Key Points
Anti–IL-6 antibody treatment modulates the proinflammatory cytokine signature in JAK2-V617F knock-in mice.
Anti–IL-6 treatment does not reduce hematocrit or splenomegaly.
Abstract
Somatic mutations in JAK2, MPL and Calreticulin and inflammation play a key role in pathophysiology of chronic myeloproliferative neoplasia (CMN). One of the most prominent cytokines elevated in serum of Polycythemia vera patients is interleukin-6 (IL-6). Currently, it is being discussed whether suppression of inflammation by anti-cytokine approaches as anti-IL-6 treatment may be therapeutically useful in CMN. We here sought to investigate the efficacy of anti-IL-6 treatment on inflammatory cytokines, hematocrit and splenomegaly in CMN like disease. JAK2-V617F knock-in mice (JAK2+/V617F) were treated for three weeks with anti-IL-6 antibody (Ab) or IgG-control. Upon anti-IL-6 Ab treatment, serum levels of CXCL2 and CXCL10 were significantly reduced. In addition, CXCL1, CCL11, M-CSF, G-CSF, IL-17, IL-12p40 and CCL2 were reduced by a factor of 0.3 -- 0.8. Partly, this was also achieved by applying high-dose IgG. Hematocrit, erythrocyte and leukocyte counts were elevated in JAK2+/V617F mice but were not reduced by anti-IL6 Ab treatment. In addition, there was no apparent amelioration of splenomegaly and spleen histopathology. In conclusion, anti-IL-6 Ab treatment did not result in improvement of hematological disease parameters but was shown to modulate the serum cytokine signature.
Introduction
Chronic myeloproliferative neoplasias (CMNs) are hematologic disorders characterized by clonal proliferation of leukocytes (white blood cells [WBCs]), erythrocytes (red blood cells [RBCs]), and/or thrombocytes. Somatic mutations in JAK2, MPL, and calreticulin constitutively activate JAK-STAT signaling and drive inflammation in the bone marrow microenvironment. In classic CMN, comprising polycythemia vera (PV), essential thrombocytosis, and primary myelofibrosis (PMF), inflammation plays a key pathophysiologic role. Thus, proinflammatory cytokines are strongly elevated in the peripheral blood of patients1,2 and contribute to a variety of constitutional symptoms.3,4 Moreover, the proinflammatory microenvironment promotes growth of the malignant clone and drives disease progression.4 In addition, systemic inflammation contributes to induction of arterial and venous thrombosis, a major cause of morbidity and mortality in CMN.5 JAK inhibition using ruxolitinib has been shown to provide clinical benefit, particularly a strong reduction of splenomegaly, in patients with PMF.3
One of the most prominent cytokines elevated in serum of patients with PV is interleukin-6 (IL-6).1,6,7 IL-6 is a master cytokine involved in many physiologic and inflammatory mechanisms.8 IL-6 and its downstream molecule STAT3 have been described as integration points linking inflammation with malignant proliferation.2,4 IL-6 stimulates the growth of multipotent hematopoietic progenitor cells and seems to play an important role in the pathogenesis of CMN.9 In JAK2-V617F–mutated CMN, IL-6 is secreted by mutated and nonmutated cells, mostly monocytes (data not shown).10
Whether the suppression of inflammation by anti-cytokine approaches may be therapeutically useful is currently under discussion.4,10-13 To date, however, there are few preclinical data addressing this approach in vivo. Importantly, lowering IL-6 serum levels with the JAK inhibitor ruxolitinib correlated with an improvement of symptom burden in PMF.3 Here we sought to investigate the efficacy of anti–IL-6 treatment in inflammation, hematocrit (Hct) level, and splenomegaly in CMN-like disease.
