Key Points
This is the largest summary to date of the management of people with hemophilia A on emicizumab prophylaxis undergoing surgery.
Major and minor surgeries were performed safely in people receiving emicizumab, regardless of factor VIII inhibitor status.
Abstract
Many people with hemophilia A (PwHA) undergo surgery in their lifetime, often because of complications of their disease. Emicizumab is the first bispecific monoclonal antibody prophylactic therapy for PwHA, and its efficacy and safety have been previously demonstrated; however, there is a need to build an evidence base on the management of PwHA on emicizumab undergoing surgery. Data from the HAVEN 1-4 phase 3 clinical trials were pooled to provide a summary of all minor and major surgeries in PwHA with or without factor VIII (FVIII) inhibitors who were receiving emicizumab prophylaxis. Overall, 233 surgeries were carried out during the HAVEN 1-4 trials: 215 minor surgeries (including minor dental and joint procedures, central venous access device placement or removal, and endoscopies) in 115 PwHA (64 with FVIII inhibitors) and 18 major surgeries (including arthroplasty and synovectomy) in 18 PwHA (10 with FVIII inhibitors). Perioperative hemostatic support was at the discretion of the treating physician. Overall, the median (interquartile range [IQR]) age was 33.5 (13.0-49.0) years and the median (IQR) emicizumab exposure time before surgery was 278.0 (177.0-431.0) days. Among the 215 minor surgeries, 141 (65.6%) were managed without additional prophylactic factor concentrate, and of those, 121 (85.8%) were not associated with a postoperative bleed. The majority (15 of 18 [83.3%]) of major surgeries were managed with additional prophylactic factor concentrate. Twelve (80.0%) of these 15 surgeries were associated with no intraoperative or postoperative bleeds. The data demonstrate that minor and major surgeries can be performed safely in PwHA receiving emicizumab prophylaxis. These trials are registered at www.clinicaltrials.gov as #NCT02622321, #NCT02795767, #NCT02847637, and #NCT03020160.
Introduction
People with hemophilia A (PwHA) often require surgical procedures during their lifetime. While some procedures may be unrelated to the disease, issues arising because of the condition itself, such as the requirement for central venous access devices (CVADs) for clotting factor administration and the treatment of joint damage because of hemarthrosis, provide additional reasons for surgeries in this population. Compared with people without hemophilia, PwHA are at increased risk of bleeding when undergoing surgery, as well as other complications such as poor wound healing and infection, and require management with hemostatic treatment during the perioperative period.1-3 Surgery is a particular challenge in PwHA with factor (F)VIII inhibitors, in whom FVIII replacement therapy is ineffective. Before the advent of recombinant activated FVII (rFVIIa) and activated prothrombin complex concentrate (aPCC) bypassing agents (BPAs), many procedures would have been contraindicated in this population.4,5 As a result of years of experience of surgeries being performed on PwHA with the use of FVIII and bypassing agents to achieve hemostasis, although there is no uniform approach to perioperative management, there now exists a wealth of expert guidance, and even major surgeries can be safely performed on PwHA with or without FVIII inhibitors.3,4,6-8
A more recent addition to the armamentarium for bleed prevention in PwHA is emicizumab. This is a bispecific humanized monoclonal antibody that bridges activated FIX and FX, substituting for the function of missing activated FVIII, with resultant downstream thrombin generation and fibrin clot formation.9 It is administered subcutaneously with high bioavailability (80.4%-93.1%) and has a long half-life (26.8 [standard deviation, 9.2] days), enabling effective steady-state plasma concentrations throughout the dosing interval.10,11 Emicizumab is indicated for routine prophylaxis in PwHA of all ages, with or without FVIII inhibitors, as 3 maintenance dosing regimens: 1.5 mg/kg weekly, 3 mg/kg every 2 weeks, and 6 mg/kg every 4 weeks.12,13
The efficacy and safety of emicizumab were demonstrated in adults, adolescents, and children with hemophilia A with or without FVIII inhibitors in the HAVEN 1-4 phase 3 clinical trials (NCT02622321, NCT02795767, NCT02847637, and NCT03020160) and long-term follow-up.14-18 However, there are limited prospective data on the management and outcomes of people receiving emicizumab prophylaxis and undergoing surgery. Such data would be of great clinical interest,19-22 and may inform the development of surgical guidelines in the future.
This pooled analysis summarizes the surgical experience of PwHA receiving emicizumab prophylaxis who underwent major and/or minor surgeries in the HAVEN 1-4 studies.
Methods
Study design and participants
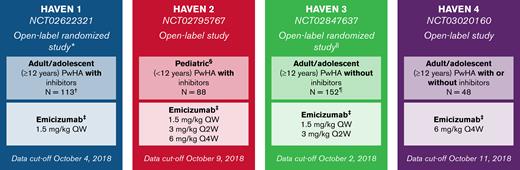
This study was performed as a post hoc subanalysis of the HAVEN 1-4 clinical trials, which were phase 3, open-label, multicenter studies (Figure 1). The designs and populations of HAVEN 1-4 have been described previously.14-17 The cutoff dates for the data included in the present analysis were in October 2018. The HAVEN studies were conducted in compliance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All study protocols were approved by the institutional review board or ethics committee at each participating center. Adult participants provided written informed consent before study entry. For participants <18 years of age, informed consent was provided by a parent or legally authorized representative, along with informed assent in those aged 8 to 17 years.
Study designs of the HAVEN 1-4 trials.14-17 ∗Participants receiving episodic BPAs before study entry were randomized to emicizumab prophylaxis (Arm A) or no emicizumab (Arm B, control), and those receiving prophylactic BPAs before study entry received emicizumab prophylaxis (Arm C). After completing the first 24 weeks of the trial, participants in the control arm (Arm B) could receive emicizumab prophylaxis. A fourth arm also receiving emicizumab prophylaxis (Arm D) comprised participants enrolled after Arms A to C closed. †One participant in HAVEN 1 assigned to an active arm discontinued before the first emicizumab treatment and was excluded from the analyses. ‡Maintenance doses. With the exception of the HAVEN 4 pharmacokinetics run-in cohort (n = 7), all maintenance doses were preceded by loading doses of 3.0 mg/kg QW for 4 weeks. §Adolescents aged 12 to 17 years were also eligible to enroll in HAVEN 2 if they weighed <40 kg; 3 participants were aged 12 to 17. ǁParticipants receiving episodic FVIII before study entry were randomized (2:2:1) to emicizumab 1.5 mg/kg QW (Arm A), emicizumab 3 mg/kg Q2W (Arm B), or no prophylaxis (Arm C, control), and those receiving prophylactic FVIII before study entry received emicizumab 1.5 mg/kg QW (Arm D). ¶One participant in HAVEN 3 assigned to no prophylaxis was lost to follow-up before switching to emicizumab and was therefore not treated; hence, they have been excluded from the analyses. F, factor; QW, once weekely; Q2W, once every 2 weeks; Q4W, once every 4 weeks.
