Figure 3).23,25
The pooled odds ratios (OR) of humoral immune responses after 2 doses of mRNA vaccines. CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation.
The pooled odds ratios (OR) of humoral immune responses after 2 doses of mRNA vaccines. CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation.
We found that patients with higher antibody response rates were not receiving active treatment. The pOR for no active treatment from 4 studies was 2.42 (1.10, 5.33), P = .029, I2 = 7% (Figure 3).23-25,27 Treatment with daratumumab (anti-CD38 antibody) was associated with lower antibody response rates, with the pOR from 5 studies of 0.42 (0.22, 0.79), P = .007, I2 = 14% (Figure 3).23-25,27,30 Treatments with immunomodulatory agents (lenalidomide, pomalidomide, and thalidomide) and proteasome inhibitors (bortezomib, carfilzomib, and ixazomib) were not associated with lower antibody response rates. The pORs of immunomodulatory agents (3 studies23,25,27) and proteasome inhibitors (3 studies23,25,27) were 0.76 (0.25, 2.31), P = .63, I2 = 64% and 0.51 (0.21, 1.24), P = .14, I2 = 29%, respectively (Figure 3).
Treatment response to MM therapy was not associated with antibody response rates. The pOR for patients with partial, very good partial, and complete response (partial-to-complete response) to treatment from 4 cohorts in 2 studies was 1.40 (0.73, 2.68), P = .310, I2 = 57% (Figure 3).23,25 The pOR for patients with less-than-partial response from 3 cohorts in 2 studies was 0.38 (0.10, 1.42), P = .15, I2 = 35 (Figure 3).24,25 Receiving ≥ 3 lines of treatment was not associated with lower antibody response rates, with a pOR from 2 studies of 0.38 (0.07, 1.97), P = .250, I2 = 71 (Figure 3).23,25
Cellular immune response
Three studies reported cellular immune response after 2 doses of mRNA COVID-19 vaccines among patients with MM (Table 2). The cellular immune response was evaluated by 2 main methods: functional T-lymphocyte response by ELISpot and in vitro T-helper cell type 1–associated cytokine release using ELISA.25,28,29 The average rate of positive functional T-lymphocyte response was 44.2% (range 34.2%-48.5%).25,28,29 Enßle et al. reported a significantly lower median of CD19+ B lymphocytes among antibody nonresponders than responders.25
Sensitivity analysis and publication bias
The pORs for male sex, less-than-partial response to treatment, and immunomodulatory agents (no significant association with antibody response rates) remained consistent by sensitivity analyses. The pOR for high-risk cytogenetics became insignificant after removing Avivi et al 2021.23 The pOR for daratumumab became insignificant when removing Enßle et al25 from the analysis. The pOR for proteasome inhibitors became significantly associated with lower antibody response rates after removing Avivi et al 2021,23 with a pOR of 0.24 (0.07, 0.85), P = .027. The pOR for no active treatment became insignificant after removing Enßle et al25 or Greenberg et al.27 The pOR for partial-to-complete treatment response became significantly associated with higher immune response rates when removing the partial response cohort of Enßle et al,25 with a pOR of 1.90 (1.11, 3.25), P = .019. The pOR for receiving ≥3 lines of treatment became significantly associated with lower antibody response rates after removing Enßle et al,25 with a pOR of 0.16 (0.04, 0.61), P = .007.
We did not find evidence of publication bias by the Egger’s test and inspection of the funnel plots in following factors: male sex, partial-to-complete response to treatment, less-than-partial response to treatment, and use of proteasome inhibitors. We cannot evaluate publication bias in high-risk cytogenetics and patients receiving ≥ 3 lines of treatment owing to limited numbers of included studies.
Discussion
This is the systematic review and meta-analysis summarizing the accumulating data regarding the cellular and humoral immune responses of COVID-19 vaccines and risk factors contributing to the poor humoral antibody response in patients with MM. The average antibody response rates increased from 43% to 78% after the second dose of mRNA COVID-19 vaccines but were still lower than rates reported in the general population.40 One study reported a humoral immune response of 85% after 3 doses of mRNA vaccines.36 The average cellular response rate after 2 doses of mRNA COVID-19 vaccines was 44%, which was significantly lower than rates reported for healthy controls.29 However, interpretation of the cellular response needs to be cautious as some therapies might interfere with T-cell function assays.41,42 This study underscores the importance of subsequent doses of the COVID-19 vaccine and adhering to the safety precautions among patients with MM regardless of vaccine status. As of July, 2022, the US Centers for Disease Control and Prevention has recommended that immunocompromised patients, including patients with MM, should receive 2 booster doses after the primary 3-dose mRNA vaccine series (BNT162b2 or mRNA-1273) or a total of 4 doses of Ad26.COV2.S vaccine (the primary series, an additional dose, and 2 booster doses).43
Here, we identified patients with high-risk cytogenetics and patients receiving daratumumab as less likely to have an antibody response after 2 doses of mRNA COVID-19 vaccines. It is known that high-risk cytogenetics are associated with high-risk disease characteristics and have poor prognosis owing to rapid disease progression, often necessitating more aggressive treatment.39,44 Patients with high-risk cytogenetics tend to be treated with multiple antimyeloma agents, which can potentially lead to further diminished humoral immune response. The exact mechanism linking high-risk cytogenetics and poor humoral immune response may relate to disease or treatment factors. Daratumumab targets CD38 on both cancerous and normal plasma cells, which is expected to interfere with antibody response. The finding of lower antibody response rates after treatment with daratumumab is consistent with other studies that were excluded from this analysis owing to mixed patient populations with previous COVID-19 infection or other plasma cell disorders other than MM and smoldering MM.45-47 High-risk cytogenetics and treatment with anti-CD38 antibody could be related and confounded as patients with high-risk cytogenetics are likely to receive more aggressive treatment regimen including anti-CD38 antibody.48 However, the primary studies do not provide sufficient information for further analysis. Patients with MM who were not on active treatment had a more favorable immune response.
