Key Points
ADAP-, ARP2/3-, WASp-, and PFN1-deficient MKs show reduced adhesion to collagen.
β1-integrin activation and F-actin organization is impaired in ADAP-, PFN1-, and ARPC2-deficient MKs.
Abstract
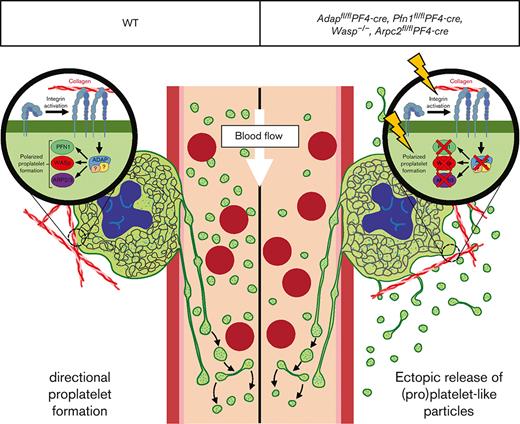
Mature bone marrow (BM) megakaryocytes (MKs) produce platelets by extending proplatelets into sinusoidal blood vessels. Defects in this process can lead to thrombocytopenia and increased risk of bleeding. Mice lacking the actin-regulatory proteins Profilin 1 (PFN1), Wiskott–Aldrich Syndrome protein (WASp), Actin Related Protein 2/3 complex (Arp2/3), or adhesion and degranulation-promoting adapter protein (ADAP) display thrombocytopenia and ectopic release of (pro)platelet-like particles into the BM compartment, pointing to an important axis of actin-mediated directional proplatelet formation. The mechanism underlying ectopic release in these mice is still not completely understood. However, we hypothesized that similar functional defects account for this observation. We analyzed WASp-, ADAP-, PFN1-, and ARPC2-knockout mice to determine the role of actin reorganization and integrin activation in directional proplatelet formation. ADAP-, ARPC2-, and PFN1-deficient MKs displayed reduced adhesion to collagen, defective F-actin organization, and diminished β1-integrin activation. WASp-deficient MKs showed the strongest reduction in the adhesion assay of collagen and altered F-actin organization with reduced podosome formation. Our results indicate that ADAP, PFN1, WASp, and ARP2/3 are part of the same pathway that regulates polarization processes in MKs and directional proplatelet formation into BM sinusoids.
Introduction
Megakaryocytes (MKs) are the largest cells in the bone marrow (BM) and are responsible for constant platelet production into the blood stream by a process known as proplatelet formation.1 Inherited thrombocytopenias are a heterogeneous group of disorders characterized by a sustained reduction in platelet count and are associated with increased bleeding risk.2 Underlying mutations responsible for thrombocytopenia are found in genes encoding proteins important for MK differentiation, maturation, proplatelet formation, platelet release, or platelet clearance.3,4 Cytoskeletal (-regulatory) proteins are particularly important for the terminal stages of platelet biogenesis.5 Microthrombocytopenias (smaller and fewer platelets in the circulation) in humans are rare and predominantly associated with mutations in genes encoding for direct or indirect interaction partners of the F-actin cytoskeleton (WAS,6ARPC1B7, ADAP8,9). In WASp-deficient as well as MK-/platelet-specific ARPC2, ADAP, and PFN1 deficient mice it was shown that the mutant MKs release (pro)platelet-like particles ((pro)PLPs) ectopically into the BM compartment, which contributes to the low platelet count in the circulation.10-13 Little is known about the regulatory processes in MKs that accomplish directional proplatelet formation into the BM sinusoids. We recently showed that impaired F-actin organization and β1-integrin activation in ADAP-deficient MKs contribute to ectopic release of (pro)PLPs and suggested that proper adhesion of MKs to extracellular matrices (especially collagen I) is important for directional proplatelet formation.10 In this comparative study, we demonstrated that the ectopic release of (pro)PLPs in ADAP-, PFN1-, WASp-, and ARPC2-deficient mice is comparable. In all tested mutant mice, we found an increased number of (pro)PLPs in the BM, a profound and selective adhesion defect of MKs on Horm collagen, and altered F-actin organization. Furthermore, β1-integrin activation was defective in ADAP-, PFN1-, and ARP2/3-deficient MKs. We speculate that ADAP, PFN1, WASp, and ARP2/3 are part of the same pathway that regulates polarization processes in MKs and directional proplatelet formation into the BM sinusoids, as described for the formation of the immunological synapse in T-cells.14
Methods
Mice
Conditional Adapfl/fl,10Pfn1fl/fl13 or Arpc2fl/fl12 mice were intercrossed with mice carrying the Cre-recombinase under the platelet factor 4 promoter.15 ADAP- and WASp-deficient were described previously.16,17 Animal studies were approved by the district government of Lower Franconia (Bezirksregierung Unterfranken).