Methods
Animal treatment
As described previously, 11- to 13-week-old JAK2-V617F knock-in mice (Vav1-Cre × JAK2-V617F [JAK2+/V617F]) were treated for 3 weeks with intraperitoneal anti–IL-6 antibody (Ab; MP5-20F3; BioXcell, West Lebanon, NH) or immunoglobulin G (IgG) control (HRPN; BioXcell).14 Anti–IL-6 treatment was started early after weaning of mice. Thus, our approach covered the early stages of disease development. All mouse experiments were performed under specific pathogen-free conditions and with approval of the regional government authority of Saxony-Anhalt (42502-2-1417).
Blood analysis
Retrobulbarly collected peripheral blood was analyzed using the ADVIA 2120i Hematology System (Siemens Healthcare Diagnostics, Erlangen, Germany). Serum was generated using BD Microtainer SSTTM tubes (BD Diagnostics, Franklin Lakes, NJ). Cytokine levels in serum were measured by Eve Technologies Corporation (Mouse Cytokine Array/Chemokine Array 31-Plex, Calgary, AB, Canada).
Bioactivity verification of treatment antibody
The analysis of bioactivity is described in supplemental Figure 1.
Histology
Paraffin-embedded sections of spleen and bone marrow were stained with hematoxylin (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) and eosin (Dr. K. Hollborn & Söhne GmbH & Co. KG, Leipzig, Germany) and analyzed by microscopy.
Statistical analysis
Data are presented as starting and end point of treatment. Statistical significance was evaluated using the nonparametric, two-tailed Mann-Whitney test and defined as P < .05 (nonsignificant, P > .05). All analyses were conducted using GraphPad Prism software (version 7.04).
Results and discussion
To investigate the feasibility and efficacy of anti–IL-6 treatment, we used a JAK2-V617F knock-in mouse model that exhibits a PV-like phenotype.15 The model is characterized by increased serum levels of inflammatory cytokines, elevated blood parameters (eg, Hct), and splenomegaly.
Reduction of proinflammatory cytokine levels in serum
Elevated levels of proinflammatory cytokines such as IL-1α, CCL2, CCL5, CCL11, CXCL9, and CXCL10 were observed in JAK2+/V617F mice (supplemental Figure 1). This confirms and extends the observations of other groups,1,2,6,10 demonstrating marked inflammation in CMN.
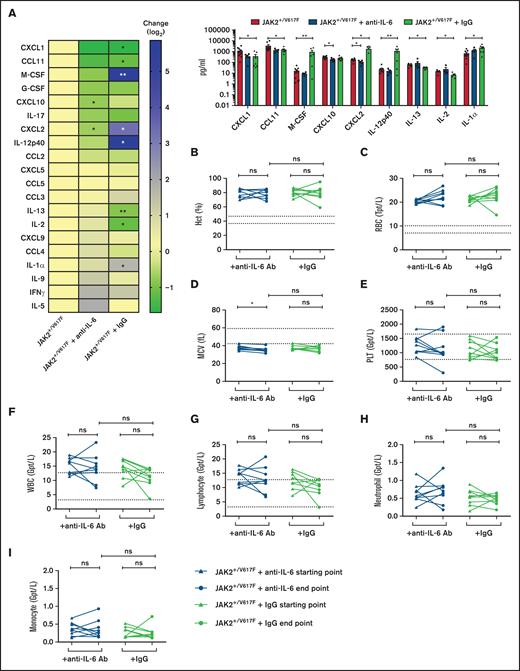
Moreover, IL-6 cytokine levels were elevated in JAK2+/V617F mice (supplemental Figure 1), demonstrating the suitability of this JAK2-V617F knock-in mouse model for our studies. Upon anti–IL-6 Ab treatment, serum levels of CXCL2 and CXCL10 were significantly reduced (P = .0280 and P = .0127, respectively). In addition, CXCL1, CCL11, macrophage colony-stimulating factor, granulocyte colony-stimulating factor, IL-17, IL-12p40, and CCL2 were reduced by a factor of 0.3 to 0.8 (Figure 1A; supplemental Table 1). Of note, CXCL2 was significantly downregulated by anti–IL-6 treatment but upregulated by IgG control (P = .0351). This is in line with the observation that expression of CXCL2 is induced by IL-6 signaling, among others.16 CXCL2 plays pivotal roles in recruiting leukocytes to inflammatory sites,17 and its serum concentration is upregulated in patients with CMN.18 Interestingly, normal isotype IgG also revealed efficacy in modification of some serum cytokine levels (Figure 1A). Immunomodulatory activity of high-dose IgG has been described, including suppression of IL-1 and TNF-α–mediated mechanisms.19,20
