Key Points
Mortality for patients with marginal zone and indolent B-cell lymphoma is largely unrelated to lymphoma in the first decade from diagnosis.
Early progression or retreatment within 24 months of diagnosis is strongly associated with increased risk of lymphoma-related mortality.
Abstract
Low-grade B-cell lymphomas other than follicular and small lymphocytic lymphoma (LGBCL) account for 10% of all B-cell non-Hodgkin lymphomas. Despite improvements in survival outcomes for these patients, little is known about cause of death (COD) in the rituximab era. For a better understanding, we studied 822 newly diagnosed patients with marginal zone, lymphoplasmacytic, and unclassifiable low-grade B-cell lymphoma prospectively enrolled in the University of Iowa/Mayo Clinic Specialized Program of Research Excellence Molecular Epidemiology Resource from 2002 to 2015. COD was assigned based on medical record review using a standard protocol. At a median follow-up of 107 months, 219 (27%) patients had died. The incidence of lymphoma-related deaths when pooling across subtypes was lower than non–lymphoma-related deaths (10-year incidence, 8.0%; 95% confidence interval [CI]: 6.2-10.4 vs 13.6%; 95% CI: 11.2-16.6). The incidence of lymphoma-related deaths varied by subtype, ranging from 3.7% at 10 years in extranodal marginal zone lymphoma to 19.3% in lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. Patients with early progression or retreatment events, defined using event-free survival at 24 months from diagnosis, had significantly higher likelihood of lymphoma-related death compared with patients without early events (10-year estimate: 19.1% vs 5.1%, respectively; P < .001), whereas the rates for non–lymphoma-related death were comparable in patients with or without early events (10-year estimates: 11.0% vs 15.3%, respectively). In conclusion, the most common COD in LGBCLs in the first decade after diagnosis was for causes other than lymphoma. Progression or retreatment within the first 2 years of diagnosis was a strong predictor for risk of lymphoma-related death.
Introduction
Indolent B-cell lymphomas are a group of neoplastic disorders that account for approximately 35% to 45% of non-Hodgkin lymphomas in the Western world.1-4 They are clinically indolent but pathologically diverse and are composed of follicular lymphoma (FL), marginal zone lymphoma (MZL, including nodal MZL [NMZL], extranodal MZL [EMZL], and splenic MZL [SMZL]), lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia (WM), and small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL).5,6 The overall prognosis is favorable for most patients, but some have an aggressive disease course associated with early progression, relapse, or histologic transformation.5,7 Advances in molecular pathology and the introduction of anti-CD20 monoclonal antibodies have significantly impacted the outcomes of these patients.8,9 Despite therapeutic advances, lymphoma remains the leading cause of death (COD) in patients with FL.10 Similarly, in patients with CLL/SLL, deaths caused by the underlying disease and disease-related complications are the principal CODs in the rituximab era.11,12
Prospective data on cause-specific mortality are limited for other indolent B-cell lymphomas. The initial studies, published between 1998 and 2000, reported modest survival outcomes in patients with NMZL with a 5-year overall survival (OS) estimate of 55% to 57%.13-16 The survival outcomes of NMZL have improved significantly over the last 2 decades, with the 5-year OS rate ranging from 70% to 90%.16-22 However, OS estimates do not provide specific information about the incidence of death from lymphoma or other causes. An analysis from the Surveillance, Epidemiology, and End Results (SEER) cancer registry database of patients aged ≥ 66 years and diagnosed from 2004 to 2013 showed higher non–lymphoma-related deaths than deaths secondary to lymphoma-related causes in patients with MZL, but mortality rates of different subtypes of MZL such as EMZL, NMZL, and SMZL were not separately reported.23 Moreover, the existing mortality data for LPL/WM are somewhat limited and conflicting. The data from the US population-based cancer registries showed higher rates of non–lymphoma-related deaths vs lymphoma-related deaths, whereas the study of patients with symptomatic WM by the Greek Myeloma Study Group showed higher WM-related deaths than non–WM-related deaths.24,25 The majority of these studies were constrained to retrospective study design and registry database analyses primarily focusing on survival outcomes.
We previously identified in a cohort of patients from our University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) that survival in non-FL low-grade B-cell lymphomas (LGBCL) was comparable to that of the general population for patients who did not progress, relapse, or require retreatment within 12 months of diagnosis.5 Conversely, early progression or retreatment of disease was associated with inferior postprogression survival.5 Similar results have been observed in a cohort of patients with MZL from the FIL-NF10 study and from a cohort of patients with mucosa-associated lymphoid tissue (MALT) lymphoma from the IELSG-19 clinical trial.26,27 In addition, early relapse was highly associated with lymphoma-related death in a cohort of patients with FL from the MER and Lyon, France, but it is unknown how early events impact the cause of death in other indolent B-cell lymphomas.10 Although there are various known clinicopathologic characteristics that are predictive of survival, factors related to COD have not been well studied in patients with LGBCL.7,18,21,26 ,, -31 Moreover, characterization of CODs in patients with LGBCL is important for delivering comprehensive care from counseling to intervention beyond lymphoma and its therapies that could improve their survival. Thus, we performed a detailed examination of COD in the cohort of patients with LGBCL prospectively enrolled in the MER.
Methods
Patient selection
The Mayo Clinic institutional review board reviewed and approved the study. All patients provided informed consent to enroll in the MER of the University of Iowa/Mayo Clinic Lymphoma SPORE.32 The MER is a prospective cohort study of lymphoma outcomes established in 2002 and includes adult patients (≥18 years) who are residents of the United States with newly diagnosed lymphoma (within 9 months of diagnosis). Newly diagnosed patients enrolled between 2002 and 2015 were eligible for this study. We included the following histopathologic diagnoses: NMZL; EMZL; SMZL; LPL/WM; and low grade B-cell lymphoma, unclassifiable (LGBCL-U).5 Patients with transformation and/or concomitant diffuse large B-cell lymphoma or other aggressive B-cell lymphoma at diagnosis were excluded.
Demographic, clinical, and pathologic data were abstracted using a standard protocol. Pathologic confirmation was done centrally by MER-affiliated expert hematopathologists using current World Health Organization criteria at the time of diagnosis. Participants were contacted prospectively every 6 months for the initial 3 years and then annually. Transformation was defined as the development of biopsy-confirmed diffuse large B-cell lymphoma or high-grade B-cell lymphoma during the follow-up period. Disease progression, retreatment, transformation, and deaths were validated by medical record review. The International Prognostic Index (IPI), Follicular Lymphoma International Prognostic Index (FLIPI), and MALT-IPI were incorporated in the study based on their prognostic utility in patients with LGBCL.5,33-35 COD was determined via a review of death certificate copy and/or medical records by MER physicians using a standard protocol and classified as the result of lymphoma (progression or transformation), treatment, unrelated cancer, other causes, or unknown. Treatment-related mortality was broadly defined and further classified into infection, cardiac, or secondary myelodysplastic syndrome/acute myeloid leukemia.
