Key Point
The use of Hu and thalidomide combination in β-thalassemia resulted in fewer transfusions and increased Hb levels.
Abstract
Transfusion-related complications and lack of resources in low-to-middle-income countries have led to a search for novel therapies to reduce the need for blood transfusions in patients with β-thalassemia. Hydroxyurea (HU) has demonstrated promising outcomes; additionally, thalidomide has also shown improvement in hemoglobin (Hb) levels for patients with β-thalassemia in some studies. This study presents the findings of a single-arm nonrandomized trial to evaluate the efficacy of combination therapy of HU and thalidomide in children with β-thalassemia. A total of 135 patients (median age, 6 [interquartile range, 3-10] years), 77 (57%) males and 58 (43%) females, were followed first using HU alone, for 6 months, and then using the combination of HU and thalidomide for another 6 months. The primary outcome was a response to therapy, as measured by the number of transfusions required and Hb levels, for patients while receiving HU alone and then while using the combination therapy. Study findings showed a significant decline in blood transfusion volume (P < .001) and a significant increase in median Hb levels within 3 and 6 months of the combination therapy (P < .001). Eighty-nine (65.93%) participants were good responders, 16 (11.85%) were responders, and 30 (22.22%) were nonresponders, whereas the responders had variable genetic mutations. A total of 38 adverse events were reported that resolved on supportive treatment or temporary hold of the intervention. The combination therapy demonstrated promising results and could be considered for a diverse patient population with β-thalassemia. This trial was registered at www.clinicaltrials.gov as #NCT05132270.
Introduction
β-thalassemia syndrome constitutes a group of genetic blood disorders caused by decreased expression of β globin chains resulting in reduced hemoglobin levels and red blood cell production, and anemia. β-thalassemia major (BTM) usually manifests at 6 to 24 months of age with failure to thrive and progressive hepatosplenomegaly. In contrast, β-thalassemia intermedia (BTI) presents after 2 years of age with milder anemia and occasional need of blood transfusions.1,2 Because of limited resources in low-and-middle-income countries, patients receiving suboptimal care can experience jaundice, pallor, growth retardation, and extramedullary hematopoiesis resulting in axial and appendicular skeletal deformities and changes.3,4 Although regular blood transfusions can sustain growth and development for up to a decade of life, the transfusion-resultant iron overload ultimately restricts children’s physical and sexual growth and negatively affects the heart, liver, and endocrine systems. Cardiac complications are the most common cause of mortality among chronically transfused patients with BTM.1,3,4 Chronic blood transfusions also increase the likelihood of acquiring transfusion-transmitted infections.5
The standard of care for BTM remains bone marrow transplantation or lifelong blood transfusions followed by iron chelation therapies.6 However, lack of access to bone marrow transplant services and safe blood in low-and-middle-income countries and other transfusion-related hazards has compelled researchers to search for alternative therapies. Various medications have been investigated for their potential to augment hemoglobin F (HbF) and reactivate γ-globin genes; the therapeutic response has been demonstrated in patients with β-thalassemia as increased hemoglobin (Hb) levels and reduced or eliminated need for transfusion.
Hydroxyurea (HU) is a US Food and Drug Administration-approved HbF inducer for sickle cell disease and is currently being used for patients with β-thalassemia with variable therapeutic responses. HU has a documented role in improving clinical and hematological outcomes among patients with thalassemia because it defers or prevents frequent blood transfusions.7-9 Since 2004, our study team has been actively involved in investigating therapies for HbF augmentation that could limit the volume of blood transfusions, spare patients from complications, and improve their life expectancy. In 2007, we evaluated the efficacy of HU in maintaining Hb levels and reducing the need for packed red cell (PRC) transfusions among our patient population, for the first time.10 Since then, we have also published a series of papers describing the genetics, metabolomics, and proteomics associated with HU use in patients with thalassemia.11-13 In our 15 years of experience with HU, 36% of our patients were complete responders and are entering the third decade of their lives with improved life quality as they maintain their Hb around or above 7 to 8 g/dL; for some, their phenotype has transformed from thalassemia major to intermedia. However, 20% demonstrated a partial response and 43.75% demonstrated no response to HU.14 This called for a search for an adjunct or alternative therapy to improve the outcomes in these patients. Thalidomide is an immune modulator that is being studied for its HbF induction potential and has demonstrated significant transfusion reduction in patients with BTM and BTI.15 Thalidomide causes suppression of nuclear factor-κB induction by inflammatory cytokines such as tumor necrosis factor, which potentially increases HbF.16 There are many studies examining the effect of HU in treating patients with β-thalassemia and a few studies reporting on the use of thalidomide; however, as per our search, there are very few that have evaluated the effect of the combination of both drugs. Our study goal was to evaluate the efficacy and safety of combination therapy of thalidomide and HU after 6 months for patients with BTM and BTI.
