TO THE EDITOR:
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare, life-threatening disorder first recognized in early 2021 that has been documented mainly in relation to adenoviral vector-based vaccines ChAdOx1 nCoV-19 (Oxford-AstraZeneca) and Ad26.COV2.S (Janssen, Johnson & Johnson) vaccination.1 VITT is characterized by severe thrombosis, often at unusual locations such as cerebral venous sinuses and splanchnic vessels, and thrombocytopenia, occurring due to the development of platelet-activating anti-platelet factor 4 (PF4) antibodies. More recently, a likely case of ultra-rare messenger RNA (mRNA)-1273–associated VITT, also mediated by anti-PF4 antibodies, has been documented.2 Acute management generally incorporates the use of intravenous immunoglobulins, often with corticosteroids, as well as nonheparin anticoagulation,3 and is generally effective in treating acute VITT. However, some patients continue to have a refractory course, requiring additional interventions.4 Given the rarity and relatively recent discovery of this disorder, little is known about potential long-term consequences and considerations for management. Several groups have documented long-term persistence (of several months) of enzyme-linked immunosorbent assay (ELISA)-reactive anti-PF4 antibodies in both the ChAdOx1 nCoV-19 and Ad26.COV2.S-associated VITT settings.5-8 Although several hundred patients with ChAdOx1 nCoV-19-associated VITT9 have been reported in the medical literature, there are far fewer reports of Ad26.COV2.S-associated VITT, with fewer than 60 cases reported in the United States as of August 2021.10
Although recent reports demonstrate the safety of mRNA COVID-19 booster vaccination in the more common VITT setting, after ChAdOx1 nCoV-19 vaccination,5,6,11 information on the safety of mRNA vaccine boosters in patients with Ad26.COV2.S-associated VITT is lacking. Due to the high morbidity and mortality of VITT, and the possibility (albeit very low probability) of VITT after nonadenoviral vector vaccines, there is hesitance among clinicians and patients alike to consider alternate COVID-19 vaccination in the Ad26.COV2.S-associated VITT setting. Booster vaccinations may be critical in protecting against recurrent COVID-19 surges with constantly evolving SARS-CoV-2 variants. This study reports on a patient with persistent strong positive VITT serology who received an mRNA COVID-19 vaccine booster. It was approved by the Mayo Clinic Institutional Review Board and performed according to the Declaration of Helsinki.
Briefly, a 48-year-old previously healthy male presented with bilateral lower extremity pain and chest pain 19 days after receiving the Ad26.COV2.S vaccine.12 On presentation, he had a platelet count of 74 × 109/L (the last known prevaccine platelet count from a couple of years prior was 177 × 109/L) and a d-dimer of 15 109 ng/mL fibrinogen equivalent units (≤499 ng/mL fibrinogen equivalent units). He was found to have bilateral, occlusive, and extensive deep vein thrombosis and multiple acute bilateral pulmonary emboli. A hypercoagulable workup did not reveal any abnormalities. After a presumptive diagnosis of VITT was made, he was treated empirically with 1 g/kg of intravenous immunoglobulin G (IVIG) for 2 days, 1 mg/kg of prednisone, and intravenous argatroban infusion drip. His anti-PF4 ELISA (LIFECODES PF4 immunoglobulin G [IgG], Immucor), performed on a sample collected prior to initiating IVIG therapy, demonstrated a strongly positive result of 3.323 optical density (OD) units (reference interval <0.399), consistent with a diagnosis of VITT. Subsequent testing in a recently described novel, specific VITT test that uses uncomplexed PF4 targets demonstrated a high OD of 3.22.7 Functional testing in the serotonin release assay and PF4-dependent P-selectin expression assay (PEA) were also positive.7 His course was complicated by recurrence of thrombocytopenia, which was refractory to additional IVIG infusion, which improved with corticosteroid dose adjustment.12
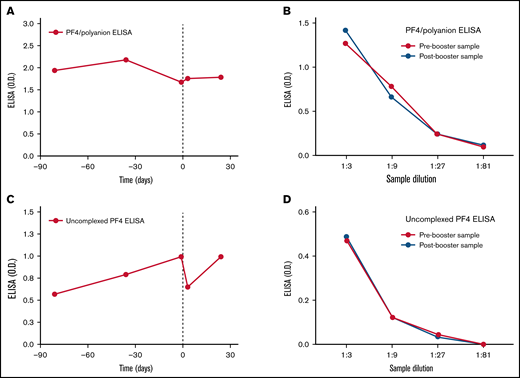