Statistical analyses
Event-free survival (EFS) was defined as the time from diagnosis to the first of progression, relapse, transformation, initiation of a new line of treatment for lack of efficacy, or death from any cause. The cut-point EFS24 was defined based on EFS status at 24 months from diagnosis. Early event was defined as a nondeath event within 24 months of diagnosis (EFS24). All-cause survival was defined as the time from diagnosis until death from any cause or last known follow-up for those alive. COD was first examined by the 5 major categories (lymphoma, treatment, unrelated cancer, other causes, or unknown) and subsequently collapsed into 3 groups for analysis as per previous publication; lymphoma-related (defined as lymphoma or treatment related), unrelated (other malignancies and other causes), and unknown.10 If COD was unclear, consensus opinion was obtained from 2 other investigators. All-cause survival was summarized by Kaplan-Meier curves. Cumulative incidence for the competing risks of COD and tests of equality for COD between groups were calculated using the cuminc function from the cmprsk package in R.36 For early event analysis, time to death was defined as the time since the risk defining event (24-month time point for patients who achieved EFS24 and time since the first event for patients who did not achieve EFS24). Survival from transformation was calculated from the date of pathologic confirmation of transformation. All analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC) and R (version 4.0.3). 95% confidence intervals (CIs) are reported for point estimates; P values are not adjusted for multiple comparisons.
Results
Baseline characteristics and initial management strategies
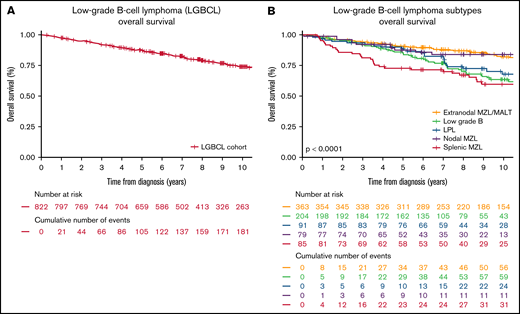
A total of 822 patients with newly diagnosed LGBCLs enrolled in the MER from 2002 to 2015 were included in this study. Baseline characteristics of the study population are shown in Table 1. The most common subtype was EMZL (n = 363; 44%), followed by LGBCL-U (n = 204; 25%), LPL/WM (n = 91; 11%), SMZL (n = 85; 10%), and NMZL (n = 79; 10%). The median age at diagnosis was 64 years (range, 18-92 years), with 60% (n = 490) of patients at age > 60 years. Most patients (n = 501; 63%) had stage III to IV disease. FLIPI risk category was low risk in n = 375 patients (46%), whereas 195 patients (24%) had high-risk FLIPI. MALT-IPI categorized patients slightly differently, with 208 patients (25%) in the low-risk category and 238 (29%) in the high-risk category.
Baseline characteristics of patients with LGBCL
| Characteristics . | EMZL (N = 363) . | LGBCL-U (N = 204) . | LPL (N = 91) . | NMZL (N = 79) . | SMZL (N = 85) . | Total (N = 822) . |
|---|---|---|---|---|---|---|
| Age group, n (%) | ||||||
| ≤60 | 163 (44.9) | 69 (33.8) | 37 (40.7) | 30 (38.0) | 33 (38.8) | 332 (40.4) |
| 61-70 | 105 (28.9) | 57 (27.9) | 31 (34.1) | 32 (40.5) | 23 (27.1) | 248 (30.2) |
| 70+ | 95 (26.2) | 78 (38.2) | 23 (25.3) | 17 (21.5) | 29 (34.1) | 242 (29.4) |
| Sex, n (%) | ||||||
| Male | 154 (42.4) | 119 (58.3) | 61 (67.0) | 39 (49.4) | 41 (48.2) | 414 (50.4) |
| Female | 209 (57.6) | 85 (41.7) | 30 (33.0) | 40 (50.6) | 44 (51.8) | 408 (49.6) |
| PS group, n (%) | ||||||
| <2 | 345 (96.6) | 193 (96.0) | 82 (92.1) | 69 (89.6) | 74 (88.1) | 763 (94.4) |
| ≥2 | 12 (3.4) | 8 (4.0) | 7 (7.9) | 8 (10.4) | 10 (11.9) | 45 (5.6) |
| Missing | 6 | 3 | 2 | 2 | 1 | 14 |
| LDH group, n (%) | ||||||
| ≤Normal | 265 (88.0) | 122 (79.2) | 57 (83.8) | 46 (76.7) | 49 (64.5) | 539 (81.8) |
| >Normal | 36 (12.0) | 32 (20.8) | 11 (16.2) | 14 (23.3) | 27 (35.5) | 120 (18.2) |
| Missing | 62 | 50 | 23 | 19 | 9 | 163 |
| HGB group, n (%) | ||||||
| ≥12 g/dL | 240 (79.2) | 93 (55.4) | 26 (32.5) | 46 (75.4) | 27 (33.3) | 432 (62.3) |
| <12 g/dL | 63 (20.8) | 75 (44.6) | 54 (67.5) | 15 (24.6) | 54 (66.7) | 261 (37.7) |
| Missing | 60 | 36 | 11 | 18 | 4 | 129 |
| Ann Arbor stage group, n (%) | ||||||
| I-II | 221 (61.6) | 35 (18.4) | 10 (11.1) | 24 (31.6) | 7 (8.4) | 297 (37.2) |
| III-IV | 138 (38.4) | 155 (81.6) | 80 (88.9) | 52 (68.4) | 76 (91.6) | 501 (62.8) |
| Missing | 4 | 14 | 1 | 3 | 2 | 24 |
| IPI group, n (%) | ||||||
| IPI 0-1 | 234 (64.5) | 65 (31.9) | 31 (34.1) | 30 (38.0) | 14 (16.5) | 374 (45.5) |
| IPI 2 | 72 (19.8) | 88 (43.1) | 39 (42.9) | 36 (45.6) | 41 (48.2) | 276 (33.6) |
| IPI 3 | 46 (12.7) | 41 (20.1) | 16 (17.6) | 9 (11.4) | 26 (30.6) | 138 (16.8) |
| IPI 4-5 | 11 (3.0) | 10 (4.9) | 5 (5.5) | 4 (5.1) | 4 (4.7) | 34 (4.1) |
| FLIPI group, n (%) | ||||||
| FLIPI 0-1 | 239 (65.8) | 68 (33.3) | 22 (24.2) | 33 (41.8) | 13 (15.3) | 375 (45.6) |
