Key points
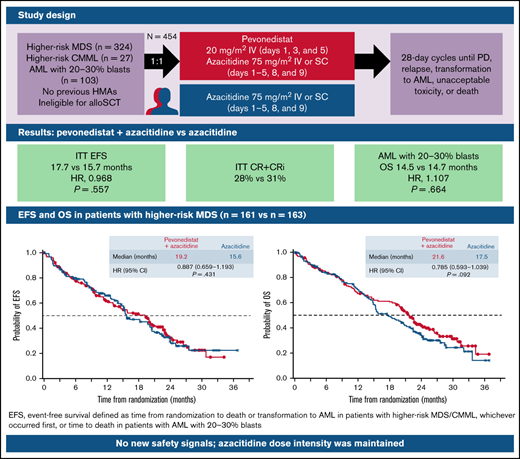
In the phase 3 PANTHER trial, EFS was similar between arms in the intent-to-treat population and in patients with higher-risk MDS.
A signal for improved overall survival for pevonedistat+azacitidine was seen in higher-risk MDS, especially with >3 treatment cycles.
Abstract
PANTHER is a global, randomized phase 3 trial of pevonedistat+azacitidine (n = 227) vs azacitidine monotherapy (n = 227) in patients with newly diagnosed higher-risk myelodysplastic syndromes (MDS; n = 324), higher-risk chronic myelomonocytic leukemia (n = 27), or acute myeloid leukemia (AML) with 20% to 30% blasts (n = 103). The primary end point was event-free survival (EFS). In the intent-to-treat population, the median EFS was 17.7 months with pevonedistat+azacitidine vs 15.7 months with azacitidine (hazard ratio [HR], 0.968; 95% confidence interval [CI], 0.757-1.238; P = .557) and in the higher-risk MDS cohort, median EFS was 19.2 vs 15.6 months (HR, 0.887; 95% CI, 0.659-1.193; P = .431). Median overall survival (OS) in the higher-risk MDS cohort was 21.6 vs 17.5 months (HR, 0.785; P = .092), and in patients with AML with 20% to 30% blasts was 14.5 vs 14.7 months (HR, 1.107; P = .664). In a post hoc analysis, median OS in the higher-risk MDS cohort for patients receiving >3 cycles was 23.8 vs 20.6 months (P = .021) and for >6 cycles was 27.1 vs 22.5 months (P = .008). No new safety signals were identified, and the azacitidine dose intensity was maintained. Common hematologic grade ≥3 treatment emergent adverse events were anemia (33% vs 34%), neutropenia (31% vs 33%), and thrombocytopenia (30% vs 30%). These results underscore the importance of large, randomized controlled trials in these heterogeneous myeloid diseases and the value of continuing therapy for >3 cycles. The trial was registered on clinicaltrials.gov as #NCT03268954.
Introduction
Myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML), and acute myeloid leukemia (AML) are bone marrow neoplasms that share some common foundational biology, clinical features, and genetic mutations and exist on a disease continuum.1-5 These conditions are characterized by cytopenia and progressive bone marrow failure, transfusion dependence, and a low chance of cure without allogeneic stem cell transplantation (allo-SCT).6,7 Patients with higher-risk MDS/CMML or AML with 20% to 30% blasts in the bone marrow are typically older, with a median age at diagnosis of ∼70 to 75 years,8,9 and usually have comorbid conditions.5,10 Consequently, only a minority of patients are considered for curative treatment with intensive chemotherapy and/or allo-SCT11,12 ; most are treated as outpatients with less-intensive, noncurative chemotherapy, primarily based on a hypomethylating agent (azacitidine, decitabine) backbone.11-14 When treated, median survival for patients with higher-risk MDS is ∼12 to 24 months, as ultimately almost all patients relapse; only recipients of allografts are apt to achieve long-term survival.4,12,14 -17 Therefore, there is an unmet need for novel agents that improve outcomes in combination with azacitidine.
Pevonedistat is a selective inhibitor of NEDD8-activating enzyme,18,19 which is required for ubiquitination and degradation of select proteins to maintain protein homeostasis.20,21 Inhibition of NEDD8-activating enzyme by pevonedistat prevents degradation of proteins affecting DNA repair, cell cycle, and cell survival pathways, thereby disrupting protein homeostasis and leading to cancer cell death.18,19,21,22 In a randomized, proof-of-concept, phase 2 study (registered on clinicaltrials.gov as #NCT02610777), pevonedistat+azacitidine demonstrated encouraging clinical efficacy vs azacitidine alone in patients with higher-risk MDS and AML with 20% to 30% blasts, with nearly double the complete remission (CR) rate, almost triple the duration of response, and improved event-free survival (EFS) in patients with higher-risk MDS.23 Pevonedistat+azacitidine had a safety profile comparable to that of azacitidine alone, with no increase in myelosuppression.23 We now report findings from a global, open-label, multicenter, randomized phase 3 trial of pevonedistat+azacitidine vs azacitidine alone in patients with newly diagnosed, higher-risk MDS/CMML or AML with 20% to 30% blasts.
Methods
Patients
Patients aged ≥18 years with morphologically confirmed higher-risk MDS, nonproliferative CMML (white blood cell count, <13 000/µL), or AML with 20% to 30% bone marrow blasts by French-American-British or World Health Organization classification24 were eligible. Patients with MDS/CMML had very high-, high-, or intermediate-risk disease according to the revised International Prognostic Scoring System (IPSS-R)25 ; patients with intermediate-risk disease according to IPSS-R (>3-4.5 points) had ≥5% bone marrow myeloblasts. Patients eligible for intensive chemotherapy and/or allo-SCT and those previously treated with chemotherapy or other antineoplastic agents, including decitabine or azacitidine, were excluded. Full eligibility criteria are in the supplemental Appendix.
Trial design and treatment
The trial was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Council for Harmonisation. The study was conducted at 141 sites in 20 countries. The study protocol was approved by local ethics committees and institutional review boards at all participating sites. All patients provided written informed consent before participation in the study. During the study, the sponsor and the trial-monitoring component of the contract research organization remained blinded to the efficacy data. The sponsor analyzed the data, and all authors had access to the primary clinical trial data.
Patients were randomized (1:1) to receive pevonedistat 20 mg/m2 via a 60 ±10-minute intravenous infusion (full details in the supplemental Appendix) on days 1, 3, and 5, with IV or subcutaneous azacitidine 75 mg/m2 on days 1 to 5, 8, and 9 (azacitidine was administered before pevonedistat on days 1, 3, and 5), or azacitidine alone in 28-day cycles. The randomization scheme was generated by an independent statistician, with treatment assignment via an Interactive Web Response System. Patients were stratified into 4 categories: very high-, high-, and intermediate-risk (IPSS-R) MDS/CMML or AML with 20% to 30% blasts. Patients received treatment until unacceptable toxicity, relapse, progressive disease (PD), or transformation to AML (defined according to World Health Organization classification as ≥20% blasts in blood or marrow and ≥50% increase in blast count from baseline).24 Patients with PD based only on blast count (without AML transformation in patients with higher-risk MDS or CMML) could continue to receive treatment if, in the judgement of the investigator, they were continuing to derive clinical benefit and if their disease had not transformed to AML.