Data analysis
Results are mean ± standard deviation. Differences between control and knockout samples were statistically analyzed using the Mann-Whitney U test or 2-way analysis of variance test. P-values < .05 were considered as statistically significant: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Results with a P-value > .05 were considered as not significant. Adjustments of brightness/contrast of images (cryosections) as well as analysis of mean fluorescence intensities and number of podosomes-like structures/area of spread MKs of unmodified images were performed using ImageJ. The adjustments were applied linearly to the entire image. Flow cytometry data were analyzed using FlowJo software.
MK and (pro)PLPs analysis using flow cytometry, MK cultivation, MK adhesion assay, determination of α2 expression in MKs, and immunofluorescence are described in detail in the supplemental Methods.
Results and discussion
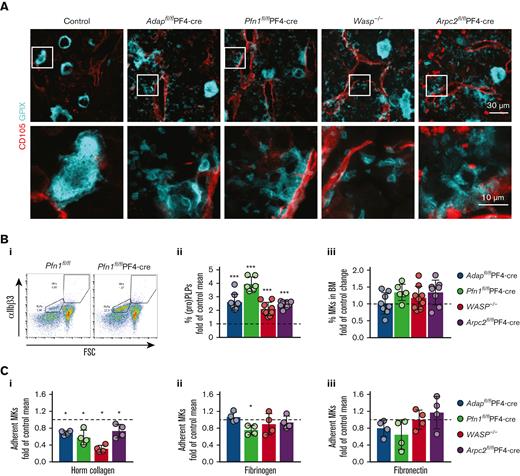
To investigate the extent and pattern of the ectopic release of (pro)PLPs in the BM of ADAP-, PFN1-, WASP-, and ARP2/3-deficient mice, we compared intact BM by cryosectioning and subsequent staining of MKs/(pro)platelets and sinusoids. Intact mature MKs, proplatelets, and PLPs were found in the BM of all the mutant mouse lines (Figure 1A). The appearance of ectopically released (pro)PLPs in the vessels and BM cavity was comparable based on BM cryosections. In addition, the abundance of (pro)PLPs in the BM of all knockout mice was significantly higher than that in the control BM (Figure 1Bi-ii, supplemental Figure 1Ai-v), whereas the MK number was not altered (Figure 1Biii, supplemental Figure 1Bi-v). These data suggest that common mechanisms may be responsible for ectopic release in those knockout mice.
Reduced adhesionof BM-derived ADAP-, PFN1-, WASp-, and ARP2/3-deficient MKs on ECM proteins. (A) Cryosections of BM from control (Adapfl/fl), ADAP-, PFN1-, WASp-, and ARP2/3-deficient mice stained for GPIX (cyan) to visualize MKs, and (pro)PLPs and CD105 (red) to identify sinusoids. The images from the bottom panel are magnified from the regions indicated in the upper panel. Scale bars represent 30 μm (upper panel) and 10 μm (lower panel). (Bi) Gating strategy for the detection of (pro)PLPs and MKs in BM samples based on the size and expression of αIIbβ3. Shown is the dot plot of a Pfn1fl/fl-PF4-cre BM sample and the corresponding control (Pfn1fl/fl). (Bii) Quantification of (pro)PLPs in the BM as percentage of the control mean. The percentage of (pro)PLPs in the BM was calculated and normalized to the mean of their respective control MKs. The controls are indicated by dashed lines. (Biii) Quantification of MKs in the BM as percentage of the control mean. The percentage of MKs in the BM was calculated and normalized against the mean of the respective control MKs. The controls are indicated by dashed line. Each data point represents a single mouse. N = 3-9. (C) Normalized adhesion assay of in vitro cultured BM-derived MKs from the respective controls, ADAP-, PFN1-, WASp-, and ARP2/3-deficient mice on Horm collagen (Ci), fibrinogen (Cii), and fibronectin (Ciii). The percentage of adherent mutant MKs after 3.5 hours incubation was calculated and normalized against the mean of their respective control MKs. Controls are indicated with a dashed line. Each dot represents a technical replicate. The experiment was performed at least twice.