| FLIPI 2 | 90 (24.8) | 74 (36.3) | 30 (33.0) | 31 (39.2) | 27 (31.8) | 252 (30.7) |
| FLIPI 3-5 | 34 (9.4) | 62 (30.4) | 39 (42.9) | 15 (19.0) | 45 (52.9) | 195 (23.7) |
| MALTIPI group, n (%) | ||||||
| MALTIPI 0 | 153 (42.1) | 29 (14.2) | 9 (9.9) | 14 (17.7) | 3 (3.5) | 208 (25.3) |
| MALTIPI 1 | 150 (41.3) | 91 (44.6) | 53 (58.2) | 45 (57.0) | 37 (43.5) | 376 (45.7) |
| MALTIPI 2-3 | 60 (16.5) | 84 (41.2) | 29 (31.9) | 20 (25.3) | 45 (52.9) | 238 (29.0) |
| Initial treatment, n (%) | ||||||
| Observation | 117 (32.2) | 95 (46.6) | 24 (26.4) | 35 (44.3) | 41 (48.2) | 312 (38.0) |
| R-Mono therapy | 50 (13.8) | 27 (13.2) | 18 (19.8) | 8 (10.1) | 7 (8.2) | 110 (13.4) |
| ImmunoChemo therapy | 43 (11.8) | 44 (21.6) | 36 (39.6) | 24 (30.4) | 8 (9.4) | 155 (18.9) |
| Radiation therapy | 82 (22.6) | 9 (4.4) | 3 (3.3) | 6 (7.6) | 1 (1.2) | 101 (12.3) |
| Other | 71 (19.6) | 29 (14.2) | 10 (11.0) | 6 (7.6) | 28 (32.9) | 144 (17.5) |
| Primary cause of death, n (%) | ||||||
| Related to lymphoma | 15 (20.5) | 21 (30.4) | 17 (54.8) | 4 (33.3) | 8 (23.5) | 65 (29.7) |
| Not related to lymphoma | 43 (58.9) | 33 (47.8) | 10 (32.3) | 7 (58.3) | 19 (55.9) | 112 (51.1) |
| Unknown | 15 (20.5) | 15 (21.7) | 4 (12.9) | 1 (8.3) | 7 (20.6) | 42 (19.2) |
| Characteristics . | EMZL (N = 363) . | LGBCL-U (N = 204) . | LPL (N = 91) . | NMZL (N = 79) . | SMZL (N = 85) . | Total (N = 822) . |
|---|---|---|---|---|---|---|
| Age group, n (%) | ||||||
| ≤60 | 163 (44.9) | 69 (33.8) | 37 (40.7) | 30 (38.0) | 33 (38.8) | 332 (40.4) |
| 61-70 | 105 (28.9) | 57 (27.9) | 31 (34.1) | 32 (40.5) | 23 (27.1) | 248 (30.2) |
| 70+ | 95 (26.2) | 78 (38.2) | 23 (25.3) | 17 (21.5) | 29 (34.1) | 242 (29.4) |
| Sex, n (%) | ||||||
| Male | 154 (42.4) | 119 (58.3) | 61 (67.0) | 39 (49.4) | 41 (48.2) | 414 (50.4) |
| Female | 209 (57.6) | 85 (41.7) | 30 (33.0) | 40 (50.6) | 44 (51.8) | 408 (49.6) |
| PS group, n (%) | ||||||
| <2 | 345 (96.6) | 193 (96.0) | 82 (92.1) | 69 (89.6) | 74 (88.1) | 763 (94.4) |
| ≥2 | 12 (3.4) | 8 (4.0) | 7 (7.9) | 8 (10.4) | 10 (11.9) | 45 (5.6) |
| Missing | 6 | 3 | 2 | 2 | 1 | 14 |
| LDH group, n (%) | ||||||
| ≤Normal | 265 (88.0) | 122 (79.2) | 57 (83.8) | 46 (76.7) | 49 (64.5) | 539 (81.8) |
| >Normal | 36 (12.0) | 32 (20.8) | 11 (16.2) | 14 (23.3) | 27 (35.5) | 120 (18.2) |
| Missing | 62 | 50 | 23 | 19 | 9 | 163 |
| HGB group, n (%) | ||||||
| ≥12 g/dL | 240 (79.2) | 93 (55.4) | 26 (32.5) | 46 (75.4) | 27 (33.3) | 432 (62.3) |
| <12 g/dL | 63 (20.8) | 75 (44.6) | 54 (67.5) | 15 (24.6) | 54 (66.7) | 261 (37.7) |
| Missing | 60 | 36 | 11 | 18 | 4 | 129 |
| Ann Arbor stage group, n (%) | ||||||
| I-II | 221 (61.6) | 35 (18.4) | 10 (11.1) | 24 (31.6) | 7 (8.4) | 297 (37.2) |
| III-IV | 138 (38.4) | 155 (81.6) | 80 (88.9) | 52 (68.4) | 76 (91.6) | 501 (62.8) |
| Missing | 4 | 14 | 1 | 3 | 2 | 24 |
| IPI group, n (%) | ||||||
| IPI 0-1 | 234 (64.5) | 65 (31.9) | 31 (34.1) | 30 (38.0) | 14 (16.5) | 374 (45.5) |
| IPI 2 | 72 (19.8) | 88 (43.1) | 39 (42.9) | 36 (45.6) | 41 (48.2) | 276 (33.6) |
| IPI 3 | 46 (12.7) | 41 (20.1) | 16 (17.6) | 9 (11.4) | 26 (30.6) | 138 (16.8) |
| IPI 4-5 | 11 (3.0) | 10 (4.9) | 5 (5.5) | 4 (5.1) | 4 (4.7) | 34 (4.1) |
| FLIPI group, n (%) | ||||||
| FLIPI 0-1 | 239 (65.8) | 68 (33.3) | 22 (24.2) | 33 (41.8) | 13 (15.3) | 375 (45.6) |
| FLIPI 2 | 90 (24.8) | 74 (36.3) | 30 (33.0) | 31 (39.2) | 27 (31.8) | 252 (30.7) |
| FLIPI 3-5 | 34 (9.4) | 62 (30.4) | 39 (42.9) | 15 (19.0) | 45 (52.9) | 195 (23.7) |
| MALTIPI group, n (%) | ||||||
| MALTIPI 0 | 153 (42.1) | 29 (14.2) | 9 (9.9) | 14 (17.7) | 3 (3.5) | 208 (25.3) |
| MALTIPI 1 | 150 (41.3) | 91 (44.6) | 53 (58.2) | 45 (57.0) | 37 (43.5) | 376 (45.7) |
| MALTIPI 2-3 | 60 (16.5) | 84 (41.2) | 29 (31.9) | 20 (25.3) | 45 (52.9) | 238 (29.0) |
| Initial treatment, n (%) | ||||||
| Observation | 117 (32.2) | 95 (46.6) | 24 (26.4) | 35 (44.3) | 41 (48.2) | 312 (38.0) |
| R-Mono therapy | 50 (13.8) | 27 (13.2) | 18 (19.8) | 8 (10.1) | 7 (8.2) | 110 (13.4) |
| ImmunoChemo therapy | 43 (11.8) | 44 (21.6) | 36 (39.6) | 24 (30.4) | 8 (9.4) | 155 (18.9) |
| Radiation therapy | 82 (22.6) | 9 (4.4) | 3 (3.3) | 6 (7.6) | 1 (1.2) | 101 (12.3) |
| Other | 71 (19.6) | 29 (14.2) | 10 (11.0) | 6 (7.6) | 28 (32.9) | 144 (17.5) |
| Primary cause of death, n (%) | ||||||
| Related to lymphoma | 15 (20.5) | 21 (30.4) | 17 (54.8) | 4 (33.3) | 8 (23.5) | 65 (29.7) |
| Not related to lymphoma | 43 (58.9) | 33 (47.8) | 10 (32.3) | 7 (58.3) | 19 (55.9) | 112 (51.1) |
| Unknown | 15 (20.5) | 15 (21.7) | 4 (12.9) | 1 (8.3) | 7 (20.6) | 42 (19.2) |
ECOG PS, Eastern Cooperative Oncology Group Performance Status.