Methods
Study design and setting
A single-arm nonrandomized trial was conducted at Children’s Hospital Karachi (CHK; Karachi, Pakistan) from January 2020 to December 2020. CHK is a tertiary care hospital specializing in hematological disorders with a particular focus on the management of patients with thalassemia of all ages. Around 15 000 patients with thalassemia from all over the provinces of Sindh and Baluchistan have presented and are registered at CHK.
Patients
Participants aged between 2 and 50 years of either sex presenting with clinical manifestation and genetic diagnosis of BTM and BTI were consecutively enrolled in the study. For all participants, bone marrow transplant was not an option because of a lack of financial resources, absence of a human leukocyte antigen-matched donor, or high risk of transplant-related mortality. Furthermore, all participants were already being treated with HU but showed partial response or decline in their response. Patients with comorbidities such as liver dysfunction and married patients were excluded because of the potential of teratogenicity of the experimental drug.
HU alone was continued at the dose of 10 to 20 mg/kg once a day for 6 months and the response was recorded. At 6 months, thalidomide was added to the regimen at a dose of 2 to 5 mg/kg, orally, once a day at bedtime. An escalating dose regime was used for thalidomide, and those who did not respond to a lower dose were escalated to a higher dose with a maximum dose of 5 mg/kg. To prevent thrombosis, aspirin (2-4 mg/kg per day) was also prescribed.
A prestructured case report form was designed to record the demographic characteristics of the patients, clinical characteristics, laboratory findings, and treatment outcomes. Baseline characteristics were recorded at the time of the initiation of combination therapy. Blood transfusion and complete blood count were observed monthly and recorded at baseline, third month, and sixth month of follow-up of combination therapy, whereas safety parameters including urea, creatinine levels, and liver function tests along with liver and spleen size were recorded at baseline and sixth month of follow-up of combination therapy. The outcome was measured after 6 months of combination therapy as “good responders,” “responders,” or “nonresponders.” Good responders were defined as patients who were being transfused on HU but went off-transfusion after HU and thalidomide combination therapy. Additionally, patients who were already off transfusion on HU therapy and have shown an increment in Hb of >1 g/dL on combination therapy were also defined as good responders. Responders were defined as patients who were being transfused on HU but displayed at least a 50% reduction in PRC transfusion volume over 6 months of combination therapy. Nonresponders were defined as patients who were off transfusion on HU therapy but who did not show any further improvement in Hb levels following the combination therapy or patients who were on transfusion but experienced less than a 50% reduction in PRC transfusion with HU and thalidomide combination therapy.
Blood transfusions were given if at any point during the study the Hb was <7 g/dL or if the patients were symptomatic or unstable, irrespective of the Hb level. Patients who were taking iron chelation therapy (deferasirox, deferiprone, and/or deferoxamine) during HU-only therapy continued it during the combination therapy period. Ethical approval was obtained from the CHK’s institutional review board before conducting the study (institutional review board #CH-0420). Furthermore, written informed consent or assent was obtained in the local language from all study participants or their legal guardians before enrollment in the study. Adequate information was provided to the participants regarding benefits, treatment protocol, and possible adverse events. The data were kept anonymous and confidential.
Statistical analyses
Nonparametric tests were applied to see if the median difference of quantitative variables as normality assumptions were not fulfilled. The Kruskal-Wallis test was applied to see the median difference in laboratory characteristics among different responses, whereas the Friedman test and Wilcoxon test were applied to see the median difference in laboratory findings at different time intervals. The χ2 test was also applied to see the association of categorical predictor variables with responders. A P value ≤.05 was considered significant.