Reduced adhesionof BM-derived ADAP-, PFN1-, WASp-, and ARP2/3-deficient MKs on ECM proteins. (A) Cryosections of BM from control (Adapfl/fl), ADAP-, PFN1-, WASp-, and ARP2/3-deficient mice stained for GPIX (cyan) to visualize MKs, and (pro)PLPs and CD105 (red) to identify sinusoids. The images from the bottom panel are magnified from the regions indicated in the upper panel. Scale bars represent 30 μm (upper panel) and 10 μm (lower panel). (Bi) Gating strategy for the detection of (pro)PLPs and MKs in BM samples based on the size and expression of αIIbβ3. Shown is the dot plot of a Pfn1fl/fl-PF4-cre BM sample and the corresponding control (Pfn1fl/fl). (Bii) Quantification of (pro)PLPs in the BM as percentage of the control mean. The percentage of (pro)PLPs in the BM was calculated and normalized to the mean of their respective control MKs. The controls are indicated by dashed lines. (Biii) Quantification of MKs in the BM as percentage of the control mean. The percentage of MKs in the BM was calculated and normalized against the mean of the respective control MKs. The controls are indicated by dashed line. Each data point represents a single mouse. N = 3-9. (C) Normalized adhesion assay of in vitro cultured BM-derived MKs from the respective controls, ADAP-, PFN1-, WASp-, and ARP2/3-deficient mice on Horm collagen (Ci), fibrinogen (Cii), and fibronectin (Ciii). The percentage of adherent mutant MKs after 3.5 hours incubation was calculated and normalized against the mean of their respective control MKs. Controls are indicated with a dashed line. Each dot represents a technical replicate. The experiment was performed at least twice.
The capacity to form proplatelets is inhibited by collagen I18,19 and it was suggested that the spatial expression pattern of collagen types I and IV in the BM cavity and at sinusoids is a mechanism for how proplatelet formation is orchestrated across the endothelial barrier.20 We found an activation defect for β1-integrin in ADAP-deficient MKs on Horm collagen and speculated that it contributes to the observed phenotype of ectopically released (pro)PLPs in vivo.10 To test whether PFN1-, WASp-, and ARP2/3-deficient MKs also display an integrin defect on Horm collagen we seeded cultured control and mutant MKs on different extracellular matrix (ECM) proteins and calculated the relative number of MKs, which were able to establish a firm adhesion to the ECM. For all tested knockout MKs, we found a strong adhesion defect on Horm collagen compared with their respective control cells. However, adhesion to fibronectin was unaltered (Figure 1Ci,iii, supplemental Figure 2A-D). Interestingly, only PFN1-deficient MKs displayed an adhesion defect on fibrinogen (Figure 1Cii, supplemental Figure 2B).
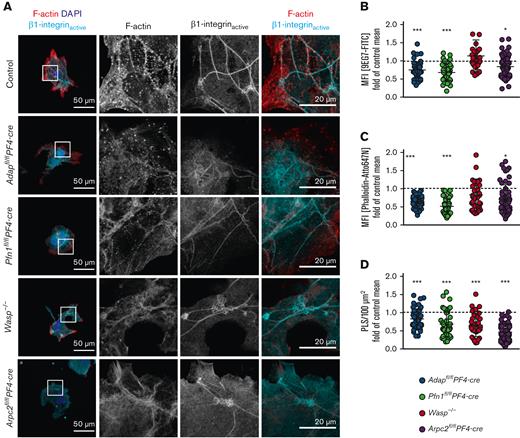
Because we observed impaired adhesion of mutant MKs from the 4 mouse lines on Horm collagen, we were interested in investigating this defect in more detail. To analyze integrin activation in MKs, we stained active β1-integrin and F-actin in MKs spread on Horm collagen (Figure 2A). Measurements of the mean fluorescence intensity of the fluorophore-labeled antibody 9EG7-FITC (recognizing active β1-integrin) revealed a significantly reduced signal in ADAP-, PFN1-, and ARP2/3-deficient MKs, whereas WASp-deficient MKs displayed normal β1-integrin activation compared with the control (Figure 2B, supplemental Figure 3Ai-iv). This was surprising, because WASp-deficient MKs showed the strongest adhesion defects on Horm collagen. To exclude the reduced expression of α2β1 integrin in cultured MKs, we analyzed the cells via flow cytometry and detected overall comparable expression profiles (supplemental Figure 4A-D, expression of α2 integrin). In addition, the expression of α2 and/or β1 integrin is not or only mildly affected in ADAP-, PFN1-, and ARP2/3–deficient platelets.10,12,13 A limitation of in vitro MK culture experiments is that although enriched for mature MKs, the MK culture is not synchronized. Therefore, we speculate that even though the MKs are mature, only a fraction of WASp-deficient MKs can activate the β1-integrin and adhere and spread on Horm collagen. Similar results were obtained when we analyzed the F-actin content in the lowest optical plane (Figure 2C, supplemental Figure 3Bi-iv). Podosomes are actin-based structures involved in cell adhesion, migration, and ECM degradation. It has been proposed that podosome formation is important for transendothelial proplatelet formation and the delivery of platelets into the bloodstream.21 Recently, a study showed that MKs use podosome-like structures (PLS) to collectively penetrate the endothelium of BM sinusoids in vivo.22 In support of this, cultured WASp-, PFN1-, ARP2/3-, and ADAP-deficient MKs displayed a strong defect in the formation of F-actin structures on Horm collagen reminiscent of podosomes (further referred to as PLS; Figure 2A,D and supplemental Figure 3Ci-iv and Spindler et al,10 Sabri et al,11 Paul et al,12 and Bender et al13). These data suggest a possible link between impaired PLS formation in vitro and platelet production defects, characterized by the ectopic release of (pro)PLPs into the BM compartment in vivo.