Study designs of the HAVEN 1-4 trials.14-17 ∗Participants receiving episodic BPAs before study entry were randomized to emicizumab prophylaxis (Arm A) or no emicizumab (Arm B, control), and those receiving prophylactic BPAs before study entry received emicizumab prophylaxis (Arm C). After completing the first 24 weeks of the trial, participants in the control arm (Arm B) could receive emicizumab prophylaxis. A fourth arm also receiving emicizumab prophylaxis (Arm D) comprised participants enrolled after Arms A to C closed. †One participant in HAVEN 1 assigned to an active arm discontinued before the first emicizumab treatment and was excluded from the analyses. ‡Maintenance doses. With the exception of the HAVEN 4 pharmacokinetics run-in cohort (n = 7), all maintenance doses were preceded by loading doses of 3.0 mg/kg QW for 4 weeks. §Adolescents aged 12 to 17 years were also eligible to enroll in HAVEN 2 if they weighed <40 kg; 3 participants were aged 12 to 17. ǁParticipants receiving episodic FVIII before study entry were randomized (2:2:1) to emicizumab 1.5 mg/kg QW (Arm A), emicizumab 3 mg/kg Q2W (Arm B), or no prophylaxis (Arm C, control), and those receiving prophylactic FVIII before study entry received emicizumab 1.5 mg/kg QW (Arm D). ¶One participant in HAVEN 3 assigned to no prophylaxis was lost to follow-up before switching to emicizumab and was therefore not treated; hence, they have been excluded from the analyses. F, factor; QW, once weekely; Q2W, once every 2 weeks; Q4W, once every 4 weeks.
Data collection and analysis
Unplanned surgeries and elective minor procedures were permitted in the HAVEN program; however, individuals with planned major surgical interventions at the time of enrollment were excluded. Surgical procedures were managed per the investigator’s discretion; no protocol-specified guidance on periprocedural hemostatic management was provided. For this analysis, data were collected and pooled across the safety populations of the HAVEN 1-4 studies, which included all enrolled participants who received emicizumab.
Procedures were categorized as minor or major, as defined by Santagostino and colleagues.23 A minor surgery was defined as an invasive procedure involving the manipulation of only skin, mucus membranes, or superficial connective tissue. A major surgery was defined as an invasive procedure that included ≥1 of the following: entering a body cavity, crossing a mesenchymal barrier, opening a fascial plane, removing an organ, or operatively altering the normal anatomy. Details of the type and number of procedures, FVIII concentrate or BPA utilization, antifibrinolytic therapy, adverse events, and postoperative bleeds were captured.14-17
Postoperative bleeds were defined as those that occurred after surgery and were categorized as “bleed because of surgery/procedure” by the treating physician. In the HAVEN studies, a treated bleed was defined as one that was directly followed by administration of FVIII concentrate or BPA (ie, no intervening bleed), irrespective of the time between the treatment and the preceding bleed. A bleed and the first treatment thereafter were deemed connected (ie, a treatment belonged to 1 bleed only); however, if multiple bleeds occurred on the same calendar day, each bleed was counted separately, but the subsequent treatment was considered to apply for each of these multiple bleeds.
Outcomes are reported using descriptive statistics. Data analyses were conducted by study statisticians who vouch for the completeness and accuracy of the analyses. Data were made available to all authors, who confirmed adherence to the protocol.
Results
Study population
Across the HAVEN 1-4 safety population, 126 (31.6%) of 399 participants who received emicizumab had ≥1 surgery, while 43 (10.8%) participants had ≥2 procedures (Table 1). For the population of PwHA who underwent a surgical procedure, the median (interquartile range) age was 33.5 (13.0-49.0) years and the median (interquartile range) emicizumab exposure time before surgery was 278.0 (177.0-431.0) days. Sixty-nine (55.6%) participants had FVIII inhibitors at study entry.
Characteristics of patients undergoing surgical procedures in the HAVEN 1-4 clinical trials
| . | HAVEN 1 n = 38 . | HAVEN 2 n = 27 . | HAVEN 3 n = 45 . | HAVEN 4 n = 16 . | Total N = 126 . |
|---|---|---|---|---|---|
| Median age (IQR), y | 31.5 (17.0-46.0) | 7.0 (5.0-8.0) | 44.0 (29.0-53.0) | 46.5 (35.5-57.5) | 33.5 (13.0-49.0) |
| Race, n (%) | |||||
| White | 27 (71.1) | 17 (63.0) | 33 (73.3) | 10 (62.5) | 87 (69.0) |
| Asian | 6 (15.8) | 4 (14.8) | 9 (20.0) | 5 (31.3) | 24 (19.0) |
| Black/African American | 2 (5.3) | 2 (7.4) | 0 | 1 (6.3) | 5 (4.0) |
| Other or unknown | 3 (7.9) | 4 (14.8) | 3 (6.7) | 0 | 10 (7.9) |
| Presence of FVIII inhibitors, n (%) | 38 (100) | 27 (100) | 0 | 4 (25.0) | 69 (54.8) |
| Median number of bleeds in 24 wk before study entry (IQR) | 9.5 (6.0-19.0) | 5.0 (4.0-9.0) | 7.0 (2.0-13.0) | 5.5 (3.0-13.0) | 7.0 (4.0-14.0) |
| Presence of target joints∗ at study entry, n (%) | 23 (62.2) | 13 (48.1) | 28 (62.2) | 11 (68.8) | 75 (60.0) |
| Patients who underwent: n (%) | |||||
| 1 surgical procedure | 21 (55.3) | 25 (92.6) | 25 (55.6) | 12 (75.0) | 83 (65.9) |
| 2 surgical procedures | 6 (15.8) | 2 (7.4) | 8 (17.8) | 2 (12.5) | 18 (14.3) |
| >2 surgical procedures | 11 (28.9) | 0 | 12 (26.7) | 2 (12.5) | 25 (19.8) |
| Median duration of emicizumab exposure (IQR), wk | 101.7 (84.1-127.1) | 79.1 (67.1-102.1) | 89.1 (80.3-97.1) | 68.1 (68.1–72.1) | 86.3 (75.1-102.1) |
| . | HAVEN 1 n = 38 . | HAVEN 2 n = 27 . | HAVEN 3 n = 45 . | HAVEN 4 n = 16 . | Total N = 126 . |
|---|---|---|---|---|---|
| Median age (IQR), y | 31.5 (17.0-46.0) | 7.0 (5.0-8.0) | 44.0 (29.0-53.0) | 46.5 (35.5-57.5) | 33.5 (13.0-49.0) |
| Race, n (%) | |||||
| White | 27 (71.1) | 17 (63.0) | 33 (73.3) | 10 (62.5) | 87 (69.0) |
| Asian | 6 (15.8) | 4 (14.8) | 9 (20.0) | 5 (31.3) | 24 (19.0) |
| Black/African American | 2 (5.3) | 2 (7.4) | 0 | 1 (6.3) | 5 (4.0) |
| Other or unknown | 3 (7.9) | 4 (14.8) | 3 (6.7) | 0 | 10 (7.9) |
| Presence of FVIII inhibitors, n (%) | 38 (100) | 27 (100) | 0 | 4 (25.0) | 69 (54.8) |
| Median number of bleeds in 24 wk before study entry (IQR) | 9.5 (6.0-19.0) | 5.0 (4.0-9.0) | 7.0 (2.0-13.0) | 5.5 (3.0-13.0) | 7.0 (4.0-14.0) |
| Presence of target joints∗ at study entry, n (%) | 23 (62.2) | 13 (48.1) | 28 (62.2) | 11 (68.8) | 75 (60.0) |
| Patients who underwent: n (%) | |||||
| 1 surgical procedure | 21 (55.3) | 25 (92.6) | 25 (55.6) | 12 (75.0) | 83 (65.9) |
| 2 surgical procedures | 6 (15.8) | 2 (7.4) | 8 (17.8) | 2 (12.5) | 18 (14.3) |
| >2 surgical procedures | 11 (28.9) | 0 | 12 (26.7) | 2 (12.5) | 25 (19.8) |
| Median duration of emicizumab exposure (IQR), wk | 101.7 (84.1-127.1) | 79.1 (67.1-102.1) | 89.1 (80.3-97.1) | 68.1 (68.1–72.1) | 86.3 (75.1-102.1) |
Out of the 126 participants who received emicizumab and had ≥1 surgery, 5 and 2 participants were patients with mild and moderate hemophilia, respectively; however, because these 7 patients had FVIII inhibitors, they were consequently considered as having severe hemophilia A phenotype.