Results
Initially, a total of 144 patients were enrolled, but after the initiation of the combination therapy, 9 patients were removed from the study because of the side effects of cytopenia (33.3%), extremity pain (33.3%), hyperbilirubinemia (22.2%), and sedation (11.1%); the remaining 135 patients were continued on the combination regimen for the complete 6-month period and thus included for the final analysis.
The median age of patients was 6 (interquartile range, 3-10) years. There were 77 (57%) males and 58 (43%) females. The median age at the time of the first transfusion and age at the start of hydroxyurea was 9 (6-15) months and 1.2 (1.0-2.5) years, respectively.
The treatment outcome at 6 months showed that 89 (65.93%) were good responders, 16 (11.85%) were responders, and 30 (22.22%) were nonresponders. The median age (P = .101) and weight (P = .075) of the patients were insignificantly higher among nonresponders and responders compared with good responders. Similarly, an insignificant median difference of age at the time of first transfusion (P = .824), age at the start of hydroxyurea (P = .252), HbA electrophoresis (P = .392), HbA2 electrophoresis (P = .606), and HbF electrophoresis (P = .851) was also observed in between 3 response categories (Table 1).
Baseline characteristics with respect to the outcome of the patients
| . | Overall Median (IQR) . | Good responder (n = 89) Median (IQR) . | Responder (n = 16) Median (IQR) . | Nonresponder (n = 30) Median (IQR) . | P . |
|---|---|---|---|---|---|
| Age, y | 6 (3-10) | 5 (3-8.5) | 6.5 (3.2-10.7) | 7 (3-12.2) | .101∗ |
| Weight, kg | 17 (12-24) | 16 (11.2-22) | 20 (16.2-26.5) | 20 (12-33) | .075∗ |
| Age at first transfusion, mo | 9 (6-15) | 9 (6-14) | 7.5 (3.2-1.6) | 9 (5.7-1.4) | .824∗ |
| Age at the start of HU, y | 1.2 (0.9-2.5) | 1 (0.9-2) | 2.1 (1-4.8) | 1.6 (0.8-4) | .252∗ |
| HbA electrophoresis, n = 12 | 5.7 (2.4-11.2) | 3.2 (2.1-10.8) | 6.6 (6.6-6.6) | 7.8 (5.1-7.8) | .392∗ |
| HbA2 electrophoresis, n = 45 | 2.6 (2-4.1) | 2.8 (2.2-4) | 2.3 (1.3-4.1) | 2.2 (1.6-4.2) | .606∗ |
| HbF electrophoresis, n = 76 | 96.4 (94-98) | 96.6 (94.1-97.9) | 96.2 (60.8-97.7) | 96.3 (92.7-97.8) | .851∗ |
| . | Overall Median (IQR) . | Good responder (n = 89) Median (IQR) . | Responder (n = 16) Median (IQR) . | Nonresponder (n = 30) Median (IQR) . | P . |
|---|---|---|---|---|---|
| Age, y | 6 (3-10) | 5 (3-8.5) | 6.5 (3.2-10.7) | 7 (3-12.2) | .101∗ |
| Weight, kg | 17 (12-24) | 16 (11.2-22) | 20 (16.2-26.5) | 20 (12-33) | .075∗ |
| Age at first transfusion, mo | 9 (6-15) | 9 (6-14) | 7.5 (3.2-1.6) | 9 (5.7-1.4) | .824∗ |
| Age at the start of HU, y | 1.2 (0.9-2.5) | 1 (0.9-2) | 2.1 (1-4.8) | 1.6 (0.8-4) | .252∗ |
| HbA electrophoresis, n = 12 | 5.7 (2.4-11.2) | 3.2 (2.1-10.8) | 6.6 (6.6-6.6) | 7.8 (5.1-7.8) | .392∗ |
| HbA2 electrophoresis, n = 45 | 2.6 (2-4.1) | 2.8 (2.2-4) | 2.3 (1.3-4.1) | 2.2 (1.6-4.2) | .606∗ |
| HbF electrophoresis, n = 76 | 96.4 (94-98) | 96.6 (94.1-97.9) | 96.2 (60.8-97.7) | 96.3 (92.7-97.8) | .851∗ |
| . | n (%) . | n (%) . | n (%) . | n (%) . | . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 77 (57) | 42 (47.2) | 6 (37.5) | 10 (33.3) | .372† |
| Female | 58 (43) | 47 (52.8) | 10 (62.5) | 20 (66.7) | |
| . | n (%) . | n (%) . | n (%) . | n (%) . | . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 77 (57) | 42 (47.2) | 6 (37.5) | 10 (33.3) | .372† |
| Female | 58 (43) | 47 (52.8) | 10 (62.5) | 20 (66.7) | |
P ≤ .05 considered significant.