Analysis of serum cytokine levels and blood parameters upon anti–IL-6 Ab treatment. JAK2+/V617F mice were treated 3 times per week over 3 weeks with 200 μg of anti-IL6 Ab per injection (MP5-20F3; BioXcell; n = 5) and IgG control (HRPN; BioXcell; n = 7). (A) The cytokine levels in serum of treated and untreated (n = 10) mice were measured by Eve Technologies Corporation (Mouse Cytokine Array/Chemokine Array 31-Plex). Cytokine level changes are shown as a heatmap according to the JAK2+/V617F level (left) and were sorted by reduction of cytokine level by anti–IL-6 Ab treatment. Additionally, cytokines with distinct changes are shown as a scatter blot (right). (B-I) Retrobulbarly collected peripheral blood of treated and untreated mice was analyzed using the ADVIA 2120i Hematology System. Samples indicating Hct (B), RBC count (C), mean corpuscular volume (MCV) (D), platelets (PLT) (E), WBC count (F), lymphocyte (G), neutrophil granulocyte (H), and monocyte counts (I) are depicted at start and end point of anti–IL-6 Ab (n = 9) and IgG (n = 9) treatment of JAK2+/V617F mice. *P < .05, **P < .01, nonsignificant (ns) P > .05 (by nonparametric, 2-tailed Mann-Whitney test). IFN, interferon; G-CSF, granulocyte colony-stimulating factor; M-CSF, macrophage colony-stimulating factor.
Analysis of serum cytokine levels and blood parameters upon anti–IL-6 Ab treatment. JAK2+/V617F mice were treated 3 times per week over 3 weeks with 200 μg of anti-IL6 Ab per injection (MP5-20F3; BioXcell; n = 5) and IgG control (HRPN; BioXcell; n = 7). (A) The cytokine levels in serum of treated and untreated (n = 10) mice were measured by Eve Technologies Corporation (Mouse Cytokine Array/Chemokine Array 31-Plex). Cytokine level changes are shown as a heatmap according to the JAK2+/V617F level (left) and were sorted by reduction of cytokine level by anti–IL-6 Ab treatment. Additionally, cytokines with distinct changes are shown as a scatter blot (right). (B-I) Retrobulbarly collected peripheral blood of treated and untreated mice was analyzed using the ADVIA 2120i Hematology System. Samples indicating Hct (B), RBC count (C), mean corpuscular volume (MCV) (D), platelets (PLT) (E), WBC count (F), lymphocyte (G), neutrophil granulocyte (H), and monocyte counts (I) are depicted at start and end point of anti–IL-6 Ab (n = 9) and IgG (n = 9) treatment of JAK2+/V617F mice. *P < .05, **P < .01, nonsignificant (ns) P > .05 (by nonparametric, 2-tailed Mann-Whitney test). IFN, interferon; G-CSF, granulocyte colony-stimulating factor; M-CSF, macrophage colony-stimulating factor.