The frontline therapeutic strategies for patients with LGBCL are summarized in Table 1 and varied between different subtypes. The most common approach was observation (38%), which ranged from 26% in LPL/WM to 48% in SMZL. Combination immunochemotherapy was used in 19% (n = 155) of patients, ranging from 9% in SMZL to 40% LPL/WM. A total of 110 study patients (13%) initially received rituximab (R) monotherapy with a range of 8% in SMZL to 20% in LPL/WM. Overall, 12% (n = 101) of patients were treated only with radiation therapy, with the highest frequency in patients with EMZL (23%) and lowest in SMZL (1%).
At a median follow-up of 8.9 years (range, 0.3-17.9 years), 31 patients (3.8%) had a transformation, and 219 patients (27%) had died. EFS24 by Kaplan-Meier was 79% (95% CI: 76-82). Lymphoma-related death was observed in 65 patients (58 because of progressive lymphoma and 7 because of therapy-related causes). The subtypes for the 7 therapy-related deaths were LPL (N = 3), EMZL (N = 3), and LGBCL-U (N = 1); class of therapy at the time of death was anthracycline-based immunochemotherapy (N = 2), CD20-bendamustine (N = 2), Bruton's tyrosine kinase inhibitor (N = 1), mechanistic target of rapamycin inhibitor (N = 1), and proteosome inhibitor (N = 1). Non–lymphoma-related deaths occurred in 112 patients (51.1%), and cause of death was unknown in 42 (19.2%) patients. Most common causes for non–lymphoma-related deaths (supplemental Table 1) were medical comorbidities (35%) and other malignancies (35%).
Cumulative incidence and distribution of COD overall and by subtype
Ten-year all-cause OS in the cohort was 73.8% (95% CI: 70.4-77.3; Figure 1A), which ranged from 59.6% in SMZL to 83.3% in NMZL (Figure 1B). There were 219 deaths with 51% (112 of 219) non–lymphoma-related causes and 30% (65 of 219) lymphoma-related causes; and in 19% (42 of 219), the COD was unknown.
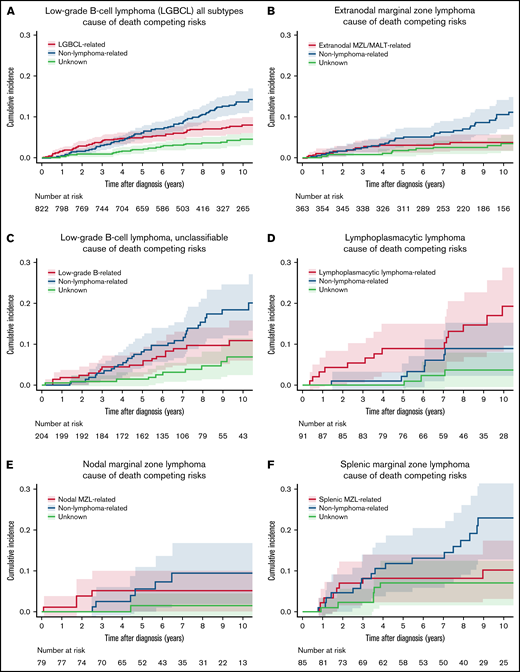
The 10-year estimate for lymphoma-related death in the overall study population was 8.0% (95% CI: 6.2-10.4), which was lower than the non–lymphoma-related death rate of 13.6% (95% CI: 11.2-16.6; Figure 2A; Table 2). COD by subtypes is shown in Figure 2B-F. The highest lymphoma-related death incidences were in LPL/WM (10-year estimate, 19.3%; 95% CI: 11.7-31.8) and LGBCL-U (10-year estimate, 11.0%; 95% CI: 7.0-17.2), and the lowest incidence was in EMZL (10-year estimate, 3.7%; 95% CI: 2.2-6.4; Table 2). The incidence for non–lymphoma-related death was highest in SMZL (10-year estimate, 23.0%; 95% CI: 14.9-35.4), followed by LGBCL-U (10-year estimate, 18.5%; 95% CI: 13.2-25.9); the lowest incidence was observed in LPL/WM (10-year estimate, 8.9%; 95% CI: 4.4-18.3).
Cumulative incidence for the competing risks of cause of death by lymphoma subtype. (A) LGBCL all subtypes. (B) EMZL. (C) LGBCL-U. (D) LPL. (E) NMZL. (F) SMZL.
Cumulative incidence for the competing risks of cause of death by lymphoma subtype. (A) LGBCL all subtypes. (B) EMZL. (C) LGBCL-U. (D) LPL. (E) NMZL. (F) SMZL.
