Key Points
SVT is a potentially life-threatening disease associated with a substantial risk of recurrence and bleeding.
Rivaroxaban appears to be a reasonable alternative to standard anticoagulation for the treatment of SVT in patients without cirrhosis.
Abstract
Heparins and vitamin K antagonists are the mainstay of treatment of splanchnic vein thrombosis (SVT). Rivaroxaban is a potential alternative, but data to support its use are limited. We aimed to evaluate the safety and efficacy of rivaroxaban for the treatment of acute SVT. In an international, single-arm clinical trial, adult patients with a first episode of noncirrhotic, symptomatic, objectively diagnosed SVT received rivaroxaban 15 mg twice daily for 3 weeks, followed by 20 mg daily for an intended duration of 3 months. Patients with Budd-Chiari syndrome and those receiving full-dose anticoagulation for >7 days prior to enrollment were excluded. Primary outcome was major bleeding; secondary outcomes included death, recurrent SVT, and complete vein recanalization within 3 months. Patients were followed for a total of 6 months. A total of 103 patients were enrolled; 100 were eligible for the analysis. Mean age was 54.4 years; 64% were men. SVT risk factors included abdominal inflammation/infection (28%), solid cancer (9%), myeloproliferative neoplasms (9%), and hormonal therapy (9%); 43% of cases were unprovoked. JAK2 V617F mutation was detected in 26% of 50 tested patients. At 3 months, 2 patients (2.1%; 95% confidence interval, 0.6-7.2) had major bleeding events (both gastrointestinal). One (1.0%) patient died due to a non–SVT-related cause, 2 had recurrent SVT (2.1%). Complete recanalization was documented in 47.3% of patients. One additional major bleeding event and 1 recurrent SVT occurred at 6 months. Rivaroxaban appears as a potential alternative to standard anticoagulation for the treatment of SVT in non-cirrhotic patients. This trial was registered at www.clinicaltrials.gov as #NCT02627053 and at eudract.ema.europa.eu as #2014-005162-29-36.
Introduction
Splanchnic vein thrombosis (SVT) is an unusual site manifestation of venous thromboembolism (VTE). Of the different sites of SVT, portal vein thrombosis (PVT) is the most frequently detected, with a gender-specific annual incidence of 3.78 cases per 100 000 inhabitants in men and 1.73 per 100 000 inhabitants in women.1 Most common predisposing factors for SVT include liver cirrhosis, solid abdominal cancer, myeloproliferative neoplasms, abdominal surgery, and intraabdominal infection or inflammatory diseases.2
SVT is a potentially life-threatening disease, with a broad range of clinical presentations, including abdominal infarction or gastrointestinal bleeding. The complex balance between bleeding risk and the risk of thrombus extension or recurrence makes the treatment of SVT a clinical challenge. Without anticoagulation, the estimated rates of major bleeding, extension of SVT, and recurrent VTE were 16%, 15%, and 14%, respectively.3 Compared with no treatment, the use of anticoagulation significantly reduced the risk of thrombosis progression and major bleeding by 76% and 27%, respectively; it also increased the rate of recanalization by more than 2-fold,3 which is likely the main driver for the observed reduction in bleeding in anticoagulated patients with SVT.
In the absence of major contraindications, anticoagulant therapy is therefore recommended for all patients presenting with acute symptomatic SVT, starting with either low-molecular weight heparin (LMWH) or unfractionated heparin (UFH) with a transition to vitamin K antagonists (VKAs) in most cases.4,5 Similar to the recommendations for deep vein thrombosis (DVT) of the lower limbs or pulmonary embolism (PE), it is generally recommended that anticoagulant treatment of SVT be continued for at least 3 months or indefinitely if underlying persistent prothrombotic factors are identified.4
The direct oral anticoagulants (DOACs) have now become the treatment of choice for most patients with DVT or PE, and their advantages over VKAs can be relevant for patients with unusual site VTE. A recent guidance document from the International Society on Thrombosis and Haemostasis (ISTH) suggested the possibility of prescribing DOACs in patients without cirrhosis with acute symptomatic SVT, but evidence to support this statement remains scant.6 In particular, no interventional studies have sufficiently investigated the role of DOACs in the acute phase treatment of SVT.
In this pilot study, we aimed to assess the safety and efficacy of the oral direct inhibitor of factor Xa, rivaroxaban, for the treatment of acute SVT in patients without cirrhosis.
Methods
Study design and oversight
The RIVA-SVT 100 study was an international, single group assignment, open-label, prospective cohort study aiming to treat portal, mesenteric, and splenic vein thrombosis with rivaroxaban.
The study was sponsored by the University of Insubria in Varese, Italy, and partially financed by Bayer AG, Germany, and the Canadian Venous Thromboembolism Research Network (CanVECTOR). A steering committee was responsible for the design and conduct of the study and analysis of the data. A total of 18 centers from Italy, Canada, France, and Germany participated in the study. The coordinating center in Varese collected the data. Local data managers and a member of the steering committee (N.R.) regularly monitored and reviewed the data for completeness and generated queries sent to all participating centers in case of inconsistencies or missing information. All study outcomes were reviewed and adjudicated by an independent adjudication committee, which periodically reviewed study outcomes and adverse events and ensured the integrity of the trial. All authors had full access to primary clinical trial data.
Local institutional review boards or ethics committees approved the study at each participating center. The study was performed in accordance with the declaration of Helsinki as well as with the International Conference on Harmonization guidelines on Good Clinical Practice.
Study population
Patients aged 18 years or older with a first episode of symptomatic, objectively diagnosed PVT, mesenteric vein thrombosis, or splenic vein thrombosis were eligible for inclusion. Signed informed consent was required before inclusion in the study. SVT was defined as symptomatic if it was clinically suspected in the presence of any typical sign or symptom (eg, abdominal pain, gastrointestinal bleeding, and jaundice).
Patients were excluded in the presence of known liver cirrhosis (biopsy proven or with clinical, laboratory, or imaging evidence of chronic liver disease, within a context of chronic alcoholism, viral hepatitis, autoimmunity, Wilson’s disease, and iron overload); alanine aminotransferase level that was 3 times the upper limit of the normal range or higher at the time of enrollment; Budd-Chiari syndrome; previous or ongoing variceal bleeding; portal vein cavernoma at the time of diagnosis; anticipated abdominal surgical procedure; known bleeding diathesis; platelet count <100 000 mm3; creatinine clearance <30 mL/min (Cockroft-Gault formula); life expectancy of <3 months; expected inability to take oral medications; concomitant treatment with azole antimycotics and human immunodeficiency virus protease inhibitors; treatment with therapeutic doses of LMWH or UFH for >7 days; ongoing treatment with a VKA; pregnancy or lactation.
Study procedures
All patients presenting with objectively diagnosed acute SVT were assessed for eligibility. Objective diagnosis was obtained by computed tomography (CT), magnetic resonance imaging, and/or Doppler ultrasound.
At the first study visit, the following clinical evaluations were conducted: eligibility assessment (inclusion/exclusion criteria); collection of patient demographics (age, sex, weight, and race); collection of medical and surgical history, including the presence of possible risk factors for SVT (known hematologic or solid cancer; intraabdominal inflammation or infection, including inflammatory bowel disease, pancreatitis, diverticulitis, appendicitis, cholecystitis