IQR, interquartile range.
Kruskal-Wallis test applied.
χ2 test applied.
A significant upsurge of median Hb level was observed at 3 months from baseline (P ≤ .001), at 6 months from baseline (P < .001), in between 3 and 6 months (P ≤ .001), and among 0, 3, and 6 months (P ≤ .001). Specifically, the median change in the Hb level at 3 months was 1.6 (0.6-2.8) g/dL, whereas at 6 months it was 2.3 (1.0-3.4) g/dL. Ninety (66.7%) patients in our study had Hb above 8 g/dL after 6 months period of the HU and thalidomide combination therapy with a median level of 9.35 (8.65-10.30) g/dL.
A significant median difference of neutrophils was also observed at 3 months (P = .005), 6 months (P ≤ .001), in between 3 and 6 months (P = .009), and among 0, 3, and 6 months (P ≤ .001). Moreover, a significant decline in serum ferritin level (P ≤ .001), liver size (P = .041), and spleen size (P ≤ .001) through ultrasound measurement was observed at 6 months (Table 2).
Median change in laboratory values with respect to time (n = 135)
| . | Baseline . | 3 mo . | 6 mo . | Median change in 3 mo (IQR) . | Median change in 6 mo (IQR) . | P∗ . | P† . | P‡ . | P§ . |
|---|---|---|---|---|---|---|---|---|---|
| Hb, g/dL | 6.5 (5.8-7.3) | 8 (7.1-9.3) | 8.7 (7.5-9.8) | 1.6 (0.6-2.8) | 2.3 (1-3.4) | <.001 | <.001 | <.001 | <.001 |
| TLC | 8.3 (6-11.7) | 7.8 (6-10.6) | 7.9 (5.8-11) | 1.4 (0.6-3.4) | 1.8 (0.7-3.6) | .328 | .097 | .243 | .548 |
| Neutrophils | 3.2 (2.4-4.4) | 3 (2.1-4) | 2.5 (2-3.8) | 0.5 (0.3-1.2) | 0.9 (0.5-1.7) | <.001 | .005 | <.001 | .009 |
| Platelets | 270 (186-405) | 309 (237-429) | 309 (237-429) | 62 (29-136) | 89 (35-191.5) | .015 | .002 | .045 | .377 |
| Urea | 20 (16-26) | — | 20 (16-26) | — | 5 (2-10) | — | — | .764 | — |
| Creatinine | 0.4 (0.3-0.6) | — | 0.3 (0.3-0.5) | — | 0.1 (0.1-0.3) | — | — | .206 | — |
| Total bilirubin | 1.7 (1.1-2.5) | — | 1.6 (1.1-2) | — | 0.7 (0.3-1.2) | — | — | .122 | — |
| Direct bilirubin | 0.5 (0.4-1) | — | 0.5 (0.4-0.8) | — | 0.5 (0.3-1) | — | — | .895 | — |
| Indirect bilirubin | 0.9 (0.4-1.4) | 1.1 (0.6-1.4) | 0.4 (0.1-0.7) | — | — | .207 | — | ||
| SGPT | 35 (24-52) | — | 40 (24-55) | — | 14 (5-26) | — | — | 156 | — |