Note that percentage totals may not total 100% because of rounding.
FVIII, factor VIII; IQR, interquartile range.
Target joints were defined according to the International Society on Thrombosis and Haemostasis as major joints (eg, hip, elbow, wrist, shoulder, knee, and ankle) with ≥3 bleeds during the 24-wk period before study enrollment.39
Overview of all surgeries
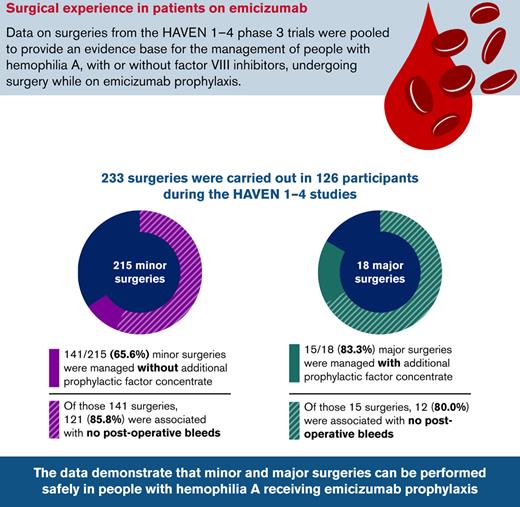
Overall, 233 surgeries were carried out during the HAVEN 1-4 trials: 215 minor surgeries (including dental, CVAD insertion/removal [including peripherally inserted central catheters, Hickman lines, and Port-a-Cath procedures], endoscopic, joint, and other) in 115 PwHA, and 18 major surgeries in 18 PwHA (Table 2). Further information on the specific major surgical procedures can be found in supplemental Table 1 in the data supplement.
Types of surgeries
| . | Surgeries/procedures . |
|---|---|
| Surgeries or procedures, N | 233 |
| Minor surgeries or procedures | 215 |
| Dental,∗ n (%) | 62 (28.8) |
| CVAD,† n (%) | 36 (16.7) |
| Endoscopic,‡ n (%) | 30 (14.0) |
| Joint,§ n (%) | 25 (11.6) |
| Other,ǁ n (%) | 62 (28.8) |
| Major surgeries or procedures¶ | 18 |
| Arthroplasty, n (%) | 5 (27.8) |
| Synovectomy, n (%) | 4 (22.2) |
| Other, n (%) | 9 (50.0) |
| . | Surgeries/procedures . |
|---|---|
| Surgeries or procedures, N | 233 |
| Minor surgeries or procedures | 215 |
| Dental,∗ n (%) | 62 (28.8) |
| CVAD,† n (%) | 36 (16.7) |
| Endoscopic,‡ n (%) | 30 (14.0) |
| Joint,§ n (%) | 25 (11.6) |
| Other,ǁ n (%) | 62 (28.8) |
| Major surgeries or procedures¶ | 18 |
| Arthroplasty, n (%) | 5 (27.8) |
| Synovectomy, n (%) | 4 (22.2) |
| Other, n (%) | 9 (50.0) |
n refers to the number of surgeries.
The most common dental procedures were tooth extraction, endodontic procedure, dental implantation, and dental prosthesis placement.
The most common CVAD procedures were central venous catheter removal, central venous catheterization, and catheter placement.
The most common endoscopic procedures were upper gastrointestinal endoscopy, esophagogastroduodenoscopy, colonoscopy, biopsy or biopsy colon, large intestinal polypectomy, cystoscopy, and sigmoidoscopy.
The most common joint procedures were joint injection, synoviorthesis, and joint aspiration.
The most common other procedures were aspiration of seroma, suture removal, biopsy or biopsy skin, ear tube insertion, hematoma evacuation, mole excision, nasal septal operation, and retinal laser coagulation.
Major surgeries are listed in supplemental Table 1.
Minor surgeries
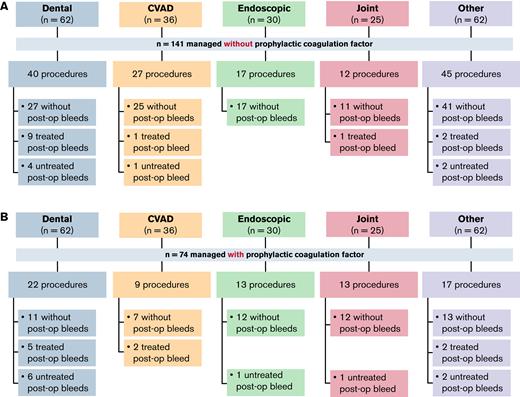
Among the 215 minor surgeries, 141 (65.6%) were managed without additional prophylactic factor concentrate. Of those, 121 (85.8%) were not associated with a postoperative bleed (Figure 2A). Of the 20 postoperative bleeds, 13 (65.0%) were treated. Of the 74 procedures managed with additional prophylactic factor concentrate, 55 (74.3%) were not associated with a postoperative bleed (Figure 2B). Of the 19 postoperative bleeds that occurred, 9 (47.4%) were treated with additional factor concentrate.
Summary of minor surgeries or procedures managed without or with prophylactic factor concentrate. (A) Minor surgeries or procedures performed without prophylactic factor concentrate. (B) Minor surgeries or procedures performed with prophylactic concentrate. n refers to the number of surgeries and procedures. CVAD, central venous access device; post-op, post-operative.
