TO THE EDITOR:
Systemic mastocytosis (SM) is a clonal disorder of proliferation and/or accumulation of neoplastic mast cells (MCs) in various organs. SM is divided into 5 subgroups: indolent SM (ISM), smoldering SM (SSM), aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and MC leukemia (MCL)1; the latter 3 subtypes are subgrouped as advanced SM (advSM) because of shortened survival.2 The signs and symptoms of SM result from the release of multiple MC mediators (mostly in ISM) or direct tissue injury (mostly in advSM).3-5 Bone marrow (BM) is the most common organ involved in SM (90%), followed by the gastrointestinal tract (GIT) (20%-80%).4,6,7 However, this can be biased since these organs are the most commonly biopsied. Establishing an organ injury advances patient clinical category from ISM/SSM to advSM.
Liver involvement in SM is likely underestimated. Currently, the elevation of liver function tests (LFTs) and hypoalbuminemia are considered evidence of liver injury by SM.2,8 Imaging of the abdomen reveals information about the size of the liver, spleen, and signs of portal hypertension. However, LFTs and images do not provide direct evidence of MC presence in the liver, and/or MC-induced liver damage, or presence of associated liver fibrosis. As expected, only a few patients with SM undergo a liver biopsy, which hinders our understanding of the accurate incidence and histopathology of liver involvement in SM. Magnetic resonance elastography (MRE), a noninvasive method of measuring liver stiffness, is accepted as the current most accurate method to detect and stage liver fibrosis in various liver diseases.9,10 However, there is no available study demonstrating the assessment of liver stiffness with MRE in patients with SM. MRE findings, confirmed by liver biopsies, in our patients with SM were extremely helpful in identifying and monitoring liver involvement during therapy.
Patients with advSM treated with avapritinib at Rush University were included in this Institutional Research Board-approved study.
A 63-year-old female with a history of urticaria pigmentosa (UP) diagnosed in 2012 presented 6 years later with diarrhea, elevated tryptase concentration of 400 μg/L, and bicytopenia (platelets of 125 × 109/L and white blood cells of 1.25 × 109/L). LFTs were within normal limits (total bilirubin of 1.2 mg/dL, aspartate aminotransferase [AST] of 15 U/L, and alanine aminotransferase [ALT] of 12 U/L) (supplemental Table 1; supplemental Figure 1 in the data supplement). A BM biopsy showed diffuse infiltrates of atypical KIT D816V mutated MCs (60%). The patient was diagnosed with ASM; there was no AHN. Interferon-α was initially effective but discontinued because of thrombocytopenia. Her symptoms (eg, abdominal cramping with diarrhea, severe flushing, night sweats, and weight loss) flared. An endoscopic evaluation with biopsies of upper and lower GIT showed the involvement of SM. Midostaurin effectively improved symptoms but was not tolerated.
The patient was enrolled in the PATHFINDER trial (NCT04241796), an open-label, phase 2 study for the treatment of ASM. Physical exam revealed hepatosplenomegaly (HSM) and telangiectasia macularis eruptive perstans while tryptase was highly elevated (691 μg/L) and LFTs were normal (total bilirubin, 1.4 mg/dL; alkaline phosphatase [ALP], 124 U/L; AST, 16 U/L; and ALT, 16 U/L). Her BM biopsy revealed spindle-shaped MCs (60%). MRE of the liver showed a mean liver stiffness of 5.25 kilopascals (kPa), corresponding to stage 4 fibrosis (Figure 1; supplemental Figure 1). The liver biopsy showed approximately 5% of the liver parenchyma was infiltrated by MC and stage 3 bridging fibrosis (Figure 2). The patient had significant improvement in her signs and symptoms of SM in 2 months. Over time, serum tryptase, ALP, and BM MCs continued to improve (supplemental Table 1). MRE of the liver showed improved stiffness at 3.6 kPa (corresponding to stage 1-2 fibrosis) (Figure 1). At 15 months, a repeat liver biopsy revealed less MCs (1%) and decreased fibrosis (Figure 2).
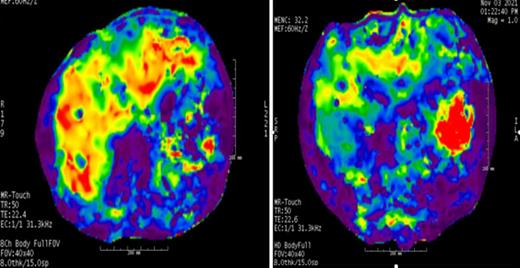
Magnetic resonance (MR) color elastogram with a 0 to 8 kPa scale in case 1 demonstrates increased liver stiffness with red and orange regions that show increased stiffness values instead of blue and purple regions with lower stiffness values. In the quantitative analyses, mean liver stiffness values were observed as 5.25 kPa before treatment (A) and 3.6 kPa after treatment (B). Of note, red and orange regions are decreased in the follow-up MR elastogram. Interpretation of MR elastography results: 2.5 kPa = normal; 2.5 to 3.0 kPa = normal or inflammation; 3.0 to 3.5 kPa = stage 1 to 2 fibrosis; 3.5 to 4 kPa = stage 2 to 3 fibrosis; 4 to 5 kPa = stage 3 to 4 fibrosis; and >5 kPa = stage 4 fibrosis.