| Ferritin | 912 (399.9-1660) | 871 (400-1500) | — | 208 (41-460) | — | — | <.001 | — | |
| Liver size, cm | 10 (9.3-12.2) | — | 9.5 (9.1-12) | — | 0.5 (0.1-1.1) | — | — | .041 | — |
| Spleen size, cm (n = 89) | 9.8 (8.1-12.5) | — | 9.6 (8-12) | — | 0.7 (0.2-1.6) | — | — | <.001 | — |
| . | Baseline . | 3 mo . | 6 mo . | Median change in 3 mo (IQR) . | Median change in 6 mo (IQR) . | P∗ . | P† . | P‡ . | P§ . |
|---|---|---|---|---|---|---|---|---|---|
| Hb, g/dL | 6.5 (5.8-7.3) | 8 (7.1-9.3) | 8.7 (7.5-9.8) | 1.6 (0.6-2.8) | 2.3 (1-3.4) | <.001 | <.001 | <.001 | <.001 |
| TLC | 8.3 (6-11.7) | 7.8 (6-10.6) | 7.9 (5.8-11) | 1.4 (0.6-3.4) | 1.8 (0.7-3.6) | .328 | .097 | .243 | .548 |
| Neutrophils | 3.2 (2.4-4.4) | 3 (2.1-4) | 2.5 (2-3.8) | 0.5 (0.3-1.2) | 0.9 (0.5-1.7) | <.001 | .005 | <.001 | .009 |
| Platelets | 270 (186-405) | 309 (237-429) | 309 (237-429) | 62 (29-136) | 89 (35-191.5) | .015 | .002 | .045 | .377 |
| Urea | 20 (16-26) | — | 20 (16-26) | — | 5 (2-10) | — | — | .764 | — |
| Creatinine | 0.4 (0.3-0.6) | — | 0.3 (0.3-0.5) | — | 0.1 (0.1-0.3) | — | — | .206 | — |
| Total bilirubin | 1.7 (1.1-2.5) | — | 1.6 (1.1-2) | — | 0.7 (0.3-1.2) | — | — | .122 | — |
| Direct bilirubin | 0.5 (0.4-1) | — | 0.5 (0.4-0.8) | — | 0.5 (0.3-1) | — | — | .895 | — |
| Indirect bilirubin | 0.9 (0.4-1.4) | 1.1 (0.6-1.4) | 0.4 (0.1-0.7) | — | — | .207 | — | ||
| SGPT | 35 (24-52) | — | 40 (24-55) | — | 14 (5-26) | — | — | 156 | — |
| Ferritin | 912 (399.9-1660) | 871 (400-1500) | — | 208 (41-460) | — | — | <.001 | — | |
| Liver size, cm | 10 (9.3-12.2) | — | 9.5 (9.1-12) | — | 0.5 (0.1-1.1) | — | — | .041 | — |
| Spleen size, cm (n = 89) | 9.8 (8.1-12.5) | — | 9.6 (8-12) | — | 0.7 (0.2-1.6) | — | — | <.001 | — |
P ≤ .05 considered significant.
IQR, interquartile range; SGPT, serum glutamic pyruvic transaminase; TLC, total leukocyte count.
0, 3, and 6 mo (Friedman test).
0 and 3 mo (Wilcoxon signed-rank test).
0 and 6 mo (Wilcoxon signed-rank test).
3 and 6 mo (Wilcoxon signed-rank test).
A significant decline in the transfusion volume before and after combination therapy was observed in all participants (P ≤ .001); good responders (P ≤ .001), responders (P = .003), and nonresponders (P = .002) (Table 3).