Elevated inflammatory cytokine levels have been shown to be causative for constitutional symptoms. Interestingly, in human ruxolitinib clinical trials, a link between cytokine reduction and reduced constitutional symptoms was described.3 Thus, reduction of >50% in the cytokine levels of IL-1α, CCL4, TNF-α, and IL-6 by ruxolitinib was associated with strong improvement in constitutive symptoms in patients.3 Therefore, this suggests that a change in serum cytokine levels of >50% is biologically meaningful in JAK2-V617F–induced disease. Bioactivity of the applied anti–IL-6 Ab treatment was verified by monitoring suppression of STAT3 phosphorylation (supplemental Figure 2), which correlates with STAT3 activation.21
Analysis of blood parameters
Hct, erythrocyte (RBC), and leukocyte (WBC) counts were elevated in JAK2+/V617F mice, as has been described previously.15 Hct levels remained constant in those receiving anti–IL-6 treatment (76.8% vs 77.6%; Figure 1B) as well as in IgG control mice (80.4% vs 79.8%). During anti–IL-6 treatment, RBC counts were slightly elevated (20.2 vs 22.2 Tpt/L; P = .0810; Figure 1C). Mean corpuscular volume was reduced in JAK2+/V617F mice, likely as a result of increased erythropoiesis and dysregulated iron metabolism.22 Mean corpuscular volume decreased upon anti–IL-6 treatment (38.1-35.0 fL; P = .0178; Figure 1D). Further deterioration of these parameters was most probably caused by disease progression, because the end point values were in the same range as those in the untreated JAK2+/V617F mice of the same age (data not shown). No impact of anti–IL-6 treatment on platelet (Figure 1E) or WBC counts (Figure 1F) was noted. Additionally, for lymphocyte (Figure 1G), neutrophil granulocyte (Figure 1H), and monocyte counts (Figure 1I), no apparent changes were found.
Spleen size and histopathology
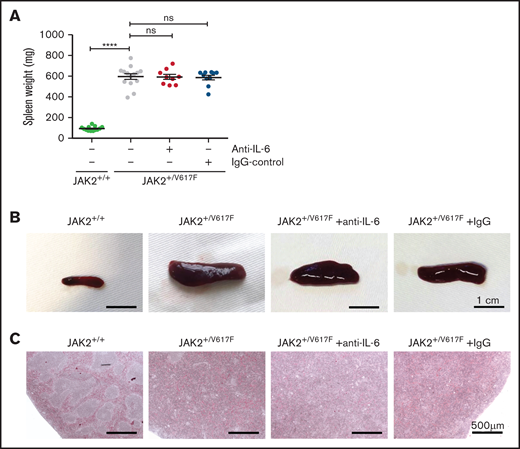
The spleen weight of JAK2+/V617F mice was increased as compared with JAK2+/+ mice (596 vs 93 mg; P = .0007). Upon anti–IL-6 Ab treatment, there was no apparent change in mean weight (592 mg) or size (Figure 2A-B). These data indicate that IL-6 has no impact on the maintenance of splenomegaly. However, Croker et al23 reported an IL-6–dependent induction of mild splenomegaly. Thus, high levels of IL-6 in CMN may promote the development of splenomegaly.
Spleen analysis of untreated and treated JAK2+/V617F mice. (A) Comparison of splenic weight measurement of untreated healthy JAK2+/+ mice (n = 13), untreated JAK2+/V617F mice (n = 14), and anti–IL-6– (n = 9) and IgG-treated JAK2+/V617F mice (n = 10), respectively. Data are shown as mean ± standard error of the mean. JAK2+/+ vs JAK2+/V617F data are our laboratory’s internal control and are also partly reported in a manuscript by Müller et al.24 (B) Representative images of the isolated spleens of untreated healthy JAK2+/+, untreated JAK2+/V617F, and anti–IL-6 Ab– and IgG-treated JAK2+/V617F mice are shown (scale bar, 1 cm). (C) Representative images of histopathologic spleen sections are shown (scale bar, 500 μm). Isolated spleens of untreated JAK2+/+ and JAK2+/V617F mice as well as anti–IL-6– and IgG-treated JAK2+/V617F mice were stained with hematoxylin and eosin. DM6000 B Microscope (Leica Microsystems, Wetzlar, Germany) and LAS (version 3.8) software (Leica Microsystems) were used for micrographs at room temperature. ****P < .0001 by nonparametric, 2-tailed Mann-Whitney test.