Magnetic resonance (MR) color elastogram with a 0 to 8 kPa scale in case 1 demonstrates increased liver stiffness with red and orange regions that show increased stiffness values instead of blue and purple regions with lower stiffness values. In the quantitative analyses, mean liver stiffness values were observed as 5.25 kPa before treatment (A) and 3.6 kPa after treatment (B). Of note, red and orange regions are decreased in the follow-up MR elastogram. Interpretation of MR elastography results: 2.5 kPa = normal; 2.5 to 3.0 kPa = normal or inflammation; 3.0 to 3.5 kPa = stage 1 to 2 fibrosis; 3.5 to 4 kPa = stage 2 to 3 fibrosis; 4 to 5 kPa = stage 3 to 4 fibrosis; and >5 kPa = stage 4 fibrosis.
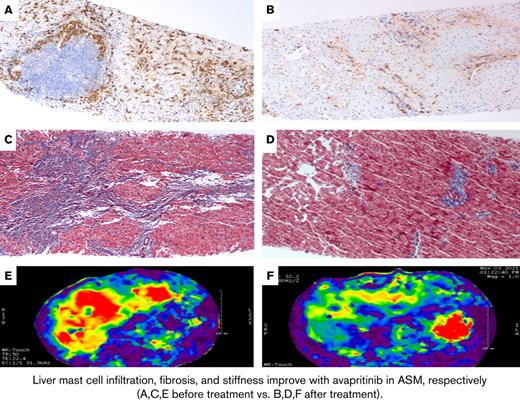
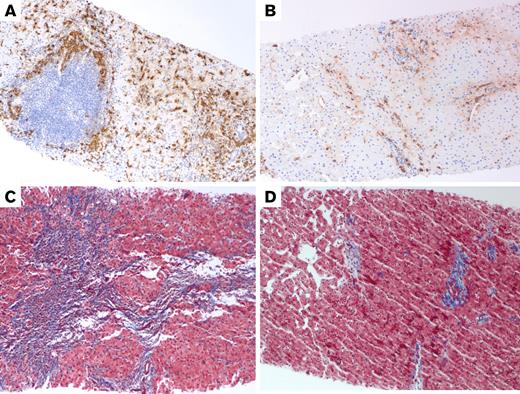
Tryptase and trichrome stains of the liver in Case 1. (A) Pretreatment liver biopsy (100×) shows clustered MCs around a lymphoid nodule in the portal tract and increased spindled MCs in the lobule on tryptase immunostain. MCs constituted approximately 5% of the tissue. (B) Posttreatment liver biopsy (100×) shows much fewer MCs on tryptase immunostain (approximately 1% of the tissue). (C) Trichrome stain of the liver (100×) before treatment shows bridging fibrosis (with collagen staining blue) and early nodularity. (D) After treatment, trichrome stain of the liver (100×) shows improvement in fibrosis, now limited mainly to the portal tracts.
Tryptase and trichrome stains of the liver in Case 1. (A) Pretreatment liver biopsy (100×) shows clustered MCs around a lymphoid nodule in the portal tract and increased spindled MCs in the lobule on tryptase immunostain. MCs constituted approximately 5% of the tissue. (B) Posttreatment liver biopsy (100×) shows much fewer MCs on tryptase immunostain (approximately 1% of the tissue). (C) Trichrome stain of the liver (100×) before treatment shows bridging fibrosis (with collagen staining blue) and early nodularity. (D) After treatment, trichrome stain of the liver (100×) shows improvement in fibrosis, now limited mainly to the portal tracts.