Transfusion characteristics of the patients
| . | Median (IQR) . | Mean (SD) . | P . |
|---|---|---|---|
| Overall (n = 135) | |||
| Transfusion volume before combination therapy | 950 (0-2100) | 1106.4 (1041.8) | <.001 |
| Transfusion volume after combination therapy | 0 (0-300) | 315.6 (616.7) | |
| Good responder (n = 89) | |||
| Transfusion volume before combination therapy | 900 (55-1750) | 991.6 (897.9) | <.001 |
| Transfusion volume after combination therapy | 0 (0-0) | 168.9 (484.7) | |
| Responders (n = 16) | |||
| Transfusion volume before combination therapy | 1575 (555-2362) | 1677.5 (1394.2) | .003 |
| Transfusion volume after combination therapy | 350 (0-975) | 618.7 (748.9) | |
| Nonresponders (n = 30) | |||
| Transfusion volume before combination therapy | 1050 (0-2100) | 1142.3 (1158.1) | .002 |
| Transfusion volume after combination therapy | 0 (0-1400) | 589 (748.5) | |
| . | Median (IQR) . | Mean (SD) . | P . |
|---|---|---|---|
| Overall (n = 135) | |||
| Transfusion volume before combination therapy | 950 (0-2100) | 1106.4 (1041.8) | <.001 |
| Transfusion volume after combination therapy | 0 (0-300) | 315.6 (616.7) | |
| Good responder (n = 89) | |||
| Transfusion volume before combination therapy | 900 (55-1750) | 991.6 (897.9) | <.001 |
| Transfusion volume after combination therapy | 0 (0-0) | 168.9 (484.7) | |
| Responders (n = 16) | |||
| Transfusion volume before combination therapy | 1575 (555-2362) | 1677.5 (1394.2) | .003 |
| Transfusion volume after combination therapy | 350 (0-975) | 618.7 (748.9) | |
| Nonresponders (n = 30) | |||
| Transfusion volume before combination therapy | 1050 (0-2100) | 1142.3 (1158.1) | .002 |
| Transfusion volume after combination therapy | 0 (0-1400) | 589 (748.5) | |
∼35 of 135 (ie, 25.9% of the patients) were already off transfusion of hydroxyurea only. ∼97 of 135 (ie, 71.9% of the patients) were off transfusion after 6 mo of combination therapy.
IQR, interquartile range.
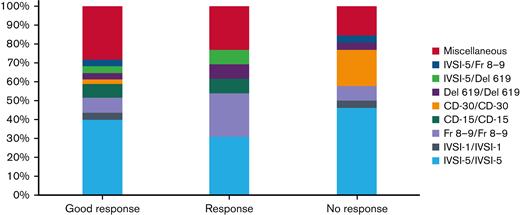
The distribution of mutations with respect to the treatment outcome is almost similar in all response categories. (Figure 1), whereas the XmnI polymorphism status was available for 133 patients. Of these, negative/negative cases were predominantly higher (ie, 108/133 [81.2%]), followed by positive/negative cases in 14 (10.5%), and positive/positive cases in 11 (8.3%) (Table 4).
Comparison of XmnI polymorphism with respect to the outcome (n = 133)
| XmnI . | Overall n (%) . | Good responder (n = 88) n (%) . | Responder (n = 15) n (%) . | Nonresponder (n = 30) n (%) . |
|---|---|---|---|---|
| Positive/positive | 11 (8.3) | 6 (6.8) | 0 (0) | 5 (16.7) |
| Negative/negative | 108 (81.2) | 72 (81.8) | 13 (86.7) | 23 (76.7) |
| Positive/negative | 14 (10.5) | 10 (11.4) | 2 (13.3) | 2 (6.7) |
| XmnI . | Overall n (%) . | Good responder (n = 88) n (%) . | Responder (n = 15) n (%) . | Nonresponder (n = 30) n (%) . |
|---|---|---|---|---|
| Positive/positive | 11 (8.3) | 6 (6.8) | 0 (0) | 5 (16.7) |
| Negative/negative | 108 (81.2) | 72 (81.8) | 13 (86.7) | 23 (76.7) |
| Positive/negative | 14 (10.5) | 10 (11.4) | 2 (13.3) | 2 (6.7) |
Sixty-seven (49.6%) patients were prescribed 2 to 3 mg/kg dose of thalidomide, whereas 35 (25.9%) and 33 (24.4%) were prescribed 3.1 to 4 mg/kg and 4.1 to 5 mg/kg doses of thalidomide, respectively. A significant mean difference of Hb was observed in various doses categories (P < .001) (Table 5).