Spleen analysis of untreated and treated JAK2+/V617F mice. (A) Comparison of splenic weight measurement of untreated healthy JAK2+/+ mice (n = 13), untreated JAK2+/V617F mice (n = 14), and anti–IL-6– (n = 9) and IgG-treated JAK2+/V617F mice (n = 10), respectively. Data are shown as mean ± standard error of the mean. JAK2+/+ vs JAK2+/V617F data are our laboratory’s internal control and are also partly reported in a manuscript by Müller et al.24 (B) Representative images of the isolated spleens of untreated healthy JAK2+/+, untreated JAK2+/V617F, and anti–IL-6 Ab– and IgG-treated JAK2+/V617F mice are shown (scale bar, 1 cm). (C) Representative images of histopathologic spleen sections are shown (scale bar, 500 μm). Isolated spleens of untreated JAK2+/+ and JAK2+/V617F mice as well as anti–IL-6– and IgG-treated JAK2+/V617F mice were stained with hematoxylin and eosin. DM6000 B Microscope (Leica Microsystems, Wetzlar, Germany) and LAS (version 3.8) software (Leica Microsystems) were used for micrographs at room temperature. ****P < .0001 by nonparametric, 2-tailed Mann-Whitney test.
Extramedullary hematopoiesis presenting as splenomegaly occurs along with damage to the splenic structure, as described by Mullally et al.15 Distinct areas of red and white splenic pulp could not be differentiated in JAK2+/V617F mice, and there was no histologic improvement with anti–IL-6 treatment (Figure 2C). Additionally, there was no apparent change in histopathologic appearance of bone marrow sections. Megakaryocytes of treated and untreated JAK2+/V617F mice showed increased frequency of emperipolesis and atypical nuclear features (supplemental Figure 3), consistent with earlier reports.15
Taken together, these data show that anti–IL-6 Ab treatment does not significantly affect hematologic disease parameters or splenomegaly in JAK2+/V617 mice. However, anti–IL-6 treatment slightly reduced serum levels of several proinflammatory cytokines; of note, CXCL2 and CXCL10 were significantly suppressed. This was also partially achieved by administering high-dose IgG. This suggests that general suppression of JAK-STAT signaling in mutant and nonmutant cells is not a prerequisite for modulating inflammation in JAK2-V617F+ CMN. Because anti-inflammatory therapy for CMN is currently a widely discussed topic of debate, we propose further evaluation of anti-cytokine treatment other than with IL-6 (eg, anti-CXCL10, anti-TNFR1, anti-TNFR2, high-dose IgG, and others) in patients with CMN with high inflammatory activity. This may open a novel therapeutic avenue, likely in combination with the well-introduced JAK inhibitor treatment.
Acknowledgments
The authors thank Anja Sammt and Corinna Fahldieck for expert technical assistance.
This work was supported by grants from the German Research Council (SFB854, 2018-2021; T.F.) and Europäischer Fonds für Regionale Entwicklung (Autonomie im Alter: PhytoHäm, 2019-21; T.F.).
Authorship
Contribution: T.F., C.K.B., P.M., and C.G. designed experiments; T.F., C.K.B., P.M., T.R.H., S.A.-F., and J.L. performed experiments; C.K.B., C.G., and T.F. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Fischer, Department of Hematology and Oncology, Medical Center, Otto-von-Guericke University, Leipziger Str. 44, Building 39, 39120 Magdeburg, Germany; e-mail: thomas.fischer@med.ovgu.de.
References
Author notes
Requests for data sharing may be submitted to Thomas Fischer (thomas.fischer@med.ovgu.de).
The full-text version of this article contains a data supplement.