A 39-year-old male presented with worsening abdominal pain, diarrhea, thrombocytopenia (137 × 109/L), and weight loss of 30 kg in 1 year. He had UP and HSM, normal LFTs, and elevated serum tryptase (634 μg/L) (supplemental Table 1). An abdominal ultrasound confirmed HSM, with the liver measuring 22 cm in length and normal in echogenicity. No focal liver lesions were noted. The spleen was enlarged, measuring 25 × 23 × 9.6 cm (supplemental Table 1). Computed tomography of the chest, abdomen, and pelvis showed lymphadenopathy and diffusely sclerotic appearance of the bones and HSM. A BM biopsy showed increased MCs (50%) (supplemental Table 1), and KIT D816V mutation was positive. The patient was enrolled in the PATHFINDER trial for the treatment of ASM (there was no evidence of AHN). LFTs were within normal range (total bilirubin, 0.4 mg/dL; ALP, 106 U/L; AST, 11 U/L; and ALT, 9 U/L) (supplemental Table 1). MRI of the abdomen demonstrated HSM (the liver measured 28.2 cm and demonstrated normal surface contour, and the spleen measured 28.9 cm). MRE revealed the mean liver stiffness was 5.8 kPa, which is consistent with stage 4 hepatic fibrosis. The patient’s symptoms significantly improved within 2 months; over time, MC burden decreased significantly (supplemental Table 1; supplemental Figure 1). Six months later, HSM and liver stiffness improved (mean, 3.35 kPa). Fifteen months later, MRE showed mean liver stiffness continued to improve (3.1 KPa) (Figure 2; supplemental Figure 1).
Neither of the patients had a history or clinical or laboratory evidence of autoimmune, viral, drug-induced hepatitis, or impaired circulatory disorders (eg, Budd-Chiari syndrome) or hepatosteatosis by available images, serological tests, and histopathology. Neither patient received steroids during avapritinib treatment.
In these 2 patients with SM, routine imaging and LFTs failed to show the accurate depth of liver disease in SM. MRE findings indicated significant liver stiffness in both patients, and fibrosis was confirmed in a patient with liver biopsies. Similarly, a few patients with SM with portal hypertension were reported to have normal LFTs.11,12 Given that there seems to be a correlation between MC infiltration and fibrosis11 in SM and that normal MCs are involved in organ and tissue fibrosis (eg, lung, kidneys, adipose tissue, and myocardium) by promoting fibroblast growth in animal and human studies,13,14 liver fibrosis in SM might be expected. However, data are limited in liver involvement of SM because MRE, fibroscan, and liver biopsy are rarely used.15,16 In the present case report, MRE was extremely useful to demonstrate increased liver stiffness in accordance with liver fibrosis and MC infiltration and proven with liver biopsy. It is known that liver stiffness depends on different factors that affect tissue composition, such as inflammation, biliary obstruction, cholestasis, passive congestion, and increased portal venous pressure, as well as fibrosis in liver diseases.17
Diagnosing liver involvement of SM is extremely important for the appropriate treatment of patients, especially with new, effective drugs. Major improvements in SM have been achieved in the last 2 decades with the discovery of effective KIT inhibitors.2,18-20 Midostaurin and avapritinib, KIT inhibitors, have been approved by the US Food and Drug Administration for the treatment of advSM.21-23 These 2 patients had good clinical and laboratory responses to avapritinib therapy. Response in HSM was reported in both midostaurin and avapritinib21,23; however, no data has been available regarding liver stiffness increased by MC infiltration and/or associated fibrosis. Our report shows that liver stiffness and fibrosis is likely much more common than reported and perhaps a contributing factor to liver-related complications seen after allogeneic stem cell transplantation,24 a potentially curative therapy.25 More importantly, for the first time, we showed that liver fibrosis may regress with avapritinib treatment. Clearly, this is a report of 2 cases and therefore needs to be confirmed by larger studies. As of now, the exact spectrum of clinical implications of these findings is unknown: for example, how they affect metabolisms and serum concentrations of medications (eg, especially KIT inhibitors), how they vary in different SM clinical categories (ISM vs SSM vs AdvSM) or subtypes (eg, ASM, MCL, and SM-AHN), how they predict the progression to liver failure, and how they correlate with successful treatment of SM. Prospective, systematic studies will reveal the importance of MRE and liver biopsy in SM. Therefore, we highly recommend MRE be integrated into prospective studies, and liver biopsies should be considered more often when MRE is significantly abnormal for optimal prognostication and treatment of SM.
Acknowledgment: The authors greatly appreciate Meri Chen, Department of Diagnostic Radiology and Nuclear Medicine, Rush Medical College, for her help in MRE.
Contribution: C.U. conceived the idea; and C.U., K.C., S.E., C.M., F.K.K., R.I., I.I., I.M., J.S., and N.R. searched the literature, collected data, and wrote and edited the manuscript.
Conflict-of-interest disclosure: C.U. receives honorarium from Blueprint and Novartis for participating in educational activities and advisory boards. The remaining authors declare no competing financial interests.
Correspondence: Celalettin Ustun, Division of Hematology–Oncology and Cellular Therapy, Department of Medicine, Rush University, 1725 W Harrison St, Suite 809, Chicago, IL 60612; e-mail: celalettin_ustun@rush.edu.
References
Author notes
E-mail the corresponding author for data sharing: celalettin_ustun@rush.edu.
final version published online 18 October 2022