Mean difference of hemoglobin level with respect to different doses (n = 135)
| Dose, mg/kg . | n . | Mean ± SD . | 95% CI . | P . |
|---|---|---|---|---|
| 2-3 | 67 | 9.26 ± 1.52 | 8.89-9.64 | <.001 |
| 3.1-4 | 35 | 8.29 ± 1.45 | 7.79-8.79 | |
| 4.1-5 | 33 | 7.98 ± 1.54 | 7.54-8.42 |
| Dose, mg/kg . | n . | Mean ± SD . | 95% CI . | P . |
|---|---|---|---|---|
| 2-3 | 67 | 9.26 ± 1.52 | 8.89-9.64 | <.001 |
| 3.1-4 | 35 | 8.29 ± 1.45 | 7.79-8.79 | |
| 4.1-5 | 33 | 7.98 ± 1.54 | 7.54-8.42 |
One-way analysis of variance test applied; P < .05 considered significant.
CI, confidence interval; SD, standard deviation.
A total of 38 adverse events were reported that resolved on supportive treatment or temporary hold of the intervention. Of these, 9 (23.68%) patients experienced neutropenia or leukopenia. Jaundice was observed in 4 (10.53%), diarrhea in 3 (7.89%), headache in 3 (7.89%), extremity pain in 3 (7.89%), and seizures in 3 (7.89%) patients. Thirteen patients (9.4%) experienced lethargy, bruises, vertigo, constipation, epistaxis, and irritability.
Discussion
In this current study, we have evaluated the outcomes of a combination of HU and thalidomide therapy. We found significant improvement in median Hb levels and decreased transfusion requirements in the study participants. A significant decrease in liver and spleen size, and serum ferritin level is also reported. The response is independent of any genetic mutation.
A multicenter study by Yang et al studied the effect of thalidomide and demonstrated a 2.9 g/dL increment of Hb from the baseline among the nontransfusion-dependent thalassemia patients aged between 14 and 65 years. In transfusion-dependent thalassemia patients, in addition to Hb improvement, a significant decrease in transfusion frequency was recorded.8 One of the studies that evaluated the effect of the HU and thalidomide combination in 140 transplant-ineligible patients with thalassemia aged 10 years or above reported maintenance of Hb levels ≥9 g/dL in 57.2% of the participants, 50% reduction of transfusion burden in 14.3%, and a decrease in ferritin levels among the responders.17 Another study with a cohort of 37 patients reported a significant decrease in annual transfusion requirements from 27 to 7.79 blood units each year among its patients who had transfusion-dependent thalassemia.18 Various case reports and other small sample-sized studies have also reported hematologic improvement among patients with β-thalassemia with the use of thalidomide alone as well as the combination therapy.19-22 A recently published randomized clinical trial with 100 participants has also reported an increase in Hb concentration and reduction in blood transfusion with thalidomide use.23 In our study, there has been a significant overall increase in median Hb levels within 3 and 6 months of use of HU and thalidomide combination. Two-thirds of the patients in the current study had Hb levels above 8 g/dL after 6 months of the HU and thalidomide combination therapy, with a median level of 9.35 g/dL. An overall decrease in transfusion volume was also observed during the 6 months of combination therapy, in comparison with the 6 months of sole HU therapy.
A retrospective pilot study evaluating the efficacy of the combination therapy in 25 patients reported the overall response rate to be 68.2% at 3 months, and a prospective study evaluating the efficacy of thalidomide use in 37 patients reported a response rate of 83.7%.21,22 In our study, the overall response rate to the combination therapy was 77.8%.
Hydroxyurea use has previously been shown to result in the reduction of spleen size and it has been used for supportive treatment of extramedullary hematopoiesis in β-thalassemia patients.24,25 Our current combination study has also demonstrated a significant decrease in liver and spleen size after 6 months. Furthermore, a significant decrease in serum ferritin levels was also found in this study that could be attributed to the reduced volume of PRC transfusions during the combination therapy period resulting in overall improvement of the health status of our study participants.
Our previous work with hydroxyurea use positively associated certain genetic mutations such as Cd-30, IVS1-1, Cap+1, and IVS1-5 with better clinical outcomes. Likewise, the presence of the XmnI polymorphism was also significantly associated with a higher response rate.11 Other studies have also reported the presence of the XmnI polymorphism to be positively associated with the response to HU.26,27 In the present study, to our surprise, the combination of HU and thalidomide success was not associated with any particular genetic mutation or the presence of the XmnI polymorphism. The responding patients had diverse genetic mutations and ∼80% of the responding patients had no XmnI polymorphism. These findings support the trial of the combination therapy in patients with thalassemia irrespective of the genetic mutation. The optimum response to the combination therapy was achieved at the dose of 2 to 3 mg/kg of thalidomide; however, we used an escalating dose regime for those who did not respond to the lower range, but escalating thalidomide resulted in only a minimal rise in hemoglobin.
