Key Points
D-dimer level at ALL diagnosis is associated with venous or arterial thrombosis during the first 100 days of therapy.
Future studies should include D-dimer with other known risk factors to build a risk assessment model for thrombosis in newly diagnosed ALL.
Abstract
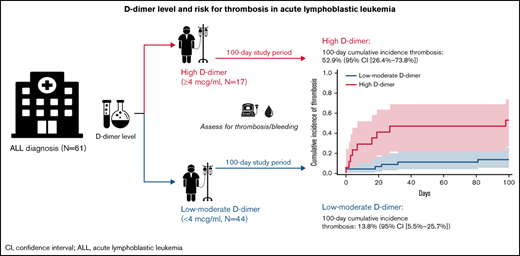
Patients with acute lymphoblastic leukemia (ALL) are at increased risk of thrombotic and/or bleeding events during early chemotherapy, especially when receiving asparaginase. D-dimer is a marker of fibrinolysis that has been associated with thrombotic risk in solid cancers and acute myeloid leukemia; however, to date, no ALL-based study has assessed D-dimer level and risk for thrombosis. We sought to examine D-dimer as a biomarker for risk of thrombosis or bleeding during ALL treatment in a retrospective cohort study at The University of Chicago. We identified 61 consecutive adult patients with ALL, gathering demographic characteristics, treatment regimens, initial biomarkers including D-dimer, and assessing occurrence of venous or arterial thrombosis and bleeding in the first 100 days after diagnosis (index). The 100-day cumulative incidence (95% confidence interval [CI]) of venous or arterial thrombosis in patients with high D-dimer (≥4 µg/mL) was 52.9% (95% CI, 26.4-73.8) compared with 13.8% (95% CI, 5.5-25.7) in patients with low to moderate D-dimer (<4 µg/mL), corresponding with a hazard ratio of 5.04 (95% CI, 1.79-14.22). When testing for potential confounders in a series of bivariate logistic regression models, the association between D-dimer and thrombosis remained after adjusting for body mass index, age, sex, asparaginase treatment, disseminated intravascular coagulation score, initial platelet level, and ALL phenotype. In conclusion, D-dimer levels at ALL diagnosis are associated with venous or arterial thrombosis at 100 days. Future studies should include D-dimer collated with other known risk factors to build a risk assessment model for thrombosis in patients with newly diagnosed ALL.
Introduction
Patients with acute lymphoblastic leukemia (ALL) are at increased risk for venous and arterial thrombotic events, particularly during induction chemotherapy.1,2 This risk is, in part, attributed to treatment for ALL, including high-dose glucocorticoids and asparaginase (l-asparaginase or pegylated asparaginase), which are known to shift the balance between procoagulant and anticoagulation factors. Other potential contributors include the frequent occurrence of infections and the inflammatory milieu of the disease itself.3 Rates of venous thromboembolism (VTE) in ALL vary across studies, ranging from 1% to 36%, with the majority of studies reporting rates in the 5% to 20% range over a 1- to 3-year follow-up period.3-7 Thrombosis in ALL can have a devastating impact on patients’ lives, leading to prolonged hospitalizations,1 central catheter removal,3 central nervous system complications,8 postthrombotic syndrome, and truncation of asparaginase therapy,5 which is associated with an increased risk of relapse9 and possibly an increased risk of death.4
Studies have attempted to stratify patients according to thrombotic risk by examining underlying demographic, clinical, biochemical, and genetic factors. Variables associated with risk of VTE in prior studies include older age, elevated body mass index (BMI), use of asparaginase, increased initial platelet count, T-cell ALL phenotype, and high-risk alleles.5,6,10,11 However, many of these risk factors display narrow margins, and there is considerable variability in significance of risk from one study to another.12
D-dimer is a marker of fibrinolysis harkening the presence of or risk for thrombosis, and it has been found to be a powerful predictor of thrombosis in solid cancers13 and acute myeloid leukemia (AML). According to the work of Libourel et al,14 a D-dimer level ≥4 µg/mL fibrinogen equivalent units (FEU) at time of AML diagnosis has a hazard ratio (HR) of 12.3 in terms of risk of venous or arterial thrombosis in multivariable analysis.
