TO THE EDITOR:
Over the past decade, treatment for chronic lymphocytic leukemia (CLL) has become defined by sequential regimens of targeted therapies.1,2 Currently, these targeted therapies include the inhibitors of Bruton’s tyrosine kinase (BTKi) ibrutinib and acalabrutinib, and an inhibitor of BCL2, venetoclax, which is usually administered with an anti-CD20 monoclonal antibody. Frontline studies of these agents show similar progression-free survival (PFS), and as these strategies have not yet been compared head to head in this setting, there is no clear indication for which to use first. Typically, patient choice, comorbidities, and genomic characteristics help determine which agent is preferred upfront.3-6 At relapse, limited data suggest switching classes between BTKi and venetoclax is equally appropriate.7-9
The efficacy of BTKi and venetoclax as single agents has prompted study of the two in combination.10,11 Preclinical research suggests the combination augments suppression of mitochondrial bioenergetics, and thus respiration, compared with either drug alone.12 Clinically, these combinations have produced outstanding results, with bone marrow undetectable minimal residual disease rates around 66% in the frontline setting and 36% in relapsed disease.6,13 This finding, along with the demonstrated success of venetoclax in patients resistant to ibrutinib and vice versa suggests the combination may have efficacy in patients previously treated with both single agents.7 -9,14 Given the near inevitability of disease progression with targeted therapies, the lack of effective options for patients resistant to both venetoclax and covalent BTKi,15 and need for further treatments, we performed a retrospective review of our institutional experience with combination BTKi and venetoclax in patients previously treated with each separately.
Cases of adult CLL patients treated at our institution between 1 January 2015 and 31 December 2020 were identified using a local data analytics search with the approval of the institutional review board. The study was conducted in accordance with the Declaration of Helsinki. Charts were screened for patients treated with both BTKi and venetoclax in either sequence, and subsequently treated with a combination of the two. PFS was calculated from date of combination therapy initiation to death or date disease progression as identified by International Workshop on CLL 2018 criteria; patients who were progression-free and alive at time of last contact were censored. Overall survival (OS) was calculated from date of combination therapy initiation to death due to any cause, censoring patients alive at time of last contact. Patients treated with chimeric antigen receptor T-cell therapy (CAR-T) were censored at cessation of combination therapy. Median PFS and OS were estimated using the Kaplan-Meier method.
Thirteen CLL patients were identified; characteristics can be found in Table 1. This was a very high-risk patient cohort: all were IgHV unmutated, seven (54%) had complex karyotype, defined as three or more cytogenetic abnormalities, and four (31%) had del(17p13.1) by fluorescent in situ hybridization. Of the patients with clinical next generation sequencing available for analysis, TP53 mutations were detected in three patients (27%), SF3B1 in two (20%), and NOTCH1 in one (10%) (supplemental Figure 1). Eight patients (67%) had mutations in BTK or PLCG2 associated with resistance to BTKi. BCL2 mutations were not tested in this cohort.
Patient characteristics at baseline
| Variables . | n = 13 . |
|---|---|
| Age at diagnosis, median (range) | 52 (43-70) |
| Age at combination BTKi and venetoclax initiation, median (range) | 63 (52-78) |
| Sex (%) | |
| Female | 2 (15) |
| Male | 11 (85) |
| Race | |
| White | 12 (92) |
| Other | 1 (8) |
| Total number of lines of chemo, median (range) | 8 (3-19) |
| Number of lines of chemo prior to combination start, median (range) | 6 (2-17) |
| Presence of bulky diseasea prior to combination start, n = 12b (%) | 6 (50) |
| IgHV unmutated (%) | 13 (100) |
| Stimulated karyotype complexity (%) | |
| Less than three abnormalities | 6 (46) |
| Three or more abnormalities | 7 (54) |
| FISH abnormalities (%) | |
| del(17p) | 4 (31) |
| del(11q) | 4 (31) |
| del(13q) | 2 (15) |
| Trisomy 12 | 4 (31) |
| Other FISH abnormalities | 5 (38) |