Patients with MM are affected by immune dysregulation, and MM treatment can lead to further reduced immune response and an increased risk of breakthrough SARS-CoV-2 infections despite being vaccinated.15 Wang et al reported 15.4% of patients with MM who were vaccinated with 2 doses of mRNA vaccines or 1 dose of Ad26.COV2.S developed breakthrough COVID-19 from 1 December 2020 to 8 October 2021.49 The Omicron SARS-CoV-2 variants have raised concerns about breakthrough infections in both healthy and immunocompromised individuals, with and without boosters.50,51 Our results demonstrate a low humoral immune response of 77% in patients with MM after 2 doses of mRNA vaccines, with an increase of humoral immune response to 85% after 3 doses of mRNA vaccines.
The Food and Drug Administration issued an emergency use authorization for tixagevimab/cilgavimab, a long-acting monoclonal antibody (mAb) cocktail, for the pre-exposure prevention against SARS-CoV-2 in moderate-to-severe immunocompromised patients, including those with MM, based on the data from a phase 3 trial that reported a single dose of tixagevimab/cilgavimab had efficacy for COVID-19 prevention.52,53 The Food and Drug Administration stated that COVID-19 vaccines are the best prevention against SARS-CoV-2 infection. However, some patients with MM are unable to produce an adequate antibody response after receiving the vaccines.52,54 Given the globally limited availability of tixagevimab/cilgavimab, additional questions arise as to who should be prioritized to receive mAb for pre-exposure prophylaxis, even with the patient populations identified in the emergency use authorization. Does the passive immunization from tixagevimab/cilgavimab make up for the low rates of immune response in patients with MM? The risk factors identified in this study may inform health care professionals on time-sensitive decisions about active vs passive immunization, by weighing the likelihood of benefit from vaccination compared with the likelihood of benefit from mAbs.
Limitations of our study include the small numbers of studies used in the meta-analysis owing to mixed vaccine platforms and mixed patient populations. Most of the available data are from the mRNA platform; however, many countries use other vaccine platforms owing to limited mRNA vaccine supply globally. There are very limited studies reporting immune response to 3 or more doses of COVID-19 vaccines as of August 2022. Included studies used differing SARS-CoV-2 antibody testing techniques, and there was no gold standard for antibody testing. Lastly, the clinical significance of measured humoral and cellular immune responses in patients with MM is uncertain.
In conclusion, patients with MM had impaired immune response after COVID-19 vaccinations. Anti-CD38 antibody–directed therapy and high-risk cytogenetics were associated with lower antibody response rates, whereas patients receiving no active treatment had higher antibody response rates. Further studies are needed to determine the optimal schedule for each COVID-19 vaccine platform, the efficacy of mAb for pre-exposure prophylaxis, and clinical outcomes in patients with MM who develop breakthrough COVID-19.
Acknowledgments
The authors would like to extend our gratitude to librarians at Chulalongkorn University and Johns Hopkins University School of Medicine for their contribution of retrieving full papers of studies that were not available on the databases. No funding to be disclosed for the study.
Authorship
Contribution: N.C., K.M., and N.P. designed the study, performed literature search, extracted data, performed quality assessment of the studies, analyzed the data, wrote the manuscript, and critically reviewed the manuscript; C.M. designed the study, performed literature search, wrote the manuscript, and critically reviewed the manuscript; A.S. designed the study, analyzed data, wrote the manuscript, and critically reviewed the manuscript; O.S.K., N.H., K.P., J.T., S.L., T.M., A.T., T.M., M.V.D., P.T., N.L., N.W., R.P., A.C., S.G., P.N., and T.T. wrote the manuscript and critically reviewed the manuscript; and S.N. performed literature search, wrote the manuscript, and critically reviewed the manuscript.
Conflict-of-interest disclosure: S.N. reported receiving a grant from the Fisher Center Discovery Program, Johns Hopkins University. N.W., R.P., and A.C. reported receiving grants from the Health Systems Research Institute (Thailand) and Rachadapiseksompotch Fund, Chulalongkorn University outside the submitted work. N.P. reported receiving grants and salary support from the Health Systems Research Institute (Thailand), the Fisher Center Discovery Program, Johns Hopkins University, the Cystic Fibrosis Foundation, NIH, Immune Tolerance Network, and Merck outside the submitted work. N.P. has served on the advisory board for Shionogi Inc and the Data Review Committee for Pulmocide Ltd. The remaining authors declare no competing financial interests.
Correspondence: Nitipong Permpalung, 601 N Wolfe St, Carnegie Building #340, Baltimore, MD 21287; e-mail: npermpa1@jhmi.edu; and Nipat Chuleerarux, 1611 NW 12th Ave, Miami, FL 33136; e-mail: nipat.chuleerarux@jhsmiami.org.
References
Author notes
∗K.M. and N.C. contributed equally to this study.
The data for our systematic review and meta-analysis are publicly available based on the study design.
The full-text version of this article contains a data supplement.