End points and assessments
The primary end point was EFS, defined as time from randomization to death or transformation to AML (requiring ≥50% increase in blasts to ≥20% blasts) in patients with higher-risk MDS/CMML, whichever occurred first, or time to death in patients with AML with 20% to 30% blasts. The key secondary end point was overall survival (OS). Other secondary end points included time to transformation to AML in patients with higher-risk MDS/CMML; response rate and duration of response; rate and duration of independence from red blood cell (RBC) and platelet transfusions; and safety (see supplemental Appendix for end point definitions and description of changes).
Investigators assessed responses based on the updated 2006 International Working Group response criteria for MDS for patients with higher-risk MDS/CMML, and the Revised Recommendations of the International Working Group for AML for patients with AML with 20% to 30% blasts.26,27 Response and EFS (events of transformation to AML) were independently assessed by a blinded Independent Review Committee (IRC). Adverse events (AEs) were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03. Detailed assessment timing and mutational and cytogenetic analyses are described in the supplemental Appendix.
Statistical analysis
Two interim analyses (IAs) and a final analysis (FA) were planned. At the prespecified IA2, for the primary end point of EFS (n = 147 events; higher-risk MDS), ∼202 OS events had also occurred at the FA for the key secondary end point of OS, and so IA2/FA were combined into the single analysis reported herein (data cutoff: 28 May 2021). EFS and OS were designed to be tested sequentially in the intent-to-treat (ITT) population (patient populations are defined in the supplemental Appendix) and higher-risk MDS cohort using separate hierarchical testing procedures with a total 1-sided α = .025 for each procedure, with subsequent testing in the AML with cohorts with 20% to 30% blasts. The Cui-Hung-Wang weighted log-rank test statistic was used to maintain a strong control of type 1 error, and an unadjusted stratified Cox model was used to estimate hazard ratios (HRs) and confidence intervals (CIs). The total number of patients (N = 450) was calculated based on maintaining an 83% power to test a difference in OS of ∼12 months in patients with higher-risk MDS at a 1-sided α = .025, as well as to ensure sufficient representation of patients with AML with 20% to 30% blasts. The study was also adequately powered to test EFS in the ITT population and in the higher-risk MDS cohort, as well as to test OS in the ITT population. See the supplemental Appendix and supplemental Figure 1 for a detailed description of the statistical cascade, assumptions for determining sample size, and re-estimation of event size. Because EFS differences were not significant, no statistical testing could be performed on secondary end points, and the P-values provided are descriptive only.
Results
Patients
Between 18 December 2017 and 2 December 2019, 454 patients were enrolled, 324 patients with higher-risk MDS (n = 161 pevonedistat+azacitidine; n = 163 azacitidine), 103 patients with AML with 20% to 30% blasts (n = 50 pevonedistat+azacitidine; n = 53 azacitidine), and 27 patients with higher-risk CMML (n = 16 pevonedistat+azacitidine; n = 11 azacitidine; supplemental Figure 2). At data cutoff, 31 patients in the pevonedistat+azacitidine arm and 23 patients in the azacitidine arm continued receiving study treatments.
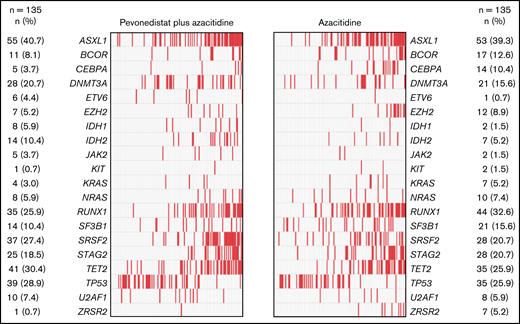
Baseline patient demographics and disease characteristics were generally well balanced between treatment arms. For patients with higher-risk MDS, median age was 73 in the pevonedistat+azacitidine arm and 74 years in the azacitidine arm, 58% and 63%, respectively, were male and 42% and 37% were female, and there was an equal distribution of patients across the IPSS-R risk categories per stratification (Table 1). There was also a fairly balanced distribution of poor prognostic and frequently mutated genes between study arms, the most common being ASXL1 (41% and 39%, respectively), RUNX1 (26% and 33%), SRFS2 (27% and 21%), STAG2 (19% and 21%), TET2 (30% and 26%), and TP53 (29% and 26%) (Figure 1). Of the patients with AML with 20% to 30% blasts, a lower proportion treated with pevonedistat+azacitidine had secondary disease (36% vs 51%), adverse European LeukemiaNet risk classification (60% vs 70%), and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 2 (14% vs 25%) vs those treated with azacitidine alone (Table 1).
Patient demographics and baseline characteristics
| . | Pevonedistat+ azacitidine n = 227 . | Azacitidine alone n = 227 . |
|---|---|---|
| Higher-risk MDS | n = 161 | n = 163 |
| Median age, years (range) | 73 (36-87) | 74 (41-92) |
| Male/female, n (%) | 93 (58)/68 (42) | 103 (63)/60 (37) |
| Disease type, n (%) | ||
| De novo | 150 (93) | 154 (94) |
| Secondary | 9 (6) | 9 (6) |
| Missing/unknown | 2 (1) | 0 |
| WHO tumor classification, n (%) | ||
| Refractory anemia with excess blasts-1 | 50 (31) | 52 (32) |
| Refractory anemia with excess blasts-2 | 63 (39) | 77 (47) |
| CMML-2 | 1 (<1) | 2 (1) |
| Not available | 2 (1) | 1 (<1) |
| Missing data | 45 (28) | 31 (19) |
| IPSS-R category, n (%) | ||
| Intermediate risk | 38 (24) | 41 (25) |
| High risk | 60 (37) | 64 (39) |
| Very high risk | 63 (39) | 58 (36) |
| ECOG PS, n (%) | ||
| 0-1 | 145 (90) | 143 (88) |
| 2 | 14 (9) | 20 (12) |
| Missing data | 2 (1) | 0 |
| TP53 mutation yes/no, n (%) | 39 (24)/122 (76) | 35 (21)/128 (79) |