Although D-dimer has proved a useful test for thrombosis risk prediction in AML, to date no ALL-based study has examined D-dimer as a predictor of thrombosis.14 Considering the ongoing need for thrombosis risk stratification in ALL, we aimed to assess the association between D-dimer levels at ALL diagnosis and subsequent venous or arterial thrombosis.
Materials and methods
Patients and sample
This retrospective cohort study was approved by The University of Chicago Internal Review Board and conducted in full accordance with all applicable research policies and procedures of The University of Chicago.
In this study, we identified adult patients (≥18 years of age) with newly diagnosed ALL from a single center between January 2008 and March 2020. Patients were included in the study if they met the following criteria: new diagnosis of ALL, receiving induction therapy at The University of Chicago, and available D-dimer level at time of ALL diagnosis (defined as study index). Records were followed up from index date up until 100 days’ postindex. Subjects receiving therapeutic-dose anticoagulation at study index were excluded. Patients were censored for primary outcome, death, loss to follow-up, or end of follow-up period, whichever came first. Data were extracted manually from the electronic medical record, de-identified, and securely stored using REDCap electronic data capture tools hosted at The University of Chicago.15
Variables and measurement
Patient demographic details, including ALL-related variables and risk factors for thrombosis or bleeding, were collected at index based on prior studies5,6,10,14 as detailed in Table 1. Laboratory variables at index were documented, with local reference ranges shown in Table 1. The D-dimer assay is ordered routinely at diagnosis of ALL at The University of Chicago Medical Center as a plasma assay and performed using photometric immunoassay analysis on a STA R Max analyzer (Stago) within 6 hours of blood draw. D-dimer tests were only considered acceptable if ordered routinely as part of the diagnostic workup for ALL within 7 days of ALL diagnosis (allowing for completion of workup among patients transferred to The University of Chicago). The disseminated intravascular coagulation (DIC) score according to the International Society on Thrombosis and Haemostasis (ISTH) was calculated at index.16
Patient characteristics, stratified for D-dimer level
| Variable (at index*) . | All . | D-dimer level . | |
|---|---|---|---|
| High† . | Low to moderate† . | ||
| No. of patients (% of all) | 61 (100) | 17 (28) | 44 (72) |
| Age, median (range), y | 36 (18-84) | 39 (19-84) | 36 (18-80) |
| Female | 32 (52) | 9 (53) | 23 (52) |
| BMI ≥ 25 kg/m2 | 43 (70) | 11 (65) | 32 (73) |
| ALL subgroup | |||
| B-cell ALL | 52 (85) | 13 (76) | 39 (89) |
| T-cell ALL | 5 (8) | 3 (18) | 2 (5) |
| Mixed phenotype ALL | 4 (7) | 1 (6) | 3 (7) |
| Ph+ALL | 12 (20) | 3 (18) | 9 (20) |
| Ph-like ALL | 5 (8) | 2 (12) | 3 (13) |
| Asparaginase-based treatment‡ | 41 (67) | 12 (71) | 29 (66) |
| Previous venous thrombosis | 1 (2) | 0 (0) | 1 (2) |
| Previous arterial thrombosis | 2 (3) | 0 (0) | 2 (5) |
| D-dimer, median (range), µg/mL§ | 2.1 (0.26-20) | 9.1 (5.5-20) | 1.2 (0.26-3.98) |
| Platelet count, median (range) (×109/L)§ | 71 (7-445) | 50 (9-445) | 87 (7-286) |
| Fibrinogen, median (range), mg/dL§ | 417 (126-974) | 457 (126-974) | 410 (249-840) |
| PT, median (range), s§ | 14 (10-25) | 16 (13-19) | 14 (10-25) |
| DIC score, median (range)ǁ | 4 (0-7) | 5 (3-7) | 4 (0-5) |
| Variable (at index*) . | All . | D-dimer level . | |
|---|---|---|---|
| High† . | Low to moderate† . | ||
| No. of patients (% of all) | 61 (100) | 17 (28) | 44 (72) |
| Age, median (range), y | 36 (18-84) | 39 (19-84) | 36 (18-80) |
| Female | 32 (52) | 9 (53) | 23 (52) |
| BMI ≥ 25 kg/m2 | 43 (70) | 11 (65) | 32 (73) |
| ALL subgroup | |||
| B-cell ALL | 52 (85) | 13 (76) | 39 (89) |
| T-cell ALL | 5 (8) | 3 (18) | 2 (5) |
| Mixed phenotype ALL | 4 (7) | 1 (6) | 3 (7) |