Ten-year risk of death with 95% CI for clinicopathologic characteristics stratified by cause of death
| Clinicopathologic characteristics . | Lymphoma-related death . | Non–lymphoma-related death . | Unknown death . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10-y estimate . | 95% CI . | P . | 10-y estimate . | 95% CI . | P . | 10-y estimate . | 95% CI . | P . | |
| All | 0.080 | 0.062-0.104 | 0.136 | 0.112-0.166 | 0.045 | 0.032-0.065 | |||
| Age group, y | <.001 | <.001 | <.001 | ||||||
| ≤60 | 0.041 | 0.023-0.075 | 0.055 | 0.033-0.093 | 0.017 | 0.007-0.040 | |||
| 61-70 | 0.068 | 0.042-0.109 | 0.137 | 0.096-0.196 | 0.031 | 0.015-0.065 | |||
| 70+ | 0.148 | 0.105-0.208 | 0.245 | 0.190-0.315 | 0.100 | 0.064-0.156 | |||
| Sex | .122 | .060 | .184 | ||||||
| Male | 0.097 | 0.070-0.133 | 0.146 | 0.113-0.188 | 0.050 | 0.032-0.078 | |||
| Female | 0.064 | 0.042-0.097 | 0.127 | 0.093-0.172 | 0.041 | 0.023-0.073 | |||
| LDH group | .001 | .971 | .628 | ||||||
| ≤Normal | 0.065 | 0.046-0.092 | 0.144 | 0.114-0.182 | 0.042 | 0.026-0.065 | |||
| >Normal | 0.158 | 0.103-0.243 | 0.154 | 0.095-0.251 | 0.072 | 0.033-0.157 | |||
| HGB group | .001 | .009 | .826 | ||||||
| ≥12 g/dL | 0.061 | 0.041-0.090 | 0.115 | 0.086-0.154 | 0.048 | 0.030-0.077 | |||
| <12 g/dL | 0.136 | 0.096-0.194 | 0.199 | 0.149-0.266 | 0.048 | 0.027-0.086 | |||
| Ann Arbor stage | <.001 | .013 | .050 | ||||||
| I-II | 0.022 | 0.009-0.054 | 0.094 | 0.063-0.141 | 0.031 | 0.015-0.066 | |||
| III-IV | 0.114 | 0.087-0.149 | 0.151 | 0.120-0.191 | 0.056 | 0.038-0.084 | |||
| IPI score | <.001 | <.001 | .069 | ||||||
| 0-1 | 0.038 | 0.021-0.069 | 0.081 | 0.055-0.119 | 0.028 | 0.014-0.056 | |||
| 2 | 0.093 | 0.061-0.144 | 0.159 | 0.116-0.218 | 0.054 | 0.030-0.095 | |||
| 3 | 0.147 | 0.098-0.220 | 0.195 | 0.134-0.286 | 0.060 | 0.030-0.117 | |||
| 4-5 | 0.181 | 0.086-0.379 | 0.312 | 0.169-0.576 | 0.140 | 0.041-0.471 | |||
| FLIPI group | <.001 | <.001 | .045 | ||||||
| FLIPI 0-1 | 0.023 | 0.011-0.048 | 0.085 | 0.057-0.125 | 0.030 | 0.015-0.059 | |||
| FLIPI 2 | 0.098 | 0.064-0.150 | 0.145 | 0.103-0.202 | 0.048 | 0.026-0.089 | |||
| FLIPI 3-5 | 0.168 | 0.120-0.237 | 0.223 | 0.165-0.302 | 0.073 | 0.041-0.131 | |||
| MALTIPI group | <.001 | <.001 | .001 | ||||||
| MALTIPI 0 | 0.013 | 0.003-0.052 | 0.033 | 0.015-0.073 | 0.016 | 0.005-0.048 | |||
| MALTIPI 1 | 0.054 | 0.033-0.087 | 0.160 | 0.121-0.211 | 0.038 | 0.021-0.069 | |||
| MALTIPI 2-3 | 0.180 | 0.134-0.243 | 0.190 | 0.143-0.253 | 0.084 | 0.052-0.136 | |||
| Initial treatment | .001 | .445 | .419 | ||||||
| Observation | 0.075 | 0.049-0.116 | 0.160 | 0.119-0.214 | 0.035 | 0.018-0.068 | |||
| R-Mono therapy | 0.124 | 0.070-0.219 | 0.121 | 0.071-0.209 | 0.047 | 0.020-0.112 | |||
| ImmunoChemo therapy | 0.131 | 0.082-0.208 | 0.141 | 0.089-0.222 | 0.059 | 0.030-0.116 | |||
| Radiation therapy | 0.000 | Not applicable | 0.073 | 0.033-0.161 | 0.026 | 0.006-0.105 | |||
| Other | 0.063 | 0.033-0.119 | 0.135 | 0.084-0.217 | 0.065 | 0.030-0.140 | |||
| EFS 24 | <.001 | .369 | .776 | ||||||
| Achieved | 0.051 | 0.033-0.078 | 0.153 | 0.121-0.192 | 0.046 | 0.030-0.073 | |||
| Failed | 0.191 | 0.128-0.285 | 0.110 | 0.065-0.187 | 0.050 | 0.022-0.113 | |||
| Histologic type | <.001 | .030 | .132 | ||||||
| EMZL | 0.037 | 0.022-0.064 | 0.106 | 0.076-0.149 | 0.035 | 0.019-0.064 | |||
| LGBCL-U | 0.110 | 0.070-0.172 | 0.185 | 0.132-0.259 | 0.069 | 0.037-0.131 | |||
| LPL | 0.193 | 0.117-0.318 | 0.089 | 0.044-0.183 | 0.038 | 0.012-0.117 | |||
| NMZL | 0.052 | 0.020-0.135 | 0.095 | 0.043-0.208 | 0.015 | 0.002-0.107 | |||
| SMZL | 0.103 | 0.052-0.204 | 0.230 | 0.149-0.354 | 0.071 | 0.033-0.155 | |||
| Clinicopathologic characteristics . | Lymphoma-related death . | Non–lymphoma-related death . | Unknown death . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10-y estimate . | 95% CI . | P . | 10-y estimate . | 95% CI . | P . | 10-y estimate . | 95% CI . | P . | |
| All | 0.080 | 0.062-0.104 | 0.136 | 0.112-0.166 | 0.045 | 0.032-0.065 | |||
| Age group, y | <.001 | <.001 | <.001 | ||||||
| ≤60 | 0.041 | 0.023-0.075 | 0.055 | 0.033-0.093 | 0.017 | 0.007-0.040 | |||
| 61-70 | 0.068 | 0.042-0.109 | 0.137 | 0.096-0.196 | 0.031 | 0.015-0.065 | |||
| 70+ | 0.148 | 0.105-0.208 | 0.245 | 0.190-0.315 | 0.100 | 0.064-0.156 | |||
| Sex | .122 | .060 | .184 | ||||||
| Male | 0.097 | 0.070-0.133 | 0.146 | 0.113-0.188 | 0.050 | 0.032-0.078 | |||
| Female | 0.064 | 0.042-0.097 | 0.127 | 0.093-0.172 | 0.041 | 0.023-0.073 | |||
| LDH group | .001 | .971 | .628 | ||||||
| ≤Normal | 0.065 | 0.046-0.092 | 0.144 | 0.114-0.182 | 0.042 | 0.026-0.065 | |||
| >Normal | 0.158 | 0.103-0.243 | 0.154 | 0.095-0.251 | 0.072 | 0.033-0.157 | |||
| HGB group | .001 | .009 | .826 | ||||||
| ≥12 g/dL | 0.061 | 0.041-0.090 | 0.115 | 0.086-0.154 | 0.048 | 0.030-0.077 | |||
| <12 g/dL | 0.136 | 0.096-0.194 | 0.199 | 0.149-0.266 | 0.048 | 0.027-0.086 | |||
| Ann Arbor stage | <.001 | .013 | .050 | ||||||
| I-II | 0.022 | 0.009-0.054 | 0.094 | 0.063-0.141 | 0.031 | 0.015-0.066 | |||
| III-IV | 0.114 | 0.087-0.149 | 0.151 | 0.120-0.191 | 0.056 | 0.038-0.084 | |||
| IPI score | <.001 | <.001 | .069 | ||||||