| Median modified Charlson Comorbidity Index, (range) | 1 (0-8) | 1 (0-9) |
| Median time from initial diagnosis, months (range) | 1.31 (0-98.7) | 1.61 (0.1-149.7) |
| Higher-risk CMML | n = 16 | n = 11 |
| Median age, years (range) | 69.5 (60-86) | 70 (61-85) |
| Male/female, n (%) | 10 (63)/6 (38) | 8 (73)/3 (27) |
| Disease type, n (%) | ||
| De novo | 15 (94) | 9 (82) |
| Secondary | 1 (6) | 2 (18) |
| WHO tumor classification, n (%) | ||
| CMML-1 | 12 (75) | 2 (18) |
| CMML-2 | 1 (6) | 6 (55) |
| Refractory anemia with excess blasts-1 | 1 (6) | 0 |
| Missing data | 2 (13) | 1 (9) |
| IPSS-R category, n (%) | ||
| Intermediate risk | 7 (44) | 3 (27) |
| High risk | 8 (50) | 4 (36) |
| Very high risk | 1 (6) | 4 (36) |
| ECOG PS, n (%) | ||
| 0-1 | 15 (94) | 9 (82) |
| 2 | 1 (6) | 2 (18) |
| TP53 mutation yes/no, n (%) | 2 (13)/14 (88) | 1 (9)/10 (91) |
| Median modified Charlson Comorbidity Index, (range) | 0 (0-6) | 2 (0-5) |
| Median time from initial diagnosis, months (range) | 4.04 (0.4-25.5) | 1.71 (0.3-55.7) |
| AML 20-30% blasts | n = 50 | n = 53 |
| Median age, years (range) | 75 (36-92) | 75 (54-92) |
| Male/female, n (%) | 29 (58)/21 (42) | 31 (58)/22 (42) |
| Disease type, n (%) | ||
| De novo | 31 (62) | 26 (49) |
| Secondary | 18 (36) | 27 (51) |
| Unknown | 1 (2) | 0 |
| Revised WHO classification, n (%) | ||
| AML with recurrent genetic abnormalities | 0 | 3 (6) |
| AML with myelodysplasia-related changes | 33 (66) | 38 (72) |
| Therapy-related AML | 1 (2) | 0 |
| AML not otherwise specified | 14 (28) | 11 (21) |
| Other | 2 (4) | 1 (2) |
| European LeukemiaNet risk classification, n (%) | ||
| Adverse | 30 (60) | 37 (70) |
| Intermediate | 1 (2) | 2 (4) |
| Indeterminate | 10 (20) | 8 (15) |
| Missing data | 9 (18) | 6 (11) |
| ECOG PS, n (%) | ||
| 0-1 | 43 (86) | 39 (74) |
| 2 | 7 (14) | 13 (25) |
| Missing data | 0 | 1 (2) |
| TP53 mutation yes/no, n (%) | 7 (14)/43 (86) | 11 (21)/42 (79) |
| Median time from initial diagnosis, months (range) | 0.72 (0.1-5.3) | 0.76 (0.1-10.7) |
| . | Pevonedistat+ azacitidine n = 227 . | Azacitidine alone n = 227 . |
|---|---|---|
| Higher-risk MDS | n = 161 | n = 163 |
| Median age, years (range) | 73 (36-87) | 74 (41-92) |
| Male/female, n (%) | 93 (58)/68 (42) | 103 (63)/60 (37) |
| Disease type, n (%) | ||
| De novo | 150 (93) | 154 (94) |
| Secondary | 9 (6) | 9 (6) |
| Missing/unknown | 2 (1) | 0 |
| WHO tumor classification, n (%) | ||
| Refractory anemia with excess blasts-1 | 50 (31) | 52 (32) |
| Refractory anemia with excess blasts-2 | 63 (39) | 77 (47) |
| CMML-2 | 1 (<1) | 2 (1) |
| Not available | 2 (1) | 1 (<1) |
| Missing data | 45 (28) | 31 (19) |
| IPSS-R category, n (%) | ||
| Intermediate risk | 38 (24) | 41 (25) |
| High risk | 60 (37) | 64 (39) |
| Very high risk | 63 (39) | 58 (36) |
| ECOG PS, n (%) | ||
| 0-1 | 145 (90) | 143 (88) |
| 2 | 14 (9) | 20 (12) |
| Missing data | 2 (1) | 0 |
| TP53 mutation yes/no, n (%) | 39 (24)/122 (76) | 35 (21)/128 (79) |
| Median modified Charlson Comorbidity Index, (range) | 1 (0-8) | 1 (0-9) |
| Median time from initial diagnosis, months (range) | 1.31 (0-98.7) | 1.61 (0.1-149.7) |
| Higher-risk CMML | n = 16 | n = 11 |
| Median age, years (range) | 69.5 (60-86) | 70 (61-85) |
| Male/female, n (%) | 10 (63)/6 (38) | 8 (73)/3 (27) |
| Disease type, n (%) | ||
| De novo | 15 (94) | 9 (82) |
| Secondary | 1 (6) | 2 (18) |
| WHO tumor classification, n (%) | ||
| CMML-1 | 12 (75) | 2 (18) |
| CMML-2 | 1 (6) | 6 (55) |
| Refractory anemia with excess blasts-1 | 1 (6) | 0 |
| Missing data | 2 (13) | 1 (9) |
| IPSS-R category, n (%) | ||
| Intermediate risk | 7 (44) | 3 (27) |
| High risk | 8 (50) | 4 (36) |
| Very high risk | 1 (6) | 4 (36) |
| ECOG PS, n (%) | ||
| 0-1 | 15 (94) | 9 (82) |
| 2 | 1 (6) | 2 (18) |
| TP53 mutation yes/no, n (%) | 2 (13)/14 (88) | 1 (9)/10 (91) |
| Median modified Charlson Comorbidity Index, (range) | 0 (0-6) | 2 (0-5) |
| Median time from initial diagnosis, months (range) | 4.04 (0.4-25.5) | 1.71 (0.3-55.7) |
| AML 20-30% blasts | n = 50 | n = 53 |
| Median age, years (range) | 75 (36-92) | 75 (54-92) |
| Male/female, n (%) | 29 (58)/21 (42) | 31 (58)/22 (42) |
| Disease type, n (%) | ||
| De novo | 31 (62) | 26 (49) |
| Secondary | 18 (36) | 27 (51) |
| Unknown | 1 (2) | 0 |
| Revised WHO classification, n (%) | ||
| AML with recurrent genetic abnormalities | 0 | 3 (6) |
| AML with myelodysplasia-related changes | 33 (66) | 38 (72) |
| Therapy-related AML | 1 (2) | 0 |
| AML not otherwise specified | 14 (28) | 11 (21) |
| Other | 2 (4) | 1 (2) |
| European LeukemiaNet risk classification, n (%) | ||
| Adverse | 30 (60) | 37 (70) |
| Intermediate | 1 (2) | 2 (4) |
| Indeterminate | 10 (20) | 8 (15) |
| Missing data | 9 (18) | 6 (11) |
| ECOG PS, n (%) | ||
| 0-1 | 43 (86) | 39 (74) |
| 2 | 7 (14) | 13 (25) |
| Missing data | 0 | 1 (2) |
| TP53 mutation yes/no, n (%) | 7 (14)/43 (86) | 11 (21)/42 (79) |
| Median time from initial diagnosis, months (range) | 0.72 (0.1-5.3) | 0.76 (0.1-10.7) |
WHO, World Health Organization.
Distribution of poor prognostic and frequently mutated genes in patients with higher-risk MDS by treatment arm. Mutational analysis was conducted on 270 bone marrow aspirate samples collected at screening (n = 135 samples from each treatment arm).
Distribution of poor prognostic and frequently mutated genes in patients with higher-risk MDS by treatment arm. Mutational analysis was conducted on 270 bone marrow aspirate samples collected at screening (n = 135 samples from each treatment arm).