| Ph+ALL | 12 (20) | 3 (18) | 9 (20) |
| Ph-like ALL | 5 (8) | 2 (12) | 3 (13) |
| Asparaginase-based treatment‡ | 41 (67) | 12 (71) | 29 (66) |
| Previous venous thrombosis | 1 (2) | 0 (0) | 1 (2) |
| Previous arterial thrombosis | 2 (3) | 0 (0) | 2 (5) |
| D-dimer, median (range), µg/mL§ | 2.1 (0.26-20) | 9.1 (5.5-20) | 1.2 (0.26-3.98) |
| Platelet count, median (range) (×109/L)§ | 71 (7-445) | 50 (9-445) | 87 (7-286) |
| Fibrinogen, median (range), mg/dL§ | 417 (126-974) | 457 (126-974) | 410 (249-840) |
| PT, median (range), s§ | 14 (10-25) | 16 (13-19) | 14 (10-25) |
| DIC score, median (range)ǁ | 4 (0-7) | 5 (3-7) | 4 (0-5) |
Data are expressed as n (%) unless otherwise indicated.
Ph, Philadelphia chromosome; PT, prothrombin time.
Study index defined as date of ALL diagnosis.
D-dimer level at index: high, ≥4 µg/mL FEU; low to moderate, <4 µg/mL FEU.
Defined as at least 1 dose of asparaginase during 100 days after study index, before censorship.
Reference range for laboratory variables: D-dimer, <0.40 µg/mL FEU; platelet count, 150 to 450 × 109/L; fibrinogen, 180 to 409 mg/dL; and PT, 11.8 to 14.4 seconds.
Calculated using the ISTH scoring system from Taylor et al.16
Patients were stratified into 2 groups according to index D-dimer level using a cutoff of ≥4 µg/mL FEU (high) and <4 µg/mL (low to moderate) based on previous work examining D-dimer and thrombosis in AML14 and the cutoff used in the DIC score.16 The primary outcome was thrombosis, defined as a composite of first VTE at any site (not including superficial vein thrombosis) or first arterial thrombosis (ie, myocardial infarction, limb arterial thromboembolism, ischemic stroke, transient ischemic attack) within 100 days’ postindex. Thrombotic events had to present clinically and be supported by imaging studies, except for stroke, transient ischemic attack, and myocardial infarction, which could be diagnosed based on clinical symptoms, signs, and auxiliary tests. Secondary outcomes included death from any cause, major bleeding,17 clinically relevant nonmajor bleeding (CRNMB) according to ISTH classification,18 and thrombotic events excluding upper extremity deep vein thrombosis (DVTs) (as these may be associated with a lower risk of pulmonary embolism compared with lower extremity DVTs19 ).
Statistical analysis
Data are presented as median (interquartile range) for continuous variables and frequency (%) for categorical variables. The cumulative incidence and corresponding 95% confidence intervals (CIs) of the primary outcome were calculated for high and low to moderate D-dimer groups and for the full cohort. Crude rates of secondary outcomes were calculated. Two types of statistical analyses were performed. A Cox proportional hazards regression model was used to analyze time to thrombosis and to calculate the hazard ratio (HR) with 95% CI for the primary outcome in the high D-dimer group compared with the low to moderate group. This analysis censored 2 patients who died or were transitioned to hospice before 100 days. Two separate sensitivity analyses of the primary outcome were performed excluding patients with the following: a history of thrombosis or anticoagulation between index and censorship, or D-dimer levels measured on the same day as the thrombotic event. Logistic regression and receiver-operating characteristic curve (ROC) analysis were used to analyze the binary outcome of whether a thrombotic event occurred within the first 100 days from index. In this analysis, the 2 patients who died/transitioned to hospice before 100 days were excluded.
A limited sample size and number of events precluded multivariable analysis of variables associated with thrombosis. We therefore developed 7 distinct bivariate models in the logistic regression analyses, each including D-dimer plus one other potential confounder: BMI, age, sex, asparaginase treatment, DIC score, initial platelet count, and prior thrombosis. D-dimer was also assessed as a continuous variable in terms of risk of thrombosis using logistic regression.