| 0-1 | 0.038 | 0.021-0.069 | 0.081 | 0.055-0.119 | 0.028 | 0.014-0.056 | |||
| 2 | 0.093 | 0.061-0.144 | 0.159 | 0.116-0.218 | 0.054 | 0.030-0.095 | |||
| 3 | 0.147 | 0.098-0.220 | 0.195 | 0.134-0.286 | 0.060 | 0.030-0.117 | |||
| 4-5 | 0.181 | 0.086-0.379 | 0.312 | 0.169-0.576 | 0.140 | 0.041-0.471 | |||
| FLIPI group | <.001 | <.001 | .045 | ||||||
| FLIPI 0-1 | 0.023 | 0.011-0.048 | 0.085 | 0.057-0.125 | 0.030 | 0.015-0.059 | |||
| FLIPI 2 | 0.098 | 0.064-0.150 | 0.145 | 0.103-0.202 | 0.048 | 0.026-0.089 | |||
| FLIPI 3-5 | 0.168 | 0.120-0.237 | 0.223 | 0.165-0.302 | 0.073 | 0.041-0.131 | |||
| MALTIPI group | <.001 | <.001 | .001 | ||||||
| MALTIPI 0 | 0.013 | 0.003-0.052 | 0.033 | 0.015-0.073 | 0.016 | 0.005-0.048 | |||
| MALTIPI 1 | 0.054 | 0.033-0.087 | 0.160 | 0.121-0.211 | 0.038 | 0.021-0.069 | |||
| MALTIPI 2-3 | 0.180 | 0.134-0.243 | 0.190 | 0.143-0.253 | 0.084 | 0.052-0.136 | |||
| Initial treatment | .001 | .445 | .419 | ||||||
| Observation | 0.075 | 0.049-0.116 | 0.160 | 0.119-0.214 | 0.035 | 0.018-0.068 | |||
| R-Mono therapy | 0.124 | 0.070-0.219 | 0.121 | 0.071-0.209 | 0.047 | 0.020-0.112 | |||
| ImmunoChemo therapy | 0.131 | 0.082-0.208 | 0.141 | 0.089-0.222 | 0.059 | 0.030-0.116 | |||
| Radiation therapy | 0.000 | Not applicable | 0.073 | 0.033-0.161 | 0.026 | 0.006-0.105 | |||
| Other | 0.063 | 0.033-0.119 | 0.135 | 0.084-0.217 | 0.065 | 0.030-0.140 | |||
| EFS 24 | <.001 | .369 | .776 | ||||||
| Achieved | 0.051 | 0.033-0.078 | 0.153 | 0.121-0.192 | 0.046 | 0.030-0.073 | |||
| Failed | 0.191 | 0.128-0.285 | 0.110 | 0.065-0.187 | 0.050 | 0.022-0.113 | |||
| Histologic type | <.001 | .030 | .132 | ||||||
| EMZL | 0.037 | 0.022-0.064 | 0.106 | 0.076-0.149 | 0.035 | 0.019-0.064 | |||
| LGBCL-U | 0.110 | 0.070-0.172 | 0.185 | 0.132-0.259 | 0.069 | 0.037-0.131 | |||
| LPL | 0.193 | 0.117-0.318 | 0.089 | 0.044-0.183 | 0.038 | 0.012-0.117 | |||
| NMZL | 0.052 | 0.020-0.135 | 0.095 | 0.043-0.208 | 0.015 | 0.002-0.107 | |||
| SMZL | 0.103 | 0.052-0.204 | 0.230 | 0.149-0.354 | 0.071 | 0.033-0.155 | |||
Patterns of COD by demographic and clinical characteristics
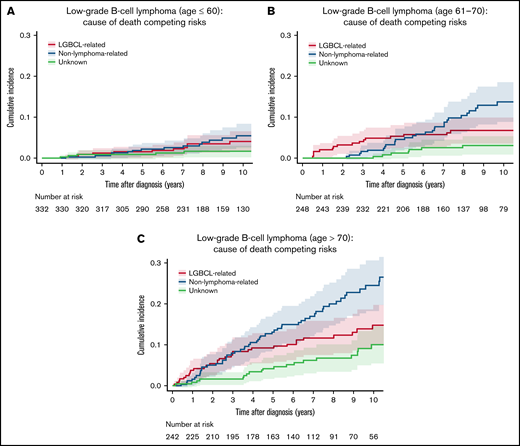
The patterns of COD by key demographic and clinical characteristics are shown in Table 2. The incidence of both lymphoma-related and non–lymphoma-related deaths increased with age at diagnosis. The 10-year estimate for lymphoma-related deaths was 4.1% (95% CI: 2.3-7.5) vs 6.8% (95% CI: 4.2-10.9) vs 14.8% (95% CI: 10.5-20.8) in patients diagnosed at age ≤ 60 years vs 61-70 years vs > 70 years, respectively (P < .001). The respective 10-year estimate of non–lymphoma-related mortality was 5.5% (95% CI: 3.3-9.3) vs 13.7% (95% CI: 9.6-19.6) vs 24.5% (95% CI: 19.0-31.5; P < .001; Figure 3A-C). Notably, the cumulative incidence of non–lymphoma-related deaths was higher than that of lymphoma-related deaths in all age groups. There were no clinically significant differences in either lymphoma-related deaths (10-year estimate, 9.7%; 95% CI: 7.0-13.3 for men vs 6.4%; 95% CI: 4.2-9.7 for women; P = .12) or non–lymphoma-related deaths (10-year estimate, 14.6%; 95% CI: 11.3-18.8 for men vs 12.7%; 95% CI: 9.3-17.2 for women; P = .06). Cause of death patterns were similar between the subset of patients diagnosed between 2002 and 2008 and the subset of patients diagnosed between 2009 and 2015 (supplemental Figure 3).
Cumulative incidence for the competing risks of cause of death by age. (A) Cumulative incidence by cause of death for patients age ≤ 60 years. (B) Cumulative incidence by cause of death for patients ages 61 to 70 years. (C) Cumulative incidence by cause of death for patients age > 70 years.
Cumulative incidence for the competing risks of cause of death by age. (A) Cumulative incidence by cause of death for patients age ≤ 60 years. (B) Cumulative incidence by cause of death for patients ages 61 to 70 years. (C) Cumulative incidence by cause of death for patients age > 70 years.
The presence of adverse clinical characteristics was broadly associated with an increase in lymphoma-related deaths. Patients with an elevated lactate dehydrogenase (LDH) at diagnosis had significantly higher incidence of lymphoma-related deaths (10-year estimate, 15.8%; 95% CI: 10.3-24.3) compared with those without LDH elevation (6.5%; 95% CI: 4.6-9.2; P = .001), with similar rates for non–lymphoma-related deaths (15.4% vs 14.4% at 10 years; P = .97). Patients with hemoglobin (HGB) < 12 g/dL had higher incidence of both lymphoma-related deaths (10-year estimate, 13.6%; 95% CI: 9.6-19.4) and non–lymphoma-related deaths (10-year estimate, 19.9%; 95% CI: 14.9-26.6) compared with patients with HGB ≥ 12 g/dL (10-year estimates, 6.1%, 95% CI: 4.1-9.0; P = .001, and 11.5%; 95% CI: 8.6-15.4; P = .009, respectively). Similarly, patients with stage III to IV disease had a higher incidence of both lymphoma-related deaths (10-year estimate, 11.4%; 95% CI: 8.7-14.9) and non–lymphoma-related deaths (10-year estimate, 15.1%; 95% CI: 12.0-19.1) compared with patients with stage I/II disease (10-year estimate, 2.2%; 95% CI: 0.9-5.4; P < .001, and 9.4%; 95% CI: 6.3-14.1; P = .013, respectively).