Efficacy
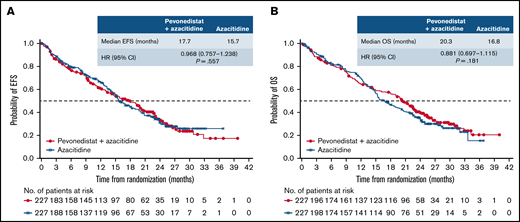
The median follow-up in the ITT population was 26.2 months in the pevonedistat+azacitidine arm and 25.7 months in the azacitidine-alone arm. In the ITT population, median EFS was 17.7 months with pevonedistat+azacitidine vs 15.7 months with azacitidine (HR, 0.968; 95% CI, 0.757-1.238; P = .557; Figure 2A); 97 vs 94 patients had died, and 38 vs 32 had AML transformation in the pevonedistat+azacitidine and azacitidine arms, respectively. Median OS was 20.3 months with pevonedistat+azacitidine vs 16.8 months with azacitidine (HR, 0.881; 95% CI, 0.697-1.115; P = .181; Figure 2B).
EFS and OS in the ITT population. Kaplan-Meier curve of EFS (A) and Kaplan-Meier curve of OS (B). ITT, intent-to-treat.
EFS and OS in the ITT population. Kaplan-Meier curve of EFS (A) and Kaplan-Meier curve of OS (B). ITT, intent-to-treat.
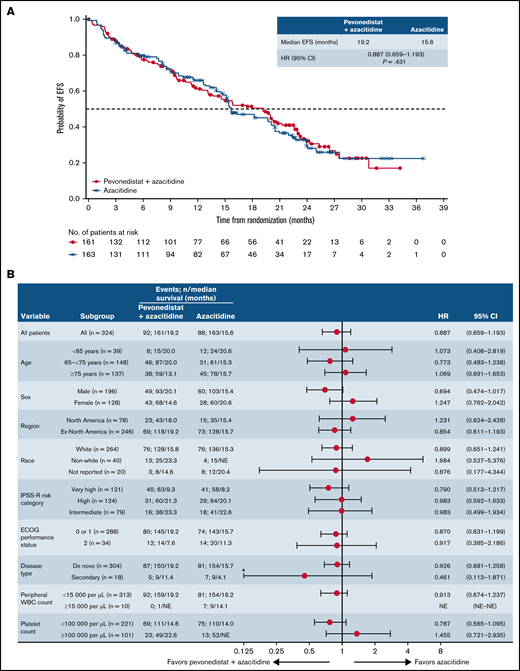
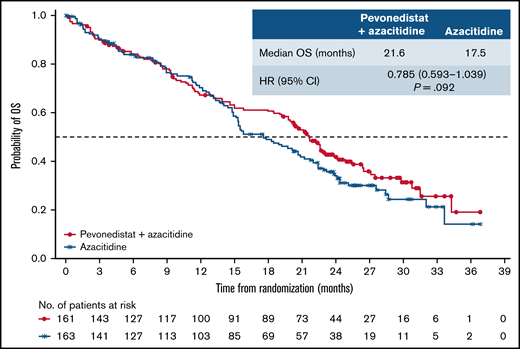
In the higher-risk MDS cohort, median EFS was 19.2 months with pevonedistat+azacitidine vs 15.6 months with azacitidine alone (HR, 0.887; 95% CI, 0.659-1.193; P = .431; Figure 3A); 55 vs 57 patients had died and 37 vs 31 had AML transformation, in the pevonedistat+azacitidine and azacitidine-alone arms, respectively. In an analysis of EFS by prespecified subgroups in the higher-risk MDS population, most subgroups had HRs close to 1, except for patients aged <65 years, male patients, and those with secondary disease, in which the HRs favored pevonedistat+azacitidine (Figure 3B). Subgroup analyses of EFS and OS by IPSS-R cytogenetic risk subgroups based on IRC assessment in patients with higher-risk MDS showed no significant difference between study arms (supplemental Figure 3). In patients with higher-risk MDS, median OS was numerically longer at 21.6 months with pevonedistat+azacitidine vs 17.5 months with azacitidine (HR, 0.785; 95% CI, 0.593-1.039; P = .092; Figure 4). The proportion of patients receiving subsequent therapies was generally well balanced between treatment arms in patients with higher-risk MDS (29% vs 23% received ≥1 subsequent antineoplastic therapy): 10 patients receiving pevonedistat+azacitidine and 11 patients receiving azacitidine alone received subsequent venetoclax-containing therapy; 8 patients treated with pevonedistat+azacitidine and 12 patients with azacitidine alone received a transplant after discontinuing study treatment. In a sensitivity analysis in which patients who received a transplant were censored, median OS was consistent with the ITT analysis, with medians of 21.6 months with pevonedistat+azacitidine vs 17.7 months with azacitidine (P = .092), respectively.
EFS in the higher-risk MDS cohort. Kaplan-Meier curve of EFS (A) and forest plot of subgroup analysis of EFS (B). *Lower confidence interval is truncated at 0.125. NE, not estimable.
EFS in the higher-risk MDS cohort. Kaplan-Meier curve of EFS (A) and forest plot of subgroup analysis of EFS (B). *Lower confidence interval is truncated at 0.125. NE, not estimable.
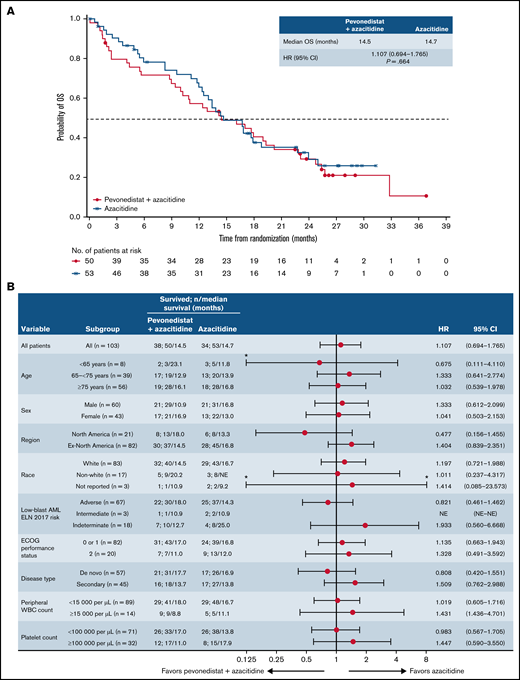
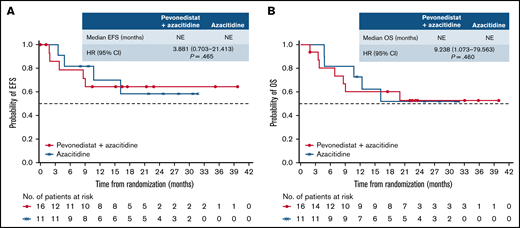
There was no difference in OS between arms in patients with AML with 20% to 30% blasts (HR, 1.107; 95% CI, 0.694-1.765; P = .664; median, 14.5 vs 14.7 months), which was reflected across patient subgroups (Figure 5). In patients with higher-risk CMML, the Kaplan-Meier curves for EFS and OS were similar between treatment arms, albeit with a small number of patients (Figure 6).
OS in patients with AML with 20% to 30% blasts. Kaplan-Meier curve of OS (A) and forest plot of subgroup analysis of OS (B). *Upper confidence interval is truncated at 8 and lower confidence interval is truncated at 0.125. ELN, European LeukemiaNet; NE, not estimable; WBC, white blood cell.