Summary of minor surgeries or procedures managed without or with prophylactic factor concentrate. (A) Minor surgeries or procedures performed without prophylactic factor concentrate. (B) Minor surgeries or procedures performed with prophylactic concentrate. n refers to the number of surgeries and procedures. CVAD, central venous access device; post-op, post-operative.
Minor dental surgeries
There were 62 minor dental surgeries included in the analysis. The most common procedures were tooth extraction (n = 29), endodontic procedures (n = 15), and dental implantations (n = 7). Twenty-two (35.5%) of the dental procedures were managed with additional prophylactic factor concentrate (Figure 2B). In the participants with FVIII inhibitors, 5 (17.2%) of the 29 procedures were managed with prophylactic rFVIIa, with a single dose administered in 4 (80.0%) of these cases (Table 3). In the participants without FVIII inhibitors, 14 (42.4%) and 2 (6.1%) of the 33 procedures were managed with standard half-life (SHL) or extended half-life (EHL) FVIII concentrate, respectively. Of these, 11 (78.6%) and 2 (100.0%) procedures, respectively, were each managed with a single dose (Table 3).
Details of prophylactic doses and treatment for postoperative bleeds in patients undergoing minor surgeries or procedures
| . | PwHA with FVIII inhibitors (n = 64) rFVIIa . | PwHA without FVIII inhibitors (n = 51) . | |
|---|---|---|---|
| SHL FVIII . | EHL FVIII . | ||
| Dental | |||
| Procedures, n | 29 | 33 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 5 (17.2) | 14 (42.4) | 2 (6.1) |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 4 (80.0) | 11 (78.6) | 2 (100) |
| Median prophylactic doses per surgery (range) | 1.0 (1-3) | 1.0 (1-3) | 1.0 (1-1) |
| Median prophylactic cumulative dose per surgery (IQR) | 83.4 μg/kg (83.4-106.8) | 25.4 IU/kg (24.8-38.9) | 42.1 IU/kg (36.8-47.4) |
| Procedures associated with treatment for postoperative bleeds, n (%) | 6 (20.7) | 6 (18.2) | 2 (6.1) |
| Median days postoperative bleeds were treated (range) | 1.5 (1-4) | 2.0 (1-2) | 1.0 (1-1) |
| CVAD | |||
| Procedures, n | 35 | 1 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 9 (25.7) | 0 | 0 |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 6 (66.7) | — | — |
| Median prophylactic doses per surgery (range) | 1.0 (1-3) | — | — |
| Median prophylactic cumulative dose per surgery (IQR) | 119.0 μg/kg (86.5-168.7) | — | — |
| Procedures associated with treatment for postoperative bleeds, n (%) | 3 (8.6) | 0 (0.0) | 0 (0.0) |
| Median days postoperative bleeds were treated (range) | 1.0 (1-3) | — | — |
| Endoscopic | |||
| Procedures, n | 13 | 17 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 2 (15.4) | 10 (58.8) | 1 (5.9) |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 1 (50.0) | 6 (60.0) | 0 |
| Median prophylactic doses per surgery (range) | 1.5 (1-2) | 1.0 (1-12) | 3.0 |
| Median prophylactic cumulative dose per surgery (IQR) | 90.3 μg/kg (62.0-118.6) | 30.0 IU/kg (23.3-52.4) | 122.4 IU/kg |
| Procedures associated with treatment for postoperative bleeds, n (%) | 0 | 0 | 0 |
| Median days postoperative bleeds were treated (range) | — | — | — |
| Joint | |||
| Procedures, n | 3 | 22 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 2 (66.7) | 9 (40.9) | 2 (9.1) |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 1 (50.0) | 9 (100) | 2 (100) |
| Median prophylactic doses per surgery (range) | 1.5 (1-2) | 1.0 (1-1) | 1.0 (1-1) |
| Median prophylactic cumulative dose per surgery (IQR) | 156.2 μg/kg (85.1-227.3) | 21.2 IU/kg (21.1-29.8) | 21.3 IU/kg (20.3-22.4) |
| Procedures associated with treatment for postoperative bleeds, n (%) | 0 | 1 (4.5) | 0 (0.0) |
| Median days postoperative bleeds were treated (range) | — | 10.0 (10-10) | — |
| Other | |||
| Procedures, n | 27 | 35 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 3 (11.1) | 13 (37.1) | 0 |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 1 (33.3) | 7 (53.8) | — |
| Median prophylactic doses per surgery (range) | 2.0 (1-2) | 1.0 (1-14) | — |
| Median prophylactic cumulative dose per surgery (IQR) | 78.2 μg/kg (62.4-154.8) | 42.6 IU/kg (33.3-138.2) | — |
| Procedures associated with treatment for postoperative bleeds, n (%) | 0 | 4 (11.4) | 0 |
| Median days postoperative bleeds were treated (range) | — | 1.0 (1-9) | — |
| . | PwHA with FVIII inhibitors (n = 64) rFVIIa . | PwHA without FVIII inhibitors (n = 51) . | |
|---|---|---|---|
| SHL FVIII . | EHL FVIII . | ||
| Dental | |||
| Procedures, n | 29 | 33 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 5 (17.2) | 14 (42.4) | 2 (6.1) |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 4 (80.0) | 11 (78.6) | 2 (100) |
| Median prophylactic doses per surgery (range) | 1.0 (1-3) | 1.0 (1-3) | 1.0 (1-1) |
| Median prophylactic cumulative dose per surgery (IQR) | 83.4 μg/kg (83.4-106.8) | 25.4 IU/kg (24.8-38.9) | 42.1 IU/kg (36.8-47.4) |
| Procedures associated with treatment for postoperative bleeds, n (%) | 6 (20.7) | 6 (18.2) | 2 (6.1) |
| Median days postoperative bleeds were treated (range) | 1.5 (1-4) | 2.0 (1-2) | 1.0 (1-1) |
| CVAD | |||
| Procedures, n | 35 | 1 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 9 (25.7) | 0 | 0 |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 6 (66.7) | — | — |
| Median prophylactic doses per surgery (range) | 1.0 (1-3) | — | — |
| Median prophylactic cumulative dose per surgery (IQR) | 119.0 μg/kg (86.5-168.7) | — | — |
| Procedures associated with treatment for postoperative bleeds, n (%) | 3 (8.6) | 0 (0.0) | 0 (0.0) |
| Median days postoperative bleeds were treated (range) | 1.0 (1-3) | — | — |
| Endoscopic | |||
| Procedures, n | 13 | 17 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 2 (15.4) | 10 (58.8) | 1 (5.9) |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 1 (50.0) | 6 (60.0) | 0 |
| Median prophylactic doses per surgery (range) | 1.5 (1-2) | 1.0 (1-12) | 3.0 |
| Median prophylactic cumulative dose per surgery (IQR) | 90.3 μg/kg (62.0-118.6) | 30.0 IU/kg (23.3-52.4) | 122.4 IU/kg |
| Procedures associated with treatment for postoperative bleeds, n (%) | 0 | 0 | 0 |
| Median days postoperative bleeds were treated (range) | — | — | — |
| Joint | |||
| Procedures, n | 3 | 22 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 2 (66.7) | 9 (40.9) | 2 (9.1) |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 1 (50.0) | 9 (100) | 2 (100) |
| Median prophylactic doses per surgery (range) | 1.5 (1-2) | 1.0 (1-1) | 1.0 (1-1) |
| Median prophylactic cumulative dose per surgery (IQR) | 156.2 μg/kg (85.1-227.3) | 21.2 IU/kg (21.1-29.8) | 21.3 IU/kg (20.3-22.4) |
| Procedures associated with treatment for postoperative bleeds, n (%) | 0 | 1 (4.5) | 0 (0.0) |
| Median days postoperative bleeds were treated (range) | — | 10.0 (10-10) | — |
| Other | |||
| Procedures, n | 27 | 35 | |
| Procedures managed with additional prophylactic factor concentrate, n (%) | 3 (11.1) | 13 (37.1) | 0 |
| Procedures associated with a single dose of factor concentrate, n (%)∗ | 1 (33.3) | 7 (53.8) | — |
| Median prophylactic doses per surgery (range) | 2.0 (1-2) | 1.0 (1-14) | — |
| Median prophylactic cumulative dose per surgery (IQR) | 78.2 μg/kg (62.4-154.8) | 42.6 IU/kg (33.3-138.2) | — |
| Procedures associated with treatment for postoperative bleeds, n (%) | 0 | 4 (11.4) | 0 |
| Median days postoperative bleeds were treated (range) | — | 1.0 (1-9) | — |
Percentages are of the number of procedures associated with additional prophylaxis.