Married patients were excluded from the study considering the underutilization and noncompliance of contraceptives in the developing world. Nine patients who were removed from the study experienced side effects of cytopenia, extremity pain, hyperbilirubinemia, and sedation that subsided when the drug was discontinued but emerged again at therapy reinitiation. Furthermore, of 135 patients who continued on the combination therapy for a complete 6-month period, 38 patients experienced at least 1 side effect of mild severity including neutropenia/leukopenia, jaundice, face swelling, diarrhea, headache, extremity pain, seizures, and constipation that subsided on temporary hold of medication or the patients recovered on symptomatic treatment. The side effects did not appear when medications were started again. In addition to the previously mentioned acute side effects, long-term side effects remain a concern with the use of thalidomide. The doses of the patients who experienced side effects were comparable to the participant without adverse events. Some of the more serious side effects such as neuropathy or thrombosis were not observed, possibly because of the small sample size, younger ages of the study participants, and short duration of the study.
Pakistan has a high disease burden of thalassemia; however, the existing blood banking system is still lagging. The high prevalence of transfusion-related viral infections is a major cause of morbidity and mortality in this patient population.28 Additionally, lack of financial resources and compliance to treatment regimen remains a barrier to effective iron chelation therapy, leading to iron overload complications and further debilitation of health in patients with thalassemia in Pakistan.
Thalidomide in combination with HU is scarcely studied. Our study adds to the existing knowledge in this area. Further, this study also analyzed the patterns of genetic mutation in patients taking the combination therapy. The strengths of this study are that it has documented not only the effect of the intervention but also correlates them with the genetic mutations and modifiers of thalassemia prevalent in Pakistan. Our study had no dropouts or losses to follow up.
The limitations of our study were the small sample size, lack of randomization, and absence of a control group. Further, some patients did not have complete Hb electrophoresis results at baseline and the test was not repeated after the intervention to study improvement in HbF levels.
The findings of this study support the use of the combination regimen over the conventional transfusion-dependent management not only in the developing world but also in the developed countries at least for patients with thalassemia major and intermedia who are maintaining Hb levels comparable to thalassemia minors. However, the efficacy and safety of the combination of thalidomide and HU for patients with thalassemia should be established further through larger randomized trials. Overall, HU and thalidomide combination use resulted in an improvement in Hb levels and decreased PRC transfusions in patients with β-thalassemia irrespective of genetic mutation and XmnI polymorphism. Large-scale randomized trials should also be conducted in parallel in 3 arms: hydroxyurea alone, HU and thalidomide, and thalidomide alone to further evaluate the efficacy of HU, thalidomide, and their combination. In future studies, quantification of HbG messenger RNA (both γ-globin genes) and HbF should also be considered to evaluate the mechanism of action of these medications in addition to optimization of dosages for the combination therapy.
Acknowledgments
The authors thank the patients who participated in this trial and their families, and Samina Ali, Ambereen Ali, and Salima Khowaja for the editorial review of the manuscript.
Authorship
Contribution: S.H.A. conceived the study, recruited the patients, and managed them clinically; I.A. wrote the protocol, oversaw data collection, and drafted the manuscript; M.W. and N.U.N.M. recruited patients and managed them clinically; S.K., A.H.A., and U.H.A. coordinated the patients and collected the data; M.Z. and Z.H. performed laboratory tests including genetic mutations; F.F. developed the protocol and manuscript; A.S. contributed to study conception, study design and performed radiologic evaluations; S.O.A. performed the statistical analysis; and all authors critically reviewed the manuscript, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Iqra Ansari, Children’s Hospital Karachi, ST 2/2, Block-5, Gulshan-e-Iqbal, Karachi, Pakistan; e-mail: ansari_iqra@yahoo.com.
References
Author notes
For data sharing, contact the corresponding author, Iqra Ansari (ansari_iqra@yahoo.com).