OS in patients with AML with 20% to 30% blasts. Kaplan-Meier curve of OS (A) and forest plot of subgroup analysis of OS (B). *Upper confidence interval is truncated at 8 and lower confidence interval is truncated at 0.125. ELN, European LeukemiaNet; NE, not estimable; WBC, white blood cell.
EFS and OS in patients with higher-risk CMML. Kaplan-Meier curve of EFS (A) and Kaplan-Meier curve of OS (B). NE, not estimable.
EFS and OS in patients with higher-risk CMML. Kaplan-Meier curve of EFS (A) and Kaplan-Meier curve of OS (B). NE, not estimable.
Overall response rates (ORR) were similar between treatment arms overall and across disease cohorts. The ORR (CR+CR with incomplete blood count recovery [CRi]+partial response [PR]) by IRC assessment for the ITT population was 28% with pevonedistat+azacitidine vs 32% with azacitidine alone. The ORR by investigator assessment was consistent with that of IRC assessment, at 32% in both treatment arms. Table 2 shows response rates, time to first response, and duration of response by disease subtype. Of the patients with higher-risk MDS, 39 (24%) achieved a CR in the pevonedistat+azacitidine arm and 52 (32%) achieved a CR in the azacitidine arm. The median time to first CR or hematologic improvement was 2.89 vs 2.92 months, and the median duration of CR was 17.1 vs 14.1 months. The ORR (CR+PR) in patients with TP53 mutations, with 17p deletions, or who were determined to be in the adverse cytogenetic risk group was 25% (13 of 52) with pevonedistat+azacitidine vs 28% (14 of 50) with azacitidine alone. In patients with higher-risk CMML, the ORR (CR+PR) was 44% vs 36%, and in patients with AML with 20% to 30% blasts, the ORR (CR+CRi+PR) was 36% vs 32% with pevonedistat+azacitidine vs azacitidine alone, respectively.
Objective response rates by IRC assessment, time to response, and DOR in patients with higher-risk MDS, CMML, or AML with 20-30% blasts
| . | Pevonedistat + azacitidine . | Azacitidine alone . |
|---|---|---|
| Higher-risk MDS | n = 161 | n = 163 |
| Objective response (CR+PR) | 39 (24) | 52 (32) |
| Objective response 2 (CR+PR+HI) | 69 (43) | 70 (43) |
| CR | 39 (24) | 52 (32) |
| mCR | 37 (23) | 32 (20) |
| PR | 0 | 0 |
| HI | 30 (19) | 18 (11) |
| Median time to first CR/PR/HI, months (range) | 2.89 (1.0-20.3) | 2.92 (1.0-13.5) |
| Median duration of ORR, months (95% CI) | 17.1 (10.71-NE) | 14.1 (7.16-29.08) |
| Median duration of ORR2, months (95% CI) | 18.9 (14.98-NE) | 18.3 (11.17-30.92) |
| Median duration of CR, months (95% CI) | 17.1 (10.71-NE) | 14.1 (6.93-29.08) |
| Patients with TP53 mutation/17p deletion/adverse-risk cytogenetics | 52 (32) | 50 (31) |
| ORR in patients with TP53 mutation/17p deletion/adverse-risk cytogenetics | 13 (25) | 14 (28) |
| Higher-risk CMML | n = 16 | n = 11 |
| Objective response (CR+PR) | 7 (44) | 4 (36) |
| Objective response 2 (CR+PR+HI) | 7 (44) | 7 (64) |
| CR | 7 (44) | 4 (36) |
| PR | 0 | 0 |
| HI | 0 | 3 (27) |
| Median time to first CR/PR/HI, months (range) | 2.63 (0.9-12.2) | 1.74 (0.9-10.4) |
| Median duration of ORR, months (95% CI) | 22.6 (3.94-NE) | NE (1.18-NE) |
| Median duration of ORR2, months (95% CI) | 29.0 (4.86-NE) | NE (1.18-NE) |
| Median duration of CR, months (95% CI) | 22.6 (3.94-NE) | NE (1.18-NE) |
| AML with 20-30% blasts | n = 50 | n = 53 |
| Objective response (CR+CRi+PR) | 18 (36) | 17 (32) |
| CR | 11 (22) | 12 (23) |
| CRi | 6 (12) | 3 (6) |
| PR | 1 (2) | 2 (4) |
| Median time to first CR/CRi/PR, months (95% CI) | 24.6 (4.80-NE) | NE (8.25-NE) |
| Median duration of CR, months (95% CI) | 15.3 (10.18-NE) | 8.0 (1.74-NE) |
| Median duration of CR/CRi, months (95% CI) | 15.0 (3.94-NE) | 8.5 (3.02-NE) |
| . | Pevonedistat + azacitidine . | Azacitidine alone . |
|---|---|---|
| Higher-risk MDS | n = 161 | n = 163 |
| Objective response (CR+PR) | 39 (24) | 52 (32) |
| Objective response 2 (CR+PR+HI) | 69 (43) | 70 (43) |
| CR | 39 (24) | 52 (32) |
| mCR | 37 (23) | 32 (20) |
| PR | 0 | 0 |
| HI | 30 (19) | 18 (11) |
| Median time to first CR/PR/HI, months (range) | 2.89 (1.0-20.3) | 2.92 (1.0-13.5) |
| Median duration of ORR, months (95% CI) | 17.1 (10.71-NE) | 14.1 (7.16-29.08) |
| Median duration of ORR2, months (95% CI) | 18.9 (14.98-NE) | 18.3 (11.17-30.92) |
| Median duration of CR, months (95% CI) | 17.1 (10.71-NE) | 14.1 (6.93-29.08) |
| Patients with TP53 mutation/17p deletion/adverse-risk cytogenetics | 52 (32) | 50 (31) |
| ORR in patients with TP53 mutation/17p deletion/adverse-risk cytogenetics | 13 (25) | 14 (28) |
| Higher-risk CMML | n = 16 | n = 11 |
| Objective response (CR+PR) | 7 (44) | 4 (36) |
| Objective response 2 (CR+PR+HI) | 7 (44) | 7 (64) |
| CR | 7 (44) | 4 (36) |
| PR | 0 | 0 |
| HI | 0 | 3 (27) |
| Median time to first CR/PR/HI, months (range) | 2.63 (0.9-12.2) | 1.74 (0.9-10.4) |
| Median duration of ORR, months (95% CI) | 22.6 (3.94-NE) | NE (1.18-NE) |
| Median duration of ORR2, months (95% CI) | 29.0 (4.86-NE) | NE (1.18-NE) |
| Median duration of CR, months (95% CI) | 22.6 (3.94-NE) | NE (1.18-NE) |
| AML with 20-30% blasts | n = 50 | n = 53 |
| Objective response (CR+CRi+PR) | 18 (36) | 17 (32) |
| CR | 11 (22) | 12 (23) |
| CRi | 6 (12) | 3 (6) |
| PR | 1 (2) | 2 (4) |
| Median time to first CR/CRi/PR, months (95% CI) | 24.6 (4.80-NE) | NE (8.25-NE) |
| Median duration of CR, months (95% CI) | 15.3 (10.18-NE) | 8.0 (1.74-NE) |
| Median duration of CR/CRi, months (95% CI) | 15.0 (3.94-NE) | 8.5 (3.02-NE) |
Data are expressed as n (%), unless stated otherwise.