There were 14 treated postoperative bleeds in participants undergoing minor dental surgeries (Figure 2). Treated postoperative bleeds occurred in 9 (22.5%) of 40 procedures managed without and 5 (22.7%) of 22 procedures managed with additional prophylactic factor concentrate. Details of the treatments can be found in Table 3.
An antifibrinolytic agent (tranexamic acid or aminocaproic acid) was used in 32 (51.6%) dental procedures in 26 participants (of whom 15 had FVIII inhibitors). Seventeen (27.4%) of the 62 minor dental surgeries were managed with tranexamic acid or aminocaproic acid as the only additional perioperative hemostatic therapy, with 10 (58.8%) of these procedures not being associated with a bleed.
Minor CVAD surgeries
Thirty-six minor CVAD procedures were included in the analysis, 29 central venous catheter removals and 7 catheter placements. Nine (25.0%) procedures were managed with and 27 (75.0%) without prophylactic factor concentrate (Figure 2). In the participants who received additional factor, all had FVIII inhibitors and were managed with rFVIIa, with a single dose administered in 6 (66.7%) of the 9 cases (Table 3).
There were 3 treated postoperative bleeds in participants undergoing minor CVAD procedures (Figure 2). These occurred in 1 (3.7%) of 27 procedures managed without additional prophylactic factor concentrate and 2 (22.2%) of 9 procedures managed with additional prophylactic factor concentrate. Two of the bleeds were treated for a single day, while the other was treated for 3 days.
An antifibrinolytic agent was used in 12 (33.3%) of the CVAD procedures. All 12 were managed with tranexamic acid or aminocaproic acid as the only additional perioperative hemostatic therapy, with 8 (66.7%) of these procedures not being associated with a bleed.
Minor endoscopic procedures
Thirty minor endoscopic procedures were included, the most common being upper gastrointestinal endoscopy (n = 7), esophagogastroduodenoscopy (n = 6), and colonoscopy (n = 4). Seventeen (56.7%) of the procedures were managed without additional prophylactic factor concentrate (Figure 2A); 4 of these were procedures that involved biopsy.
In the participants with FVIII inhibitors, 2 (15.4%) of the 13 procedures were managed with prophylactic rFVIIa, with a single dose administered in 1 (50.0%) case (Table 3). In the participants without FVIII inhibitors, 10 (58.8%) of the 17 procedures were managed with prophylactic SHL FVIII, with a single dose administered in 6 (60.0%) cases. One (5.9%) of the 17 procedures was managed with EHL FVIII, with 3 doses administered (Table 3). None of the 30 endoscopic procedures resulted in treated postoperative bleeds.
Minor joint procedures
Twenty-five minor joint procedures were performed, the most common being joint injection and synoviorthesis (n = 7 each) and joint aspiration (n = 6). Overall, 13 (52.0%) procedures were managed with and 12 (48.0%) without additional prophylactic factor concentrate (Figure 2).
In the participants with FVIII inhibitors, 2 (66.7%) of the 3 procedures were managed with prophylactic rFVIIa, with a single dose administered in 1 (50.0%) case (Table 3). In the participants without FVIII inhibitors, 9 (40.9%) and 2 (9.1%) of the procedures were managed with prophylactic SHL FVIII or EHL FVIII, respectively, with a single dose administered in all cases Table 3.
There were no treated postoperative bleeds associated with procedures managed with additional prophylaxis, and 1 (8.3%) associated with a procedure managed without additional prophylaxis (Table 3). The treated postoperative bleed was in an individual who underwent right ankle synoviorthesis. On postoperative day 5, the patient reported a bleed in the right ankle that was considered to be caused by the procedure; this was treated with SHL FVIII for 10 days.
Other minor procedures
Of the 62 other minor procedures, the most common were aspiration of right thigh seroma (n = 7), suture removal (n = 5), and biopsy or biopsy skin (n = 4). Overall, 17 (27.4%) of the 62 procedures were managed with and 45 (72.6%) without prophylactic factor concentrate (Figure 2).
In the participants with FVIII inhibitors, 3 (11.1%) of the 27 procedures were managed with prophylactic rFVIIa, with a single dose administered in 1 (33.3%) case (Table 3). In the participants without FVIII inhibitors, 13 (37.1%) of the 35 procedures were managed with prophylactic SHL FVIII, with a single dose administered in 7 (53.8%) cases.
There were 4 treated postoperative bleeds in participants who underwent other minor procedures, occurring in 2 (11.8%) of the 17 procedures managed with and 2 (4.4%) of the 45 procedures managed without additional prophylactic factor concentrate (Figure 2). All were treated with SHL FVIII (Table 3).
Major surgeries
The 18 major surgeries included 5 arthroplasties (3 hip, 1 ankle, and 1 knee) (Table 4), 4 synovectomies (Table 5), 2 muscle suture procedures, and 1 each of removal of orthopedic hardware, open reduction of fracture, appendicectomy, epidural injection, cholecystectomy, incisional hernia repair, and tonsillectomy (supplemental Table 1).