Over the subsequent 10 months, the patient demonstrated persistently strong positive anti-PF4 ELISAs that did not normalize (OD >1.5). Repeat serotonin-release assays (which can be falsely negative in VITT) were negative (data not shown). There was no recurrence of thrombosis, and platelet counts fluctuated between 130 and 160/µL. Discontinuation of anticoagulation was considered; however, given the paucity of data on the significance of persistently strong anti-PF4 antibodies, the high-risk nature of the initial thromboembolic event, as well as the patient’s low bleeding risk, anticoagulation was continued based on shared decision-making with the patient. In early 2022, during the COVID-19 omicron variant surge, the risks and benefits of COVID-19 vaccination booster were discussed with the patient. Based on early reports suggesting the safety of mRNA vaccines in patients with VITT secondary to ChAdOx1 nCoV-19 vaccination, it was felt that the risk of VITT recurrence after mRNA vaccination booster might likely be outweighed by the benefit of vaccine protection in the setting of a dramatic rise in COVID-19 cases. The patient received a BNT162b2 (Pfizer/BioNTech) vaccine booster 10 months after the initial Ad26.COV2.S vaccination while on therapeutic anticoagulation with apixaban 5 mg twice daily. He was monitored with frequent complete blood counts and tested for anti-PF4 antibodies in antigen-based and functional assays. Patient samples from ∼3 months prior to and ∼1 month after mRNA vaccine booster administration were tested in PF4/polyanion ELISA, uncomplexed PF4 ELISA, and PEA as previously described.7,13 Figure 1 demonstrates that platelet counts were stable in the ∼140 to 160 000/uL range before and up to 24 days after booster vaccination. The patient demonstrated low levels of platelet activation in the PEA (normal range, <19%) prior to vaccination, with no significant changes seen in the postvaccination setting. Patient samples were strongly positive in an IgG-specific PF4/polyanion ELISA (LIFECODES PF4 IgG, Immucor) both prior to and after vaccination (OD >1.5 at all times tested; Figure 2A). To assess for subtle changes in antibody titer, serial titrations of samples obtained 1 day prior to and 24 days post–booster vaccination were tested and demonstrated no difference in OD in the PF4/polyanion ELISA (Figure 2B). Testing in a novel, more specific VITT assay that uses uncomplexed PF4 targets demonstrated significantly lower ODs relative to the acute sample (3.22 OD, tested previously,7 vs <1.0 OD), but there was no significant change in OD in the day +24 sample relative to the day −1 sample (Figure 2C). Similar to PF4/polyanion ELISA testing, no differences were noted between these 2 samples upon titration in the uncomplexed PF4 ELISA (Figure 2D). Reactivity to uncomplexed PF4 targets correlated better with the patient’s clinical state (platelet recovered and d-dimer normalized) than PF4/polyanion ELISA, an assay designed to detect HIT anti-PF4 antibodies, that remained strongly positive (PF4/Polyanion ELISA: 1.67-2.17 OD in the samples tested [Figure 2A]; normal range <0.399 vs uncomplexed PF4 ELISA: 0.57-0.99 in the samples tested [Figure 2C]; non-VITT range: <1.007 ). Thus, its utility in monitoring the recovery of VITT patients should be evaluated. The patient did not develop any thromboembolic event over 12 weeks of follow-up postvaccination, and anticoagulation was eventually discontinued with close monitoring. The patient’s PF4/polyanion ELISA OD continues to be strongly positive at >2.0 12 months after the acute event. In summary, BNT162b2 vaccination was safely tolerated, without significant side effects. There was no downtrend in platelet counts seen on surveillance and no increase in anti-PF4 antibody titer or platelet activation potential. To the best of our knowledge, this represents the first reported case of mRNA COVID-19 vaccination in a patient with Ad26.COV2.S-associated VITT and the first reported VITT case receiving a booster in the United States. Due to the limited experience with such cases, decisions on booster vaccination should be made on a case-by-case basis after carefully weighing the individual patient’s risk-benefit ratio.