Cultured spread MKs from mutant mice exhibit reduced PLS formation, F-actin content, and β1-integrin activation. (A) Representative confocal images of cultured BM-derived MKs from control (Adapfl/fl), ADAP-, PFN1-, WASp-, and ARP2/3-deficient mice spread on Horm collagen and stained for F-actin (red), the active form of β1-integrin (cyan), and the nucleus (blue). Scale bars represent 50 μm (left) and 20 μm (right). (B) Mean fluorescence intensity of active β1-integrin, (C) F-actin, and (D) density of PLS in the lowest optical section of spread MKs on Horm collagen, normalized to their respective control cells. Controls are indicated by a dashed line (B-D). (B-D) 35 to 59 MKs per genotype were analyzed. The data points represent individual MKs.
Cultured spread MKs from mutant mice exhibit reduced PLS formation, F-actin content, and β1-integrin activation. (A) Representative confocal images of cultured BM-derived MKs from control (Adapfl/fl), ADAP-, PFN1-, WASp-, and ARP2/3-deficient mice spread on Horm collagen and stained for F-actin (red), the active form of β1-integrin (cyan), and the nucleus (blue). Scale bars represent 50 μm (left) and 20 μm (right). (B) Mean fluorescence intensity of active β1-integrin, (C) F-actin, and (D) density of PLS in the lowest optical section of spread MKs on Horm collagen, normalized to their respective control cells. Controls are indicated by a dashed line (B-D). (B-D) 35 to 59 MKs per genotype were analyzed. The data points represent individual MKs.
Platelet formation by MKs requires dynamic changes in the actin cytoskeleton. We and others10-13 observed the ectopic release of (pro)PLPs in mice lacking 1 of the 4 actin-regulatory proteins (ADAP, PFN1, WASp, and ARP2/3). Furthermore, we found defective PLS formation on collagen in MKs from all 4 mutant mouse lines. Our data indicate that similar mechanisms account for ectopic (pro)PLPs release in these 4 knockout mouse models. One explanation for the underlying mechanisms of ectopic (pro)PLPs release might be that podosome-controlled proplatelet extension into the sinusoid and, at the same time, the prevention of proplatelet formation in other areas of the cell are impaired. Another possible explanation could be deduced from the data obtained from the T cells. Interestingly, in T-cells, ADAP is phosphorylated upon T-cell receptor stimulation and is present in a complex with SLP-76, NCK, and VAV1 to regulate the F-actin cytoskeleton via PFN1, WASp, and ARP2/3. These events lead to the formation of a polarized structure called an immunological synapse, which serves as a platform for the exchange of information between antigen-presenting cells and T-cells.14,23 We speculate that similar transient protein complexes act in concert in MKs, linking signaling to the rearrangement of the actin cytoskeleton to facilitate transendothelial proplatelet formation. However, considering the small number of MKs in the BM and the formation of proplatelets, the identification of such transient protein complexes is challenging and requires further investigation.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) project number 452622720 to M.B, by the National Institutes of Health grant number 35HL144976 to W.B. and the Canadian Institutes of Health Research Foundation awarded to K.A.S. The authors are grateful to Stefanie Kliche for critically reading the manuscript and for helpful discussion.
Authorship
Contribution: M.S. performed experiments, analyzed data, and wrote the manuscript; W.B., T.E.B.S., J.Z., K.A.S., L.N., and A.R. provided mouse lines, discussed the results, and provided scientific input throughout the study; M.B. planned and supervised the research and wrote the manuscript; all the authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus Bender, Institute of Experimental Biomedicine, Chair I, University Hospital Würzburg; Josef-Schneider-Str. 2, D15, 97080 Würzburg, Germany; e-mail: Bender_M1@ukw.de.
References
Author notes
Data are available on request from the corresponding author, Markus Bender (Bender_M1@ukw.de).
The full-text version of this article contains a data supplement.