Details of the management of the five PwHA undergoing arthroplasty
| . | Knee arthroplasty (participant A) . | Hip arthroplasty24 (participant B) . | Ankle arthroplasty (participant C) . | Hip arthroplasty (participant D) . | Hip arthroplasty (participant E) . | |
|---|---|---|---|---|---|---|
| Type of prophylaxis | rFVIIa | rFVIIa | pdFVIII | SHL FVIII | EHL FVIII | rFVIIa |
| Cumulative dose | ||||||
| Preoperative | 357.7 μg/kg | 98.4 μg/kg | — | 65.9 IU/kg | 78.6 IU/kg | 89.7 μg/kg |
| Postprocedure | 7064.1 μg/kg | 3688.5 μg/kg | 751.4 IU/kg | 395.6 IU/kg | 864.3 IU/kg | 4756.4 μg/kg |
| Doses in the first 7 d postprocedure | 48 | 11 | 5 | 11 | 22 | 33 |
| Total post-op days on prophylaxis or treatment | 29 | 17 | 7 | 19 | 16 | 23 |
| Bleed because of surgery | No | Yes | No | No | No | |
| Additional medication | Antifibrinolytic | SHL FVIII; antifibrinolytic∗ | — | — | Antifibrinolytic | |
| AEs of special interest | No TE or TMA | No TE or TMA | No TE or TMA | No TE or TMA | No TE or TMA | |
| . | Knee arthroplasty (participant A) . | Hip arthroplasty24 (participant B) . | Ankle arthroplasty (participant C) . | Hip arthroplasty (participant D) . | Hip arthroplasty (participant E) . | |
|---|---|---|---|---|---|---|
| Type of prophylaxis | rFVIIa | rFVIIa | pdFVIII | SHL FVIII | EHL FVIII | rFVIIa |
| Cumulative dose | ||||||
| Preoperative | 357.7 μg/kg | 98.4 μg/kg | — | 65.9 IU/kg | 78.6 IU/kg | 89.7 μg/kg |
| Postprocedure | 7064.1 μg/kg | 3688.5 μg/kg | 751.4 IU/kg | 395.6 IU/kg | 864.3 IU/kg | 4756.4 μg/kg |
| Doses in the first 7 d postprocedure | 48 | 11 | 5 | 11 | 22 | 33 |
| Total post-op days on prophylaxis or treatment | 29 | 17 | 7 | 19 | 16 | 23 |
| Bleed because of surgery | No | Yes | No | No | No | |
| Additional medication | Antifibrinolytic | SHL FVIII; antifibrinolytic∗ | — | — | Antifibrinolytic | |
| AEs of special interest | No TE or TMA | No TE or TMA | No TE or TMA | No TE or TMA | No TE or TMA | |
AE, adverse event; pdFVIII, plasma-derived factor VIII; post-op, post-operative; TE, thromboembolic event; TMA, thrombotic microangiopathy.
Recorded as an untreated bleed; however, upon further investigation, this bleed was managed with factor concentrate.
Details of the management of the three PwHA undergoing synovectomy managed with additional prophylaxis∗
| . | Synovectomy . | Synovectomy . | Arthrofibrosis + chondroplasty + joint debridement + synovectomy . |
|---|---|---|---|
| Type of prophylaxis | SHL rFVIII | SHL rFVIII | rFVIIa |
| Cumulative dose | |||
| Preoperative | 55.0 IU/kg | 106.7 IU/kg | 170.2 μg/kg |
| Postprocedure | 192.6 IU/kg | — | 4087.8 μg/kg |
| Doses in the first 7 d postprocedure, n | 4 | — | 49 |
| Total post-op d on prophylaxis or treatment | 3 | — | 15 |
| Bleed because of surgery | No | No | Yes |
| Additional medication | — | — | — |
| AEs of special interest | No TE or TMA | No TE or TMA | No TE or TMA |
| . | Synovectomy . | Synovectomy . | Arthrofibrosis + chondroplasty + joint debridement + synovectomy . |
|---|---|---|---|
| Type of prophylaxis | SHL rFVIII | SHL rFVIII | rFVIIa |
| Cumulative dose | |||
| Preoperative | 55.0 IU/kg | 106.7 IU/kg | 170.2 μg/kg |
| Postprocedure | 192.6 IU/kg | — | 4087.8 μg/kg |
| Doses in the first 7 d postprocedure, n | 4 | — | 49 |
| Total post-op d on prophylaxis or treatment | 3 | — | 15 |
| Bleed because of surgery | No | No | Yes |
| Additional medication | — | — | — |
| AEs of special interest | No TE or TMA | No TE or TMA | No TE or TMA |
AE, adverse event; post-op, post-operative; TE, thromboembolic event; TMA, thrombotic microangiopathy.
There was 1 additional major surgical case of synovectomy, which was managed without prophylaxis.
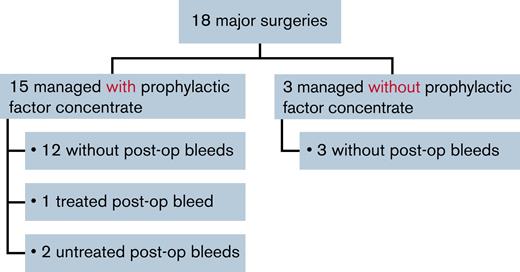
Most (15 of 18 [83.3%]) major surgeries were managed with prophylactic factor concentrate. Twelve (80.0%) of these were associated with no postoperative bleeds, 1 (6.7%) was associated with a treated postoperative bleed, and 2 (13.3%) were associated with untreated postoperative bleeds (Figure 3).
Major surgeries managed with or without prophylactic factor concentrate. post-op, post-operative.
Major surgeries managed with or without prophylactic factor concentrate. post-op, post-operative.
Three major surgeries (synovectomy, open reduction of fracture, and muscle suture) were managed without prophylactic factor concentrate; there were no postoperative bleeds following these 3 surgeries.
Arthroplasty
All 5 participants who underwent arthroplasty received pre- and postoperative factor concentrate prophylaxis. One of these individuals experienced a postoperative bleed. Details of the management of the 5 participants undergoing arthroplasty are included in Table 4.
One participant undergoing knee arthroplasty (participant A in Table 4) received 48 doses of rFVIIa prophylaxis in the first 7 days postprocedure. The individual received a further 31 doses over the following 22 days (a total of 79 doses), with no bleeds reported. The cumulative postprocedure dose was 7064 μg/kg.
One participant with FVIII inhibitors (2 BU/mL before surgery24) who had undergone a hip arthroplasty (participant B in Table 4) required postprocedure treatment with rFVIIa (11 doses of 82 μg/kg in the first 7 days after the procedure; 17 days of treatment in total; overall cumulative postprocedure dose, 3688.5 μg/kg). Following the development of a right thigh hematoma and a drop in hemoglobin concentrations despite red blood cell transfusions, the participant was treated with plasma-derived FVIII (overall cumulative postprocedure dose, 751.4 IU/kg; 7 days of treatment in total).24 Laboratory values for platelets, fibrinogen, and d-dimer around the time of surgery can be found in supplemental Table 2.
Synovectomy
One major synovectomy was managed without additional prophylaxis, with no postoperative bleed occurring. Details of the management of the 3 participants undergoing synovectomy who received additional prophylactic factor concentrate are shown in Table 5. Two of these procedures did not result in a postoperative bleed. One synovectomy was performed in conjunction with arthrofibrosis, chondroplasty, and joint debridement. This participant had a postoperative bleed and required 49 postprocedure doses of rFVIIa in the first 7 days following the procedure, with a cumulative postprocedure dose of 4087.8 μg/kg.