ORR was defined as CR+PR in higher-risk MDS/CMML and as CR+CRi+PR in AML with 20% to 30% blasts. ORR2 was defined as CR+PR+HI in higher-risk MDS/CMML and as CR+CRi+PR in patients with AML with 20% to 30% blasts.
DOR, duration of response; HI, hematologic improvement; mCR, marrow CR; NE, not estimable.
For patients with higher-risk MDS, median time to transformation to AML was not estimable with pevonedistat+azacitidine vs 35.6 months with azacitidine alone (HR, 1.037; 95% CI, 0.660-1.630). Among 129 and 140 patients in the pevonedistat+azacitidine and azacitidine-alone arms of the ITT population who were RBC/platelet transfusion–dependent at baseline, 59 (46%) and 63 (45%), respectively, achieved RBC/platelet transfusion independence (relative risk ratio, 1.02; 95% CI, 0.78-1.32; P = .899); the median duration of transfusion independence was 13.5 (95% CI, 10.38-16.66) and 12.0 (95% CI, 8.90-19.48) months, respectively. In the higher-risk MDS cohort, 39 of 86 patients (45%) and 46 of 101 patients (46%) in the pevonedistat+azacitidine and azacitidine-alone arms, respectively, achieved transfusion independence (relative risk ratio, 1.00; 95% CI, 0.73-1.36; P = .966), with a median duration of transfusion independence of 12.5 (95% CI, 10.25-21.55) and 12.0 (95% CI, 8.90-19.48) months, respectively.
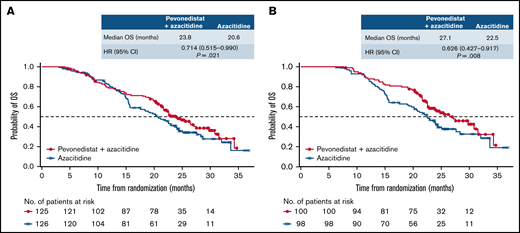
When we analyzed OS in patients with higher-risk MDS by number of cycles received, there was a late separation of the Kaplan-Meier curves in those who had received >3 cycles in favor of pevonedistat+azacitidine vs azacitidine alone (HR, 0.714; 95% CI, 0.515-0.990; P = .021; median 23.8 vs 20.6 months), and this difference was more pronounced in patients who received >6 cycles (HR, 0.626; 95% CI, 0.427-0.917; P = .008; median 27.1 vs 22.5 months; Figure 7).
OS in patients with higher-risk MDS by number of cycles received. Patients who received greater than 3 cycles (A) and patients who received greater than 6 cycles (B).
OS in patients with higher-risk MDS by number of cycles received. Patients who received greater than 3 cycles (A) and patients who received greater than 6 cycles (B).
Safety
At data cutoff, study treatment had been discontinued in 86% and 90% of patients in the pevonedistat+azacitidine and azacitidine-alone arms, respectively; the primary reasons for treatment discontinuation were AEs in 27% and 23%, and PD, in 21% and 27%, respectively. The safety population comprised 223 of 227 patients (98%) who received ≥1 dose of pevonedistat+azacitidine and 220 of 227 patients (97%) who received ≥1 dose of azacitidine. Patients received a median of 9 cycles (range, 1-41) of pevonedistat+azacitidine in the combination arm, and a median of 8 cycles of azacitidine (range, 1-37) in the azacitidine-alone arm. The median pevonedistat dose intensity was 99.4%, and median azacitidine dose intensity was 97.9% in combination with pevonedistat and 98.4% alone. The safety profiles for each treatment arm are summarized in Table 3. The most common nonhematologic treatment-emergent AEs (TEAEs) were constipation in 37% and 41%, nausea in 35% and 29%, pyrexia in 26% and 29%, and diarrhea in 28% and 24%, for the pevonedistat+azacitidine and azacitidine-alone arms, respectively. Upper respiratory tract infections were reported in 9% vs 11% of patients. Common hematologic grade ≥3 TEAEs were anemia in 33% and 34%, neutropenia in 31% and 33%, thrombocytopenia in 30% and 30%, and febrile neutropenia in 23% and 20%, for the pevonedistat+azacitidine and azacitidine-alone arms, respectively. Serious TEAEs were reported in 69% vs 65% of patients in the pevonedistat+azacitidine vs azacitidine-alone arms, respectively. Frequently reported serious TEAEs were febrile neutropenia (19% vs 16%) and pneumonia (14% vs 11%). On-study deaths occurred in 17% of patients treated with pevonedistat+azacitidine vs 16% of patients treated with azacitidine.
Overall safety profile, and most common any-grade and grade ≥3 TEAEs occurring in ≥10% (any grade) or ≥10% (grade ≥3) of patients in the safety population
| . | Pevonedistat+ azacitidine n = 223 . | Azacitidine alone n = 220 . |
|---|---|---|
| Incidence of TEAEs, n (%) | ||
| Any TEAE | 221 (99) | 219 (100) |
| Any drug-related TEAE | 179 (80) | 173 (79) |
| Any grade ≥3 TEAE | 200 (90) | 191 (87) |
| Any drug-related grade ≥3 TEAE | 130 (58) | 121 (55) |
| Any serious TEAE | 153 (69) | 142 (65) |
| Any drug-related serious TEAE | 58 (26) | 50 (23) |
| TEAE leading to discontinuation, n (%) | 67 (30) | 57 (26) |
| On-study deaths, n (%) | 38 (17) | 36 (16) |
| Most common any-grade TEAEs (≥10% of patients) | ||
| Constipation | 82 (37) | 90 (41) |
| Anemia | 83 (37) | 84 (38) |
| Neutropenia | 73 (33) | 77 (35) |
| Thrombocytopenia | 74 (33) | 76 (35) |
| Nausea | 78 (35) | 64 (29) |
| Pyrexia | 58 (26) | 64 (29) |
| Diarrhea | 63 (28) | 52 (24) |
| Vomiting | 51 (23) | 46 (21) |
| Febrile neutropenia | 51 (23) | 44 (20) |
| Asthenia | 36 (16) | 42 (19) |
| Pneumonia | 39 (17) | 38 (17) |
| Fatigue | 41 (18) | 33 (15) |
| Cough | 39 (17) | 25 (11) |
| Decreased appetite | 39 (17) | 25 (11) |
| Edema peripheral | 29 (13) | 30 (14) |
| Platelet count decreased | 33 (15) | 21 (10) |
| Dyspnea | 26 (12) | 26 (12) |
| Arthralgia | 27 (12) | 24 (11) |
| Neutrophil count decreased | 33 (15) | 18 (8) |
| Hypokalemia | 29 (13) | 19 (9) |
| Headache | 26 (12) | 20 (9) |
| Most common grade ≥3 AEs (≥5% of patients) | ||
| Anemia | 73 (33) | 75 (34) |
| Neutropenia | 70 (31) | 72 (33) |
| Thrombocytopenia | 67 (30) | 66 (30) |
| Febrile neutropenia | 51 (23) | 44 (20) |
| Pneumonia | 33 (15) | 32 (15) |
| Neutrophil count decreased | 33 (15) | 17 (8) |
| Platelet count decreased | 25 (11) | 16 (7) |
| White blood cell count decreased | 11 (5) | 11 (5) |
| Leukopenia | 10 (4) | 13 (6) |
| . | Pevonedistat+ azacitidine n = 223 . | Azacitidine alone n = 220 . |
|---|---|---|
| Incidence of TEAEs, n (%) | ||
| Any TEAE | 221 (99) | 219 (100) |
| Any drug-related TEAE | 179 (80) | 173 (79) |
| Any grade ≥3 TEAE | 200 (90) | 191 (87) |
| Any drug-related grade ≥3 TEAE | 130 (58) | 121 (55) |
| Any serious TEAE | 153 (69) | 142 (65) |
| Any drug-related serious TEAE | 58 (26) | 50 (23) |
| TEAE leading to discontinuation, n (%) | 67 (30) | 57 (26) |