Patterns of COD by baseline prognostic indices
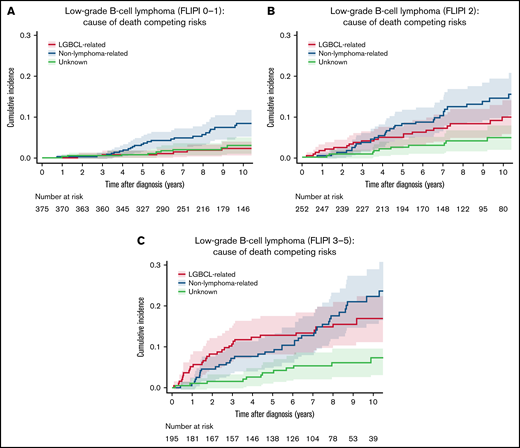
The cumulative incidence of lymphoma-related and non–lymphoma-related deaths increased with a higher FLIPI score (score 0-1 vs 2 vs 3-5) with the 10-year rates of 2.3% (95% CI: 1.1-4.8) vs 9.8% (95% CI: 6.4-15.0) vs 16.8% (95% CI: 12.0-23.7) for lymphoma-related and 8.5% (95% CI: 5.7-12.5) vs 14.5% (95% CI: 10.3-20.2) vs 22.3 (95% CI: 16.5-30.2) for non–lymphoma-related deaths, respectively (both P < .0001; Table 2; Figure 4). MALT-IPI also showed similar pattern of increasing cumulative incidence in both lymphoma-related and non–lymphoma-related death rates with increasing score but with different cumulative incidence. The 10-year cumulative incidence for lymphoma-related deaths was 1.3% (95% CI: 0.3-5.2) vs 5.4% (95% CI: 3.3-8.7) vs 18.0% (95% CI: 13.4-24.3) in patients with the MALT-IPI group of 0 vs 1 vs 2-3 (P < .001); the respective 10-year cumulative incidence for non–lymphoma-related deaths was 3.3% (95% CI: 1.5-7.3) vs 16.0% (95% CI: 12.1-21.1) vs 19.0% (95% CI: 14.3-25.3; P < .001; Table 2, supplemental Figure 1A-C). Similar patterns were observed with the IPI (Table 2). Notably, the cumulative incidence of non–lymphoma-related deaths was higher than that of lymphoma-related deaths in all IPI, FLIPI, and MALT-IPI groups.
Cumulative incidence for the competing risks of cause of death according to FLIPI score. (A) Cumulative incidence by cause of death for patients with FLIPI score 0 to 1. (B) Cumulative incidence by cause of death for patients with FLIPI score 2. (C) Cumulative incidence by cause of death for patients with FLIPI score 3 to 5.
Cumulative incidence for the competing risks of cause of death according to FLIPI score. (A) Cumulative incidence by cause of death for patients with FLIPI score 0 to 1. (B) Cumulative incidence by cause of death for patients with FLIPI score 2. (C) Cumulative incidence by cause of death for patients with FLIPI score 3 to 5.
Patterns of COD by observed outcomes
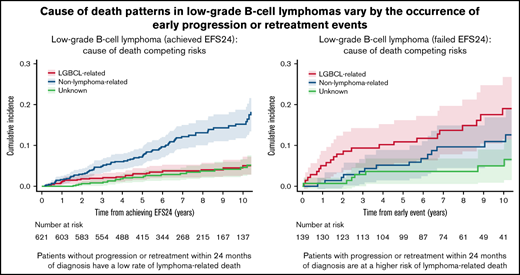
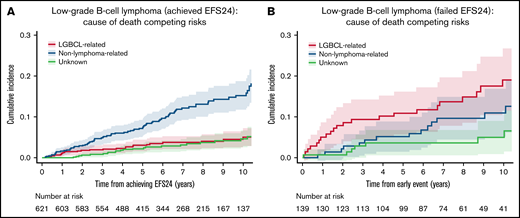
We then evaluated the pattern of COD based on transformation and EFS24. In patients with transformed disease, the rate of lymphoma-related death was higher than that of non–lymphoma-related death. The 5-year estimate of lymphoma-related death from date of transformation was 34% (95% CI: 20.3-57.9) in the 31 patients with transformation during the follow-up period, whereas non–lymphoma-related death was 6.5% at 5 years from transformation (95% CI: 1.6-25.3; supplemental Figure 2). The 621 patients who achieved EFS24 had very low rates of lymphoma-related death from achieving EFS24: 5.1% at 10 years (95% CI: 3.3-7.8; Figure 5A). In contrast, the 139 patients who failed to achieve EFS24 had significantly increased incidence of lymphoma-related deaths: 19.1% (95% CI: 12.8-28.5) at 10 years from early event (P < .001; Table 2; Figure 5B). However, death from non–lymphoma-related cause was not significantly different between patients who failed to achieve EFS24 (11.0% at 10 years; 95% CI: 6.5-18.7) compared with those who achieved EFS24 (15.3% at 10 years; 95% CI: 12.1-19.2; P = .37). Importantly, failing to achieve EFS24 identified a higher incidence of lymphoma-related vs non–lymphoma-related death (19.1% vs 11.0% at 10 years; Figure 5).
Cumulative incidence for the competing risks of cause of death by EFS24 status. (A) Cumulative incidence by cause of death for patients achieving 24 months of event-free survival from diagnosis. (B) Cumulative incidence by cause of death from early event in patients who have progression or retreatment within 24 months of diagnosis.
Cumulative incidence for the competing risks of cause of death by EFS24 status. (A) Cumulative incidence by cause of death for patients achieving 24 months of event-free survival from diagnosis. (B) Cumulative incidence by cause of death from early event in patients who have progression or retreatment within 24 months of diagnosis.
Discussion
To our knowledge, this is the largest examination of COD of a prospective cohort of patients with marginal zone, lymphoplasmacytic, or unclassifiable low-grade B-cell lymphomas with complete clinical characteristic, therapeutic, and outcomes data in the rituximab era. The existing literature on CODs in patients with LGBCLs are limited; therefore, our findings can aid in counseling and prognostication of patients.