Comparison of surgeries in PwHA according to FVIII inhibitor status
The overall HAVEN 1-4 safety population included similar numbers of participants with and without FVIII inhibitors, at 208 and 191, respectively. There were 117 surgeries (107 minor and 10 major) in 69 PwHA with FVIII inhibitors and 116 surgeries (108 minor and 8 major) in 57 PwHA without FVIII inhibitors (supplemental Table 3). The proportion of minor procedures managed with additional prophylactic factor concentrate was lower for participants with FVIII inhibitors than those without (21.5% vs 47.2%) (supplemental Table 3).
The numbers of minor dental, endoscopic, and other procedures were similar in those with and without FVIII inhibitors. For CVAD procedures, the majority were in individuals with FVIII inhibitors (Table 6). Participants with FVIII inhibitors were overrepresented in the CVAD population because patients with CVADs are usually children, and the pediatric study (HAVEN 2) only enrolled PwHA with FVIII inhibitors. For joint procedures, the majority were in the group without FVIII inhibitors (Table 6), which contained participants from the HAVEN 3 and 4 studies, in which the median ages were higher than in the 2 studies of PwHA with FVIII inhibitors (Table 1). The number of treated bleeds was low in PwHA with and without FVIII inhibitors across all surgeries (major and minor) (Table 6).
Frequency, type, and outcomes of minor and major surgeries/procedures in PwHA with and without FVIII inhibitors
| . | Minor . | Major . | ||||
|---|---|---|---|---|---|---|
| Dental . | CVAD . | Endoscopic . | Joint . | Other . | ||
| Total surgeries/procedures, n | 62 | 36 | 30 | 25 | 62 | 18 |
| Surgeries in participants with FVIII inhibitors | 29 | 35 | 13 | 3 | 27 | 10 |
| Associated with a treated bleed, n (%) | 6 (20.7) | 3 (8.6) | 0 | 0 | 0 | 1 (10.0) |
| Surgeries in participants without FVIII inhibitors | 33 | 1 | 17 | 22 | 35 | 8 |
| Associated with a treated bleed, n (%) | 8 (24.2) | 0 | 0 | 1 (4.5) | 4 (11.4) | 0 |
| . | Minor . | Major . | ||||
|---|---|---|---|---|---|---|
| Dental . | CVAD . | Endoscopic . | Joint . | Other . | ||
| Total surgeries/procedures, n | 62 | 36 | 30 | 25 | 62 | 18 |
| Surgeries in participants with FVIII inhibitors | 29 | 35 | 13 | 3 | 27 | 10 |
| Associated with a treated bleed, n (%) | 6 (20.7) | 3 (8.6) | 0 | 0 | 0 | 1 (10.0) |
| Surgeries in participants without FVIII inhibitors | 33 | 1 | 17 | 22 | 35 | 8 |
| Associated with a treated bleed, n (%) | 8 (24.2) | 0 | 0 | 1 (4.5) | 4 (11.4) | 0 |
Other adverse events
No major or minor surgery in any participant resulted in death, thrombosis, thrombotic microangiopathy (TMA), or new FVIII inhibitor development.
Discussion
This is the largest summary to date describing the management of PwHA on emicizumab prophylaxis undergoing surgery. Data from a total of 233 surgeries performed during the HAVEN 1-4 clinical trials indicate that, for some individuals undergoing certain types of minor procedures, such as CVAD removal, sufficient periprocedural hemostatic control may be achieved with emicizumab prophylaxis alone. However, the decision to employ additional factor concentrate preemptively should be made on an individual case-by-case basis. All patients should have a predetermined plan for the management of surgical bleeding, should it occur.
A high proportion of the 215 minor procedures (65.6%) were performed without additional prophylactic factor concentrate, with only 14.2% of these resulting in a treated postoperative bleed. It should be noted that as no guidelines on the management of surgeries in PwHA receiving emicizumab were available, the decision as to whether additional prophylaxis was required and at what dose was made by the treating physician.
The numbers of doses of FVIII concentrate or BPA (rFVIIa) for treatment of postoperative bleeds following minor surgery were low, suggesting that bleeds were quickly and effectively controlled. One exception was a 30-year-old male who underwent minor joint surgery (synoviorthesis) and had a postoperative bleed that was treated with 10 days of SHL FVIII concentrate. Eighteen major surgeries were performed, mostly with additional prophylactic factor concentrate, resulting in 1 treated bleed. This occurred following a synovectomy performed in conjunction with arthrofibrosis, chondroplasty, and joint debridement; it was treated with 49 postprocedure doses of rFVIIa over 14 days.
Lower proportions of surgeries in participants with FVIII inhibitors were managed with additional prophylaxis compared with those without inhibitors, in particular for minor surgeries. This may reflect concerns about the predictability of hemostasis provided by BPAs, which is in agreement with the high proportion of untreated bleeds seen in the HAVEN 1 and 2 studies in PwHA with FVIII inhibitors compared with HAVEN 3, which was in PwHA without FVIII inhibitors.25 The number of treated bleeds was low in PwHA both with or without FVIII inhibitors across both the major and minor procedures, suggesting that emicizumab provides hemostatic benefits for patients with or without FVIII inhibitors.
No major or minor surgery in any participant resulted in death, thrombosis, TMA, or new FVIII inhibitor development. Data for participants who received additional FVIII or rFVIIa during the perioperative period did not indicate any safety issues in people receiving emicizumab prophylaxis.
Data on surgical experience in PwHA on emicizumab continue to grow, and the results concur with those presented here: minor and major surgeries can be performed safely in PwHA with or without FVIII inhibitors.24,26-35 To date, there has only been a single prospective clinical trial published,31 with evidence mainly coming from reviews of medical records and observational studies.
In a phase 4 multicenter study involving PwHA on emicizumab, 11 CVAD removals and 2 dental extractions were performed.31 One participant experienced excessive bleeding during and after CVAD removal surgery, while 3 participants had postoperative bleeding. Few participants received treatment with factor concentrate either during surgery (n = 3) or postoperatively (n = 3). In a prospective study monitoring PwHA receiving emicizumab prophylaxis, 31 procedures (29 minor and 2 major) were performed on 25 participants.32 Several minor procedures were performed without additional coagulation factor and without complications, while both major surgeries (hip replacement and explorative laparotomy) were managed with additional factor.
In a multicenter observational study, 30 surgical procedures (28 minor and 2 major) were performed in 29 participants with or without FVIII inhibitors who were on emicizumab.30 Port removal was the most common procedure (n = 21). Five individuals who underwent a port removal did not receive additional prophylactic factor concentrate preoperatively. Three of the 21 PwHA who underwent port removal had swelling and hematoma at the surgical site 1 to 2 days postoperatively and subsequently received 1 to 2 doses of factor concentrate as treatment. The 2 PwHA who underwent major surgery (intracranial ventricular shunt revision and posterior spinal fusion) received multiple doses of FVIII and did not experience bleeding complications.