A ROC was generated and area under the curve calculated to assess performance of D-dimer as a predictor of thrombosis (by 100 days) in terms of sensitivity and specificity across the range of potential cutoffs. Youden’s index was used to identify the optimal cutoff for D-dimer. All data were analyzed by using STATA version 16.1 software (Stata Corp).
Results
Sample characteristics
We identified 70 consecutive eligible patients. Nine patients were excluded based on lack of availability of D-dimer level at study index, as these patients were diagnosed initially at hospitals where D-dimer analysis was not routine before transfer to The University of Chicago (where D-dimer testing is routinely ordered). The study cohort included 61 patients with ALL, of whom 17 (28%) had high (≥4 µg/mL FEU) D-dimer levels at index, and 44 (72%) had low to moderate (<4 µg/mL FEU) index D-dimer levels. The median age overall was 36 years (range, 18-84 years), with 52% female. Most patients had B-cell ALL (85%), and asparaginase–based regimens were used in 67% of patients overall. No patients received therapeutic anticoagulation before a thrombotic event and, because prophylactic anticoagulation is not routine at The University of Chicago, only 1 patient (in the low to moderate D-dimer group) received prophylactic anticoagulation (enoxaparin 40 mg daily) starting at 11 days’ postindex. Patient characteristics, stratified according to D-dimer group, are detailed in Table 1 with a few notable patterns. These patterns included a relatively higher proportion of T-cell ALL in the high D-dimer group compared with the low to moderate group (18% vs 5%), and all patients with a medical history of VTE or arterial thrombosis were in the low to moderate group (2% and 5%, respectively).
Thrombosis at 100 days
Cumulative incidence.
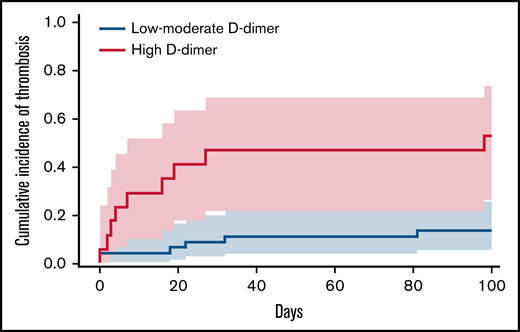
The median follow-up was 100 days. The 100-day cumulative incidence of arterial or venous thrombosis was 24.7% overall (95% CI, 14.7-36.2; n = 15) as shown in supplemental Figure 1. Most thrombotic events occurred during induction therapy with a median of 16 days (range, 0-98 days) as detailed in supplemental Figure 1. Infection was not suspected in the context of the thrombotic events. The majority of thrombotic events were venous (n = 11 [73%]) as detailed in Table 2. The 100-day cumulative incidence of thrombosis in the high D-dimer group was 52.9% (95% CI, 26.4-73.8) compared with 13.8% (95% CI, 5.5-25.7) in patients with low to moderate D-dimer, corresponding to an HR of 5.04 (95% CI, 1.79-14.22) as shown in Figure 1. Due to the low mortality rate during the study period, death was not considered a competing risk in the analysis.