| On-study deaths, n (%) | 38 (17) | 36 (16) |
| Most common any-grade TEAEs (≥10% of patients) | ||
| Constipation | 82 (37) | 90 (41) |
| Anemia | 83 (37) | 84 (38) |
| Neutropenia | 73 (33) | 77 (35) |
| Thrombocytopenia | 74 (33) | 76 (35) |
| Nausea | 78 (35) | 64 (29) |
| Pyrexia | 58 (26) | 64 (29) |
| Diarrhea | 63 (28) | 52 (24) |
| Vomiting | 51 (23) | 46 (21) |
| Febrile neutropenia | 51 (23) | 44 (20) |
| Asthenia | 36 (16) | 42 (19) |
| Pneumonia | 39 (17) | 38 (17) |
| Fatigue | 41 (18) | 33 (15) |
| Cough | 39 (17) | 25 (11) |
| Decreased appetite | 39 (17) | 25 (11) |
| Edema peripheral | 29 (13) | 30 (14) |
| Platelet count decreased | 33 (15) | 21 (10) |
| Dyspnea | 26 (12) | 26 (12) |
| Arthralgia | 27 (12) | 24 (11) |
| Neutrophil count decreased | 33 (15) | 18 (8) |
| Hypokalemia | 29 (13) | 19 (9) |
| Headache | 26 (12) | 20 (9) |
| Most common grade ≥3 AEs (≥5% of patients) | ||
| Anemia | 73 (33) | 75 (34) |
| Neutropenia | 70 (31) | 72 (33) |
| Thrombocytopenia | 67 (30) | 66 (30) |
| Febrile neutropenia | 51 (23) | 44 (20) |
| Pneumonia | 33 (15) | 32 (15) |
| Neutrophil count decreased | 33 (15) | 17 (8) |
| Platelet count decreased | 25 (11) | 16 (7) |
| White blood cell count decreased | 11 (5) | 11 (5) |
| Leukopenia | 10 (4) | 13 (6) |
Discontinuations caused by TEAEs were reported in 30% vs 26% of patients in the pevonedistat+azacitidine and azacitidine-alone arms, respectively. The most common TEAEs resulting in discontinuation were septic shock (4% vs 2%) and pneumonia (4% vs 2%). Of the patients with higher-risk MDS who received ≤3 or ≤6 cycles and who discontinued treatment, the overall proportion was similar between treatment arms (22% vs 23% and 37% vs 40%, respectively), with patients mostly frequently discontinuing because of AEs (14% vs 9% and 16% vs 12%, respectively; supplemental Table 1). In an analysis of the patients with higher-risk MDS who received ≤3 cycles in the phase 2 P-2001 study, 13% of patients treated with pevonedistat+azacitidine discontinued treatment vs 23% of patients treated with azacitidine.
Discussion
For most patients with higher-risk MDS and related malignancies, the standard backbone of therapy is based on hypomethylating agents, with a typical median response duration and survival of <2 years.28 The thrust of recent clinical therapeutic interventions has been to introduce upfront combination therapies to this patient population, in an effort to improve on the modest outcomes with monotherapy.
The P-3001 phase 3 PANTHER study is one of the largest of these types of trials. In it, we were able to demonstrate the ability to maintain azacitidine dose intensity, to conduct a large international trial with rapid accrual, and to introduce a novel end point for MDS-EFS. Unfortunately, PANTHER did not meet the primary end point of EFS in the ITT population or in patients with higher-risk MDS. No new safety signals were identified, and azacitidine dose intensity was maintained. Patient baseline demographics were generally well balanced between treatment arms for patients with higher-risk MDS, including mutational profiles and IPSS-R risk categories, and there were no significant differences in subsequent therapies received.
The efficacy results from this phase 3 study did not confirm the promising results reported in the previous phase 2 study.23 Outcomes in the combination arm were similar to those previously reported, with median EFS and OS in patients with higher-risk MDS in the present study of 19.2 and 21.6 months, respectively, compared with 20.2 and 23.9 months in the phase 2 study. In the current study, it is important to note the impressive results in the control arm, among the best reported for azacitidine in patients with higher-risk MDS. Indeed, the median OS of 17.5 months with azacitidine alone is longer than has been reported in previous studies with azacitidine monotherapy, with a median of ∼15 months.28,29
Importantly, the CR rate in the azacitidine arm (32%) was also much higher than previously observed, which may be reflective of the protocol, which strongly discouraged azacitidine dose reductions or delays for hematologic toxicities during the first 6 cycles, compared with dosing or according to the azacitidine label, which recommends that patients be treated for 4 to 6 cycles, but does not specify a minimum time before reduction of the dose.30 The CR rate with azacitidine was 17% in the phase 3 study investigating azacitidine vs conventional care,31 and in the SWOG S1117 study of azacitidine+lenalidomide or vorinostat, the CR rate was 24% with azacitidine monotherapy.28
In the ITT population, EFS and OS were not significantly different between patients treated in the combination arm and those receiving monotherapy. Interestingly, absolute differences in EFS and OS in the higher-risk MDS population were similar to that in the previous phase 2 trial, with a numerically longer OS for those receiving pevonedistat+azacitidine with a difference in medians of ∼4 months. There are 2 substantive points that can be gleaned from these findings: first that EFS is a measurable and clinically meaningful end point that can be used as a primary end point and second that randomized trials in MDS should be adequately powered to show a median OS benefit of 4 months (as opposed to the ∼12 months assumed in this study). Only when such incremental benefits can be demonstrated can combination therapies be built upon to improve future outcomes.
Continuing therapy for enough cycles to show improvement is important. When we analyzed the outcomes by treatment cycle received in the phase 3 study, OS appeared to be longer with pevonedistat+azacitidine vs azacitidine alone in patients with higher-risk MDS who received >3 cycles, and the difference was more pronounced in patients who received >6 cycles, suggesting a better control of the disease, once a response is obtained. The rate of early discontinuation (patients who received ≤3) was similar in both treatment arms in the phase 3 study; however, the rate of early discontinuation of pevonedistat+azacitidine was almost double that in the phase 2 study.23 This finding may provide some explanation of the differences in some of the outcomes in the phase 2 and 3 trials, but further analyses are needed.