Real-world data from 25 surgical procedures (20 minor and 5 major) in 22 PwHA with or without inhibitors receiving emicizumab prophylaxis have also been reported.29 Nine minor surgeries were planned with emicizumab alone, of which 4 required administration of additional hemostatic agents either during or after surgery. All major surgeries were performed with the administration of additional prophylactic factor concentrate, as planned. Overall, there were no major bleeding events and no thrombotic complications. Finally, in a review of medical records from PwHA treated with emicizumab prophylaxis at a center in the United States, 15 surgeries were undertaken in 10 PwHA (6 with FVIII inhibitors).36 The surgeries were well tolerated, with no bleeding in 11 (73.3%) procedures. Six participants were managed with additional preoperative prophylactic factor concentrate, with 4 experiencing postoperative bleeding.
It should be noted that in cases where FVIII is used in conjunction with emicizumab and coagulation monitoring is desired, chromogenic FVIII assays based on bovine proteins are considered most suitable to quantify exogenous FVIII activity. Emicizumab interferes with assays based on activated partial thromboplastin time, yielding misleading results.37,38
A limitation of this analysis is that the HAVEN 1-4 studies were not designed to analyze the management and outcomes of surgery in PwHA taking emicizumab prophylaxis, and, notably, none of the HAVEN 1-4 studies included a surgical endpoint. One consequence of this was that perioperative use of antifibrinolytics was not documented consistently and sufficiently, preventing an analysis of how this potentially affected the occurrence and outcome of bleeding. A further limitation is that conclusions on the management of some specific types of surgery are limited by the small numbers included in this analysis. Individuals with planned major surgical interventions were excluded from the HAVEN studies, reducing the amount of data collected on such procedures. The stringent inclusion and exclusion criteria of the HAVEN studies should also be considered when extrapolating the findings to real-world clinical practice.
Conclusions
The data presented here demonstrate that major and minor surgeries were performed safely in PwHA who were taking emicizumab prophylaxis as part of the HAVEN 1-4 clinical trials, regardless of FVIII inhibitor status. Taking into consideration the recommendations not to administer high doses of aPCC (>100 U/kg per day) for >24 hours in conjunction with emicizumab, procedures that required additional factor concentrate were well managed with FVIII and rFVIIa. However, further research is needed to grow the evidence base, which could eventually contribute to the establishment of surgical guidelines for this complex population.
Acknowledgments
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Alex Coulthard, and Katie Smith of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd.
The HAVEN studies were funded by F. Hoffmann-La Roche Ltd. and Chugai Pharmaceutical Co., Ltd.
Authorship
Contribution: T.C., S.C., R.H.K., M.L., and I.P.-P. contributed to the study design; R.K.-J., F.P., J.O., T.C., S.E.C., T.L., C.L.K., S.W.P., C.D., N.S.B., M.N., I.P.-P., G.Y., and V.J.-Y. contributed to study conduct, recruitment and follow-up of patients, or data collection; F.P., T.C., S.C., M.Y.D., S.E.C., C.L.K., S.W.P., R.H.K., B.T., C.D., N.S.B., M.N., M.L., I.P.-P., G.Y., and V.J.-Y. contributed to data analysis and interpretation; and all authors revised the manuscript critically and provided final approval of the version to be published; and all authors agree to be accountable for all aspects of the work.
Conflict-of-interest disclosure: R.K.-J. has received honoraria and consultancy fees from Genentech, Inc./F. Hoffmann-La Roche Ltd., CSL Behring, BioMarin, and CRISPR; has been a member of the speaker’s bureau for Genentech, Inc./F. Hoffmann-La Roche Ltd., and Sanofi; and has received research funding from Genentech, Inc. F.P. has received speaker fees for participating in educational symposia and advisory boards for F. Hoffmann-La Roche Ltd., Sanofi, SOBI, and Takeda. J.O. has received personal/consultancy fees for travel support, advisory boards, and symposia from Bayer, Biogen Idec, BioMarin, Biotest, Chugai Pharmaceutical Co., Ltd., CSL Behring, Freeline, Grifols, Novo Nordisk, Octapharma, Pfizer, F. Hoffmann-La Roche Ltd., Sanofi, Spark Therapeutics, SOBI, and Shire/Takeda; and grants from Bayer, Biotest, CSL Behring, Octapharma, and Pfizer. T.C. is an employee of Spark Therapeutics and a former employee of Genentech, Inc. S.C. is an employee and holds stocks in F. Hoffmann-La Roche Ltd. M.Y.D. is an employee of Genentech, Inc. and holds stocks in F. Hoffmann-La Roche Ltd. S.E.C. has received consultancy fees for Bayer, BioMarin, CSL Behring, HEMA Biologics, Pfizer, and Sanofi; research funding from F. Hoffmann-La Roche Ltd./Genentech, Inc.; and is a member on another entity’s Board of Directors or its advisory committees for Hemophilia Alliance and American Thrombosis and Hemostasis Network. T.L. has served as a consultant for CSL Behring, F. Hoffmann-La Roche Ltd., and SOBI. C.L.K. has received honoraria for participation in advisory boards for Sanofi US, Takeda, and Spark Therapeutics. S.W.P. has served as a consultant to Apcintex, ASC Therapeutics, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, GenVentiv, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, F. Hoffmann-La Roche Ltd./Genentech, Inc., Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, and UniQure. R.H.K. and B.T. are employees and hold stock in Genentech, Inc. C.D., N.S.B., and M.N. are employees of F. Hoffmann-La Roche Ltd. M.L. is an employee and holds stocks in F. Hoffmann-La Roche Ltd. I.P.-P. is a former employee of Genentech, Inc. G.Y. reports research funding from Genentech, Inc., Grifols, and Takeda; paid testimony from CSL Behring and Genentech, Inc.; consultancy fees from Apcintex, Bayer, BioMarin, Genentech, Inc./F. Hoffmann-La Roche, Novo Nordisk, Pfizer, Sanofi/Genzyme, Spark Therapeutics, and Takeda; and honoraria from Genentech, Inc., Sanofi, and Spark Therapeutics. V.J.-Y. has received reimbursement for attending symposia/congresses, honoraria for speaking, consulting and/or research funding from Takeda, Bayer, CSL Behring, Grifols, Novo Nordisk, SOBI, F. Hoffmann-La Roche Ltd., Octapharma, BioMarin, Sanofi, and Pfizer.
The current affiliation for T.C. is Spark Therapeutics, Inc., Philadelphia, PA.
The current affiliation for I.P.-P. is Graphite Bio, Inc., South San Francisco, CA.
Correspondence: Rebecca Kruse-Jarres, Washington Center for Bleeding Disorders, 701 Pike Street, Suite 1900, Seattle, WA 98101; e-mail: rkj@WACBD.org.
References
Author notes
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available from https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, seehttps://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
The full-text version of this article contains a data supplement.