A major finding from this study is that in contrast to recently reported data in patients with FL,10 where lymphoma was the most common cause of death during the first decade from diagnosis, the incidence of non–lymphoma-related deaths was considerably higher than lymphoma-related deaths in patients with nonfollicular LGBCL. The 10-year cumulative risk of mortality from lymphoma-related deaths and non–lymphoma-related deaths was 8.0% and 13.6%, respectively, in the current study compared with 10.3% and 5.1% at 10 years in the patients with FL.10 The higher non–lymphoma-related death rates seen in patients with NMZL, EMZL, SMZL, and LGBCL-U were similar to the data from the SEER cancer registry of patients with MZL with unspecified subtypes.23 Additionally, the most common causes of non–lymphoma-related deaths were attributed to medical comorbidities and other malignancies that are encountered routinely in patients without a diagnosis of LGBCL. We did not identify unexpected patterns of non–lymphoma-related death in our clinical review of these patients. Additional follow-up and/or larger studies would be beneficial to facilitate elucidation of particular health comorbidities. The relatively low rate of lymphoma-related death compared with death from other causes have important clinical implications, especially for patient counseling, estimating prognosis, and management planning. Because the majority of deaths are attributable to non–lymphoma-related causes, the clinical outcomes of these patients may favorably be impacted by addressing medical comorbidities, health maintenance, and increasing physical activity.37,38 These patients have intrinsic immune dysfunction rendering them at increased risk for infections and other malignancies, suggesting additional survivorship focus on immunizations, age- and sex-appropriate malignancy screening, and regular full-body skin examinations.39-43
Despite advances in the understanding of disease and therapeutic strategies, deaths related to LPL/WM could be considered remarkable and exceeded non–lymphoma-related deaths in the first decade of the rituximab era (10-year cumulative incidence, 19.3% vs 8.9%). However, LPL/WM-related death rate of 15% in our cohort is considerably lower than that of the SEER cancer registry data and the Greek Myeloma Study group data that reported a long-term LPL/WM-related death rate of ∼20% to 33%.23 -25,44 The discrepancies in mortality outcomes could be caused in part by different study populations with various disease risk profiles and diverse therapeutic strategies. LPL/WM, however, was the only LGBCL type where lymphoma-related deaths were noted to be higher than non–lymphoma-related deaths, suggesting different epidemiology and biology of disease than other MZL subtypes.
The comparison of these results in LGBCL to a similar analysis in FL provide insights on the spectrum of disease aggressiveness within the broad category of indolent lymphoma. The 10-year all-cause OS rate in the LGBCL cohort was lower than that of the FL cohort (73% vs 80%) despite inclusion of significantly older patients in LGBCL than the FL cohort (median age, 64 vs 60 and 29% vs 18% age 70+ years, respectively). This may be explained by more indolent biology of LGBCL perhaps with a better overall health status. Similar to patients with FL, age at diagnosis influenced lymphoma-related mortality rates that increased substantially in patients with an older age at diagnosis with the 10-year cumulative mortality rate of 14.8% for those diagnosed at >70 years of age.10 Likewise, the FLIPI score is strongly associated with lymphoma-related mortality in patients with LGBCL, with the 10-year cumulative mortality rate of 16.8% for those in the FLIPI score 3 to 5 group.10 However, in contrast to our previous report of patients with FL, we also observed increased risks of non–lymphoma-related death with increasing FLIPI scores in LGBCL. It is plausible that patients with a higher FLIPI score in our cohort were generally older with associated comorbidities, with subsequent increased risks of non–lymphoma-related deaths. Incorporation of genetic and molecular features may lead to development a robust model that is more specific for lymphoma-related death.45,46 Nevertheless, these findings also emphasize the need to address medical conditions other than lymphoma and promote health maintenance particularly in patients with high-risk disease.
The most clinically relevant findings in this study is that, similar to FL, patients with early events (failed to achieve EFS24) in our cohort had a markedly increased risk of lymphoma-related deaths compared with those achieving EFS24 (19.1% vs 5.1% at 10 years). This was observed without a corresponding increase in non–lymphoma-related death by EFS24 status and was the only clinical factor to identify a population at risk of lymphoma-related mortality. Early events have previously been associated with increased all-cause mortality in this cohort5 and an international prospective registry.26 This study extends those results to confirm that the observed increase in mortality is from lymphoma-related causes. This finding suggests that patients with EFS24 failure may have a different biology that confers a more aggressive tumor type and help design treatment inventions targeted to this population.47 Furthermore, although transformation was an infrequent event in our series (N = 31 patients) compared with FL, transformation was associated with a high rate of lymphoma death (34% at 5 years from transformation). Molecular studies of patients with EFS24 failure and/or transformation may provide valuable insights.
The strengths of our study include the prospective cohort design that consecutively enrolled patients with newly diagnosed lymphoma, the systematic collection of clinical data, central pathology review with accurate determination of histopathologic diagnoses, the near-complete follow-up information (particularly for disease progression and CODs), and the validation of mortality events by medical record review. The limitations include the observational study design with management being done at the discretion of the treating physicians and not driven by a protocol. However, this is more reflective of real-world practice and makes the results generalizable. Moreover, about 25% of our cohort was attributable to LGBCL-U, and further refinement of the diagnosis could be accomplished by additional molecular diagnostic techniques that were not available at the time that some of these patients were accrued (eg, the majority of patients with LPL/WM have MYD88 L265P mutation that differentiates from other indolent B-cell lymphomas).6,48 Although we are able to document the cumulative incidence of lymphoma-related deaths relative to others among patients with LGBCLs, we did not quantify cause-specific risks of death (eg, cardiovascular disease, pulmonary condition, stroke) because of limited case and event numbers. In addition, we were not able to incorporate comorbidity data, which is known to influence survival outcomes. Last, our cohort was not a population-based sample and is limited in terms of diversity with predominantly Whites; therefore, the findings may not be reflective of disease outcomes in the community.
In conclusion, the most common COD in LGBCLs at 10 years was unrelated to lymphoma, in contrast to our previous data on FL and CLL/SLL that reported lymphoma as the most common CODs at 10 years. However, like FL, patients with LGBCLs who failed to achieve EFS24 had significantly increased risk of lymphoma-related deaths. Increased efforts to address other CODs while developing novel therapeutic strategies and clinical and biologic markers that are predictive of early progression are warranted.
Acknowledgments
This work was supported in part through grants from the National Cancer Institute: Lymphoma Epidemiology of Outcomes (U01CA195568) and the University of Iowa/Mayo Clinic Lymphoma SPORE (P50CA097274).
Authorship
Contribution: A.M.T., J.R.C., T.M.H., and M.J.M. conceived and designed the study; all authors collected and/or assembled the data and wrote and reviewed the manuscript; and R.M. and M.J.M. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew J. Maurer, Department of Quantitative Health Sciences, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: maurer.matthew@mayo.edu.
References
Author notes
T.M.H. and M.J.M. contributed equally to this study.
Presented in part at the 62nd annual meeting of the American Society of Hematology, 5-8 December 2020 (virtual meeting).
The data in the study are not publicly available. Data sharing policies and the process to request the data that support the findings of this study can be found on the LEO Cohort website: https://leocohort.org/.
The full-text version of this article contains a data supplement.