| TP53 mutation (%) | 3 (27) |
| Unknown | 2 |
| SF3B1 mutation (%) | 2 (20) |
| Unknown | 3 |
| NOTCH1 mutation (%) | 1 (10) |
| Unknown | 3 |
| BTKi mutation (%) | 8 (67) |
| Unknown | 1 |
| PLCG2 hotspot mutation (%) | 2 (17) |
| Unknown | 1 |
| Other less common mutations (%) | 8 (73) |
| Unknown | 2 |
| Variables . | n = 13 . |
|---|---|
| Age at diagnosis, median (range) | 52 (43-70) |
| Age at combination BTKi and venetoclax initiation, median (range) | 63 (52-78) |
| Sex (%) | |
| Female | 2 (15) |
| Male | 11 (85) |
| Race | |
| White | 12 (92) |
| Other | 1 (8) |
| Total number of lines of chemo, median (range) | 8 (3-19) |
| Number of lines of chemo prior to combination start, median (range) | 6 (2-17) |
| Presence of bulky diseasea prior to combination start, n = 12b (%) | 6 (50) |
| IgHV unmutated (%) | 13 (100) |
| Stimulated karyotype complexity (%) | |
| Less than three abnormalities | 6 (46) |
| Three or more abnormalities | 7 (54) |
| FISH abnormalities (%) | |
| del(17p) | 4 (31) |
| del(11q) | 4 (31) |
| del(13q) | 2 (15) |
| Trisomy 12 | 4 (31) |
| Other FISH abnormalities | 5 (38) |
| TP53 mutation (%) | 3 (27) |
| Unknown | 2 |
| SF3B1 mutation (%) | 2 (20) |
| Unknown | 3 |
| NOTCH1 mutation (%) | 1 (10) |
| Unknown | 3 |
| BTKi mutation (%) | 8 (67) |
| Unknown | 1 |
| PLCG2 hotspot mutation (%) | 2 (17) |
| Unknown | 1 |
| Other less common mutations (%) | 8 (73) |
| Unknown | 2 |
BTKi, Bruton tyrosine kinase inhibitor; BTKi mutation, mutation at site known to confer resistance to BTKi; del, chromosome deletion; FISH, fluorescent in situ hybridization; IgHV, immunoglobulin heavy chain.
aBulky disease was defined as largest lymph node >5 cm on advanced radiographic imaging.
bOne patient did not have pre-combination therapy imaging available for review.
Prior to combination BTKi and venetoclax therapy, patients received a median of six lines of therapy (range 2-17). Previous BTKi was ibrutinib in six patients, acalabrutinib in one, and both in six. Median cumulative length of prior BTKi exposure was 39.4 months (range 10.0-72.1 months). Ibrutinib was stopped for progression in 11 patients, and in one patient it was stopped without documentation prior to referral to our center. Acalabrutinib was stopped for progression in six patients and clinical trial completion in the seventh. Venetoclax was given alone in 10 patients, with rituximab in two patients, and with obinutuzumab in one patient. Median duration was 13.0 months (range 0.1-35.8 months). Venetoclax was discontinued in seven patients for disease progression, three for toxicity, one due to clinical trial completion, and two for transplant or CAR-T. The BTKi used in combination with venetoclax was ibrutinib in 12 patients and acalbrutinib in one. BTKi preceded venetoclax in all but one patient. Anti-CD20 therapy was not included in combination therapy for any patient. The decision to initiate combination therapy was based on clinician determination of clinical disease progression. Treatment history and duration can be seen in supplemental Figure 2.
At start of combination treatment, median absolute lymphocyte count was 1.9 K/μL (range 0.2-60.7 K/μL), hemoglobin 11.1 g/dL (3.3-14.2 g/dL), and platelet count 97 K/μL (12-187 K/μL). Lactate dehydrogenase, β-2 macroglobulin, and bone marrow studies were not serially assessed prior to therapy change, and thus not included in this analysis.
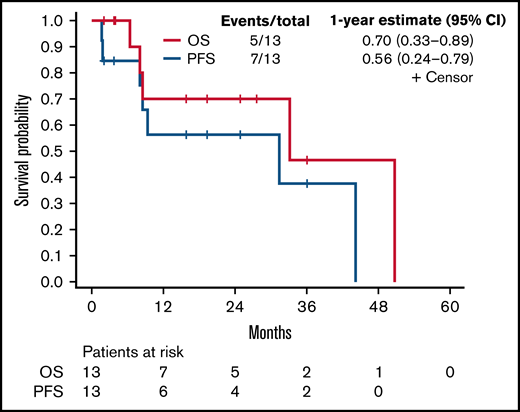
Best response to BTKi/venetoclax combination was partial response in nine, stable disease in two, and progressive disease in two. Of six patients assessed for minimal residual disease at any point, three achieved undetectable levels in the blood or bone marrow. Median duration of therapy was 9.3 months (range 1.7-44.2 months), with three patients still on the combination at the end of the assessment period. Seven patients discontinued for disease progression and two for toxicity. Two patients who had attained a response discontinued therapy and underwent CAR-T. Another two died of progressive disease. After a median follow-up among survivors of 17.6 months (range 2.0-36.1 months), the 1-year PFS and OS estimates were 56.4% (95% CI 24.4%-79.3%) and 70.0% (95% CI 32.9%-89.2%), respectively (see Figure 1). The PFS estimate was the same at 2 years.
PFS and OS. Kaplan-Meier curve for OS and PFS for all patients (n = 13).