Thrombotic or bleeding events at 100 days
| Outcome . | All (N = 61) . | D-dimer level . | |
|---|---|---|---|
| High (n = 17)* . | Low to moderate (n = 44)* . | ||
| Thrombotic events | |||
| Any thrombosis | 15 (25%) | 9 (53%) | 6 (14%) |
| UE DVT | 6 (40%) | 3 (33%) | 3 (50%) |
| Line-associated UE DVT† | 4 (27%) | 1 (11%) | 3 (50%) |
| LE DVT | 3 (20%) | 3 (33%) | 0 (0%) |
| PE without DVT | 1 (7%) | 1 (11%) | 0 (0%) |
| PE with DVT | 1 (7%) | 0 (0%) | 1 (17%) |
| Ischemic stroke‡ | 4 (27%) | 2 (22%) | 2 (33%) |
| Bleeding events | |||
| Major bleeding | 0 (0%) | 0 (0%) | 0 (0%) |
| Any CRNMB | 3 (5%) | 0 (0%) | 3 (7%) |
| Genitourinary CRNMB | 2 (67%) | 0 (0%) | 2 (67%) |
| Gastrointestinal CRNMB | 1 (33%) | 0 (0%) | 1 (33%) |
| Outcome . | All (N = 61) . | D-dimer level . | |
|---|---|---|---|
| High (n = 17)* . | Low to moderate (n = 44)* . | ||
| Thrombotic events | |||
| Any thrombosis | 15 (25%) | 9 (53%) | 6 (14%) |
| UE DVT | 6 (40%) | 3 (33%) | 3 (50%) |
| Line-associated UE DVT† | 4 (27%) | 1 (11%) | 3 (50%) |
| LE DVT | 3 (20%) | 3 (33%) | 0 (0%) |
| PE without DVT | 1 (7%) | 1 (11%) | 0 (0%) |
| PE with DVT | 1 (7%) | 0 (0%) | 1 (17%) |
| Ischemic stroke‡ | 4 (27%) | 2 (22%) | 2 (33%) |
| Bleeding events | |||
| Major bleeding | 0 (0%) | 0 (0%) | 0 (0%) |
| Any CRNMB | 3 (5%) | 0 (0%) | 3 (7%) |
| Genitourinary CRNMB | 2 (67%) | 0 (0%) | 2 (67%) |
| Gastrointestinal CRNMB | 1 (33%) | 0 (0%) | 1 (33%) |
Including events occurring within 100 days after ALL diagnosis.
LE, lower extremity; PE, pulmonary embolism; UE, upper extremity.
D-dimer level at index: high, ≥4 µg/mL FEU; low to moderate, <4 µg/mL FEU.
DVTs in the UE that were line associated.
Ischemic strokes were diagnosed radiographically (n = 3) or clinically (n = 1).
Cumulative incidence at 100 days of venous or arterial thrombosis stratified for D-dimer levels. Cumulative incidence and 95% CIs (indicated by shaded areas) of arterial or venous thrombosis throughout 100-day follow-up, stratified for index D-dimer levels. The dashed red line represents high D-dimer levels (≥4 µg/mL), and the solid blue line indicates low to moderate D-dimer levels (<4 µg/mL).
Cumulative incidence at 100 days of venous or arterial thrombosis stratified for D-dimer levels. Cumulative incidence and 95% CIs (indicated by shaded areas) of arterial or venous thrombosis throughout 100-day follow-up, stratified for index D-dimer levels. The dashed red line represents high D-dimer levels (≥4 µg/mL), and the solid blue line indicates low to moderate D-dimer levels (<4 µg/mL).
Three patients with prior venous or arterial thrombosis at a median of 5 years (range, 3-7 years) before study index, and one who received prophylactic-dose anticoagulation postindex (all in the low to moderate D-dimer group), were excluded in a sensitivity analysis. The cumulative incidence of thrombosis in the high D-dimer group in this analysis was 52.9% (95% CI, 26.4-73.8; n = 9 of 17) compared with 10.0% (95% CI, 3.1-21.7%; n = 4 of 40) in patients with low to moderate D-dimer (HR, 7.54; 95% CI, 2.31-24.58), in line with the full analysis. An additional sensitivity analysis excluding 3 patients (2 in the low to moderate D-dimer group; one in the high D-dimer group) who had D-dimer measured on the same day as the thrombotic event was also consistent with the primary analysis. In this analysis, the cumulative incidence in the high D-dimer group was 50.0% (95% CI, 23.4-71.9; n = 8 of 16) compared with 9.7% (95% CI, 3.0-21.0; n = 4 of 42) in patients with low to moderate D-dimer (HR, 7.17; 95% CI, 2.15-23.88).
An analysis of the secondary thrombotic outcome excluding upper extremity DVTs produced similar results to our original analysis, with a cumulative incidence of 35.3% (13.8%-57.8%) in the high D-dimer group vs 4.6% (0.8%-13.8%) in the low to moderate group (HR, 8.86; 95% CI, 1.79-43.94).
Risk factors for thrombosis.