There have been no new therapies approved for higher-risk MDS for more than a decade, despite multiple studies combining agents with azacitidine.7 Previous studies have often not been successful because of increased toxicity or premature discontinuation of combinations impacting azacitidine dose intensity, resulting in reduced efficacy.28,32 Encouraging response rates and outcomes have been observed in early trials of investigational agents with azacitidine in patients with higher-risk MDS.33,34 In a phase 1b study of venetoclax+azacitidine, the ORR was 77%, including 42% CR35 and in a phase 1 study of magrolimab+azacitidine, the ORR was 91% with a CR rate of 42%.33 However, our results highlight the need for long-term outcomes from large, randomized, phase 3 data sets vs the relatively immature, primarily response data reported from single-arm trials. Indeed, after encouraging results in a phase 1/2b study of eprenetapopt+azacitidine in TP53-mutant MDS or AML with 20% to 30% marrow blasts,36 the phase 3 trial failed to meet its primary end point of CR rate. Although the rate was higher with eprenetapopt+azacitidine at 33% vs 22% with azacitidine alone, this did not reach statistical significance.37 Encouraging early-phase results have been reported with venetoclax+azacitidine in patients with higher-risk MDS,35 but the data from the randomized VERONA trial are awaited (trial currently ongoing, #NCT04401748).
Higher-risk MDS, CMML, and AML with 20% to 30% blasts are heterogeneous diseases, which makes them challenging to treat.1,5,7 There are also a limited number of large, randomized, trials conducted for these patients. Phase 2 trials combining hypomethylating agents with other molecules have often shown results that were described as encouraging, but that have never been confirmed in randomized trials. This is again the case with pevonedistat. The explanations for this are probably multiple and vary according to the molecules used, but they include excessive toxicity of certain drugs or increased cytopenias. What is also important to note is the great heterogeneity of MDS and the absence, for the moment, of a clear clinical or genetic definition of subgroups in which it would be possible to demonstrate efficacy of the new molecules under evaluation. This great heterogeneity leads to variability in the results of clinical trials and difficulty in reproducing results from one study to another.
In summary, despite promising phase 2 results, this phase 3 trial did not meet the prespecified primary end point, and patients with higher-risk MDS/CMML and AML with 20% to 30% blasts have yet to benefit from an azacitidine combination therapy vs the standard of care of azacitidine alone. Signals emerged for improved OS for the combination in patients with higher-risk MDS, particularly for those who continued treatment for more than 3 cycles. Thus, in future studies, our recommendation is to investigate a more homogenous higher-risk population, excluding patients with dysplastic CMML (mainly CMML-1) and AML with 20% to 30% blasts, as their diseases have a distinct biology and therapeutic sensitivity. Mutational studies are ongoing, including longitudinal clonal evolution studies to identify the effects of various treatments on specific clones and TP53 allelic status estimation and its correlation with clinical outcomes. Our data highlight the need to investigate novel therapies and combinations and the importance of conducting larger, randomized phase 3 trials. We hope that findings from this study will improve understanding of these complex diseases and help guide development of future therapies for these underserved patient populations.
Acknowledgments
The authors thank all of the patients and their families and the investigators and staff at all clinical sites for participating in the trial; Helen Wilkinson, of Ashfield MedComms, an Ashfield Health company, for medical writing support in the development of this manuscript under the direction of the authors, which was funded by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, and complied with the Good Publication Practice 3 ethical guidelines (Battisti WP, et al. Ann Intern Med. 2015,163:461-464); and Marcel Kuttab (Takeda Pharmaceuticals, U.S.A., Inc.) for editorial support.
This study was sponsored by Takeda Development Centers Inc (TDCA; Lexington, MA).
Authorship
Contribution: L.A., M.A.S., R.J.F., and D.V.F. wrote the first draft of the manuscript; and all authors conceived and/or designed the work that led to the submission, acquired the data, and/or played a key role in interpreting the results, revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work.
Conflict-of-interest disclosure: L.A. has received research funding from Celgene and Jazz and honoraria from Jazz, Takeda, Celgene/BMS, Novartis, and AbbVie. L.G. has received research funding from Astellas Pharma, Inc. M.D.C. has been a consultant to, received honoraria from, and has held membership on the Board of Directors or advisory committees of, received research funding from, and served on the speakers’ bureau of Novartis, BMS, and Takeda Oncology. D.V. has received honoraria from Celgene/BMS, Amgen, Novartis, Jazz, Janssen, Sanofi, Pfizer, MSD, Astellas, Sobi, and Takeda. N.-A.V. has received research funding from AbbVie, Pfizer, Janssen, Sanofi, Novartis, Takeda, Sandoz, BMS, Roche, Astellas, Celgene and honoraria from Janssen, Novartis, and BMS. R.D.P.A. has been a consultant to, has received honoraria from, and has held membership on the Board of Directors or advisory committees of BMS, Takeda, and Novartis. A.S. has been a consultant to and received research funding from Astellas and MSD; has received honoraria from, been a consultant to, and has held membership on the Board of Directors or advisory committees of AbbVie, Amgen, BMS, GenesisPharma, Gilead, Janssen, Novartis, Pfizer, Roche, Takeda, Sanofi/Genzyme, and Sanofi; and has received research funding from AbbVie, Amgen, BMS, GenesisPharma, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, Takeda, Sanofi/Genzyme, Demo, WinMedica. A.A. has been involved in clinical trials for AbbVie, Sanofi, Oncopeptides, GSK, Incyte, Takeda, Amgen, Janssen, Novartis, Celgene, Roche, and Astellas. U.P. has received honoraria from Celgene/BMS, Janssen, Novartis, Geron, AbbVie, and Takeda. V.S. has held membership on the Board of Directors or advisory committees of BMS/Celgene, Geron, Gilead, Menarini, Novartis, AbbVie, and Takeda and has received honoraria from BMS/Celgene and Novartis and research funding from BMS/Celgene. R.J.F. is employed by Takeda Development Center Americas, Inc and holds equity in Takeda, BMS, Baxter, Pfizer, Gilead, Teva, Viatris, Medtronics, and Zimmer Biomet Holdings Inc. Y.Y. and S.F. are employed by Takeda Development Center Americas, Inc. D.V.F. is employed by Takeda Development Center Americas, Inc; holds stock options in Viracta Therapeutics, Inc, and Briacell, Inc; and was formerly employed and held membership on the Board of Directors or advisory committees of Viracta Therapeutics, Inc. M.A.S. has held membership on the Board of Directors or advisory committees of BMS, Takeda/Millennium, and Novartis. S.K., V.A.D., D.W., and E.C.M. declare no competing financial interests.
Correspondence: Lionel Adès, INSERM u944, Hôpital Saint Louis and University of Paris, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: lionel.ades@aphp.fr.
References
Author notes
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results of the completed study, will be made available after the publication of the final study results within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after deidentification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
The full-text version of this article contains a data supplement.