Among the six patients that were truly refractory to both BTKi and venetoclax single agents (ie, drug not stopped for toxicity, transplant, CAR-T, or trial completion), best response was partial response in four, stable disease in one, and progressive disease in one. Four patients ultimately progressed after 1.9, 9.3, 31.4, and 44.2 months of therapy. The other two patients underwent CAR-T.
Combination therapy was also effective for patients with high genomic risk. Patients with complex karyotype (n = 7) had a 1-year PFS 51.4% (95% CI 11.8%-81.3%); six patients achieved partial response as best response, and one had progressive disease. At the end of the assessment period, two patients were still on therapy, and one had undergone CAR-T while the rest progressed. All four patients with del(17p) temporarily achieved partial response, with treatment times of 24.6 and 9.4 months prior to therapy change in two, and 8.6 and 8.1 months prior to death in the others. Favorable responses were also seen in eight patients with BTK and PLGC2 mutations conferring BTKi resistance, with 1-year PFS 58.3% (95% CI 18.0%-84.4%) and two patients still on therapy at the end of the study period. Three TP53 mutated patients also did well: one progressed in 44.2 months and was still alive at 50 months follow-up, and the third was progression-free and alive at 24 months.
Overall, we found evidence that even in a heavily pretreated CLL population, specifically those already treated with both BTKi and venetoclax, a combination of the two may provide valuable disease control. Encouragingly, even poor-risk patients had meaningful responses, such as those with TP53 mutations, complex karyotypes, and unmutated IGHV. That the combination overcame molecular resistance to BTKi provides further evidence the two work effectively in combination,6,10,11,13,16 though the reason is not entirely clear. It could be due to the synergistic nature of the combination or potential drug interactions that prolong clearance or increase concentration of one or both drugs.12 We cannot rule out the possibility that, for patients in whom single agent therapy was stopped for toxicity rather than resistance/progression, the encouraging response to the combination was solely due to single-agent efficacy. While the combination in this setting is certainly not a panacea, our data, though limited by the retrospective nature and small sample size, suggests that this regimen can be used successfully as a bridge to other treatments.
To our knowledge, this is the largest dataset of its kind and provides important information in this subset of CLL patients who are not expected to do well. Our results support the finding that BTKi and venetoclax work effectively in combination, and suggest the combination may provide durable benefit for those with progressive disease on both single agents. This is important given the prolonged survival and need for several lines of treatment in CLL, warranting further prospective and controlled studies of these early and limited findings.
Acknowledgments: J.A.W. and K.A.R. are clinical scholars of the Leukemia and Lymphoma Society. This work was funded in part through R01CA177292 (J.A.W. and K.A.R.).
Contribution: J.M.H. and J.A.W. designed and performed the research; collected, analyzed, and interpreted data; and wrote and edited the manuscript; Y.H. performed statistical analysis; K.A.R., S.A.B., M.R.G., J.C.B., A.S.K., and D.J. contributed case information, helped conceptualize the project, and edited the manuscript; and C.R.M. performed research, provided research insights, and edited the manuscript.
Conflict-of-interest disclosure: S.A.B. consulted for Pharmacyclics, Janssen, Beigene, and Acerta/AstraZeneca. A.S.K. has consulted for Abbvie, Beigene, Bristol-Myers Squibb, and Janssen. D.J. has contract or project funding for BTK and NGS testing from Abbvie, Acerta, ArQule, MingSight, Novartis, and Pharmacyclics. M.R.G. served as a consultant for Astra Zeneca, Pharmacyclics, Ascerta, Axio, Innate, and EMD Serono, and served as Board Chairman for the Scientific Committee for the Hairy Cell Leukemia Foundation (no compensation), and received research funding from the Hairy Cell Leukemia Foundation in support of Patient Data Registry. C.R.M. receives research funding from AbbVie. Other investigators do not have relevant conflicts of interest. J.A.W. receives research funding from Pharmacyclics, Janssen, Karyopharm, Morphosys, and Schrodinger, and consults for Janssen, Pharmacyclics, Abbvie, AstraZeneca, Beigene, Loxo, Newave, and Genentech. K.A.R. receives research funding from Genentech, AbbVie, Novartis, and Janssen; has consulted for Genentech, AbbVie, Acerta Pharma, AstraZeneca, Innate Pharma, Pharmacyclics, and Beigene; and received travel funding from AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Jennifer A. Woyach, 445B Wiseman Hall CCC, 410 W 12th Ave, Columbus, OH 43210; e-mail: jennifer.woyach@osumc.edu.
References
Author notes
Requests for data sharing should be sent to Jennifer Woyach (e-mail: jennifer.woyach@osumc.edu).
The full-text version of this letter contains a data supplement.