High D-dimer levels at index (compared with low to moderate) were associated with arterial or venous thrombosis (odds ratio, 7.12; 95% CI, 1.97-25.7). When testing for other confounders in a series of bivariate logistic regression models, the association between D-dimer and thrombosis remained after adjusting for BMI, age, sex, asparaginase treatment, DIC score, initial platelet level, and ALL phenotype (supplemental Table 1). Analysis of D-dimer as a continuous variable (univariable analysis) indicated that the odds of thrombosis during the 100-day study period increased by 1.65 per every 3 µg/mL increase in D-dimer level (95% CI, 1.20-2.27).
ROC analysis of various D-dimer levels in association with thrombosis is shown in Figure 2, with an area under the curve of 0.80 (95% CI, 0.67-0.92) indicating good discrimination. Youden’s index identified a D-dimer level of 2.45 µg/mL as the optimal cutoff with an 80% sensitivity and 70% specificity. The predictive value of different D-dimer levels for arterial or venous thrombosis is shown in supplemental Table 2.
ROC curve of D-dimer for predicting arterial or venous thrombosis. This ROC curve shows the performance (sensitivity and specificity) of various D-dimer cutoffs in predicting arterial or venous thrombosis during the 100 days after diagnosis of ALL. Each data point in the ROC curve represents a different D-dimer level (micrograms per milliliter) taken adjacent to the diagnosis of ALL (ie, study index).
ROC curve of D-dimer for predicting arterial or venous thrombosis. This ROC curve shows the performance (sensitivity and specificity) of various D-dimer cutoffs in predicting arterial or venous thrombosis during the 100 days after diagnosis of ALL. Each data point in the ROC curve represents a different D-dimer level (micrograms per milliliter) taken adjacent to the diagnosis of ALL (ie, study index).
Bleeding at 100 days
CRNMB occurred in 3 patients (5%) during the first 100 days after diagnosis, all 3 in the low to moderate D-dimer group (7%). There were no major bleeding events. CRNMB included gastrointestinal (n = 1) and genitourinary (n = 2) bleeds as detailed in Table 2.
Mortality
Mortality was low during the follow-up period, with only 1 death on day 35 (2%) and 1 patient who transitioned to hospice care on day 33 (2%) with timing of death unknown; both were in the low to moderate D-dimer group. Neither of these patients experienced known thrombotic or bleeding events.
Discussion
In this single-center retrospective cohort study of adults with newly diagnosed ALL receiving induction therapy, high D-dimer levels (≥4 µg/mL FEU) at ALL diagnosis were associated with a 5-fold higher risk of venous or arterial thrombosis at 100 days, compared with low to moderate D-dimer levels (<4 µg/mL). Increasing D-dimer level, as a continuous variable, was also associated with thrombosis. The association between D-dimer level at index and 100-day incidence of thrombosis remained after adjusting for age, sex, BMI, ALL phenotype, asparaginase treatment, index platelet count, and DIC score in bivariate models. These results suggest that D-dimer levels at ALL diagnosis may be useful in identifying patients with a high risk of arterial or venous thrombosis.
Although multiple studies have evaluated factors associated with thrombosis in patients with acute leukemia, validated disease-specific risk prediction models are lacking. Al-Ani et al20 derived and internally validated a risk prediction model for thrombosis in AML and ALL based on medical history of thrombosis, type of leukemia (AML vs ALL), and platelet count at diagnosis (above vs below 50 × 109/L). This study did not examine D-dimer, and the majority of patients enrolled had AML.
To the best of our knowledge, this study is the first to evaluate the association between D-dimer and thrombosis in adults with ALL. Prior work showed that D-dimer is associated with arterial or venous thrombosis in patients with AML, after adjusting for confounders such as age, sex, AML phenotype, karyotype, cell counts at diagnosis, and treatment regimen.14 The authors hypothesized that the increased rate of thrombosis in the high D-dimer group suggested the presence of DIC at time of diagnosis, which previously had been overlooked in AML.14,21 In our study, D-dimer retained a trend to higher rates of thrombosis after adjusting for DIC score (which itself includes D-dimer as one of the variables). This suggests that D-dimer represents the most important component of the DIC score in this context.