Platelet count and platelet activation induced by an mRNA COVID-19 vaccine-boosted Ad26.COV2.S-associated VITT patient. Patient platelet count (red circles) and results of the PF4-dependent PEA (blue circles) are displayed on the left and right y-axes, respectively. Administration of a booster dose of mRNA BNT162b2 vaccine is represented by the vertical dashed line. Time (in days) relative to booster vaccination is shown on the x-axis.
Platelet count and platelet activation induced by an mRNA COVID-19 vaccine-boosted Ad26.COV2.S-associated VITT patient. Patient platelet count (red circles) and results of the PF4-dependent PEA (blue circles) are displayed on the left and right y-axes, respectively. Administration of a booster dose of mRNA BNT162b2 vaccine is represented by the vertical dashed line. Time (in days) relative to booster vaccination is shown on the x-axis.
VITT anti-PF4 reactivity to PF4/polyanion complexes and uncomplexed PF4 were stable after BNT162b2 booster vaccination. A Food and Drug Administration–approved in vitro diagnostic assay, LIFECODES PF4 IgG (Immucor), was used to assess VITT antibody reactivity (A) and titer (B) toward PF4/polyanion complexes prior to and after administration (vertical dashed line) of mRNA BNT162b2 vaccine booster. Similarly, a novel ELISA employing uncomplexed PF4 targets was used to define VITT antibody reactivity (C) and titer (D) to PF4 not complexed to polyanions. Time in days relative to booster vaccination is shown on the x-axes of panels A and C, and the level of sample dilution is shown on the x-axes of panels B and D.
VITT anti-PF4 reactivity to PF4/polyanion complexes and uncomplexed PF4 were stable after BNT162b2 booster vaccination. A Food and Drug Administration–approved in vitro diagnostic assay, LIFECODES PF4 IgG (Immucor), was used to assess VITT antibody reactivity (A) and titer (B) toward PF4/polyanion complexes prior to and after administration (vertical dashed line) of mRNA BNT162b2 vaccine booster. Similarly, a novel ELISA employing uncomplexed PF4 targets was used to define VITT antibody reactivity (C) and titer (D) to PF4 not complexed to polyanions. Time in days relative to booster vaccination is shown on the x-axes of panels A and C, and the level of sample dilution is shown on the x-axes of panels B and D.
Acknowledgments: This work was supported in part by National Institutes of Health (NIH), National Heart, Lung and Blood Institute grant HL158932 (A.P.).
Contribution: M.Y.A-I. and A.P. wrote the first draft of the paper; A.J.K. designed the figures; A.J.K. and N.P.S. performed the research studies; K.J.S. and K.A.M. oversaw clinical laboratory testing; and all authors edited the manuscript and approved the final version.
Conflict-of-interest disclosure: A.P. reports pending/issued patents (Mayo Clinic, Retham Technologies, and Versiti), equity ownership in Retham Technologies, and serving on the advisory board of Veralox Therapeutics. The remaining authors declare no competing financial interests.
References
Author notes
Data will be shared upon reasonable request to corresponding author: yazan.abou-ismail@hsc.utah.edu.