The small sample size precluded a multivariable analysis accounting for other potential risk factors of thrombosis. We were, however, able to show that D-dimer maintained predictive value after accounting for individual confounders, none of which was associated with thrombosis, in bivariate analysis. In previous studies, these risk factors have shown mixed results in terms of association with thrombosis in ALL. BMI was associated with thrombosis in some studies6,10 and not in others,5 whereas older age was frequently found to be a significant risk factor in most5,6,22 but not all studies.3 Asparaginase treatment has been consistently established to be associated with increased risk of thrombosis.4,5 Future ALL-based studies should use D-dimer together with other previously identified thrombosis risk factors as well as additional candidates (eg infection, immobilization, and disease burden) to generate a risk assessment model.
In our study, the cumulative incidence of thrombosis at 100 days was 52.9% in the high D-dimer group (representing 28% of all patients) and 13.8% in the low to moderate group, which is in the same order of magnitude as incidence rates in prior studies of adults with ALL.4,6,23 Most thrombotic events in our study were venous (73%) and occurred during the first month of therapy. This correlates with induction chemotherapy, which can include asparaginase, possibly explaining the burden of thrombosis early on and highlighting the importance of addressing the question of thromboprophylaxis, particularly in the beginning of ALL treatment. This is in line with prior studies such as the GRAALL-2005 study in which incidence of thrombosis during intensive chemotherapy was 16%,1,6 similar to our findings.
Bleeding events were rare in this cohort, with none occurring in the high D-dimer group. Accordingly, definitive conclusions regarding bleeding risk and D-dimer level cannot be drawn from this study and should be assessed in future work. Nonetheless, these results generate the hypothesis that D-dimer can be used to predict thrombotic events without identifying elevated bleeding risk.
The clinical implications of identifying ALL patients at high risk of thrombosis are that such patients may warrant thromboprophylaxis. In current clinical practice, the ISTH has made general recommendations on anticoagulation prophylaxis in ALL, suggesting low-molecular-weight heparin prophylaxis during induction that includes asparaginase. However, these recommendations are based on limited data, and questions remain regarding the specifics of anticoagulation dosing and use of antithrombin concentrates. Accordingly, many centers use their own institutional guidelines.22,24 Given the ongoing uncertainty about the use of anticoagulation during induction in terms of risk of bleeding vs benefit of preventing thrombosis, a more reliable method of risk-stratifying patients is needed.
We used a cutoff of D-dimer ≥4 µg/mL based on the study of Libourel et al14 in AML, which found a significant association between D-dimer and thrombosis without bleeding risk at that level. In the current study, we found associations between different D-dimer levels and thrombosis, with a 2.45 µg/mL cutoff showing the optimal balance between sensitivity and specificity. Future studies designed to identify candidates for intensification of thromboprophylaxis may use higher cutoffs, while studies aiming to reduce use thromboprophylaxis may select lower cutoffs.
Our study has several limitations in addition to bias inherent to the retrospective study design. The sample size was limited, precluding multivariable analysis, and residual confounding may have been present. Furthermore, it is possible that high D-dimer levels may have indicated underlying undiagnosed thrombosis. This concern is somewhat reconciled by the fact that D-dimer was a routine test at ALL diagnosis and was performed in 61 of the 70 consecutive eligible patients. In any event, identifying clinically unsuspected VTE is important because incidental VTEs have similar prognosis to clinically suspected VTEs.25 Finally, D-dimer may be a surrogate marker of other underlying processes (eg, inflammation); however, its association with thrombosis independent of several other covariates suggests that it is a candidate predictor worth investigating further.
In conclusion, D-dimer levels at ALL diagnosis were associated with venous or arterial thrombosis at 100 days. Future studies should include D-dimer collated with other known risk factors to build a risk assessment model for thrombosis in patients newly diagnosed with ALL.
Acknowledgment
The authors thank Tzippy Shochat (Rabin Medical Center, Israel) for her help in the statistical analyses.
Authorship
Contribution: D. R. A., W. S. and A. L. wrote the study protocol, conceptualized the study, wrote the manuscript, interpreted the data, critically reviewed the manuscript and authorized the final version; and T. G. K. planned and conducted the data analysis, interpreted the data, critically reviewed and revised the manuscript and authorized the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
Author notes
Presented in abstract form at the 62nd annual meeting of the American Society of Hematology, 5-8 December 2020 (Virtual Conference).
Requests for data sharing may be submitted to the corresponding author (e-mail: avileader@yahoo.com).
The full-text version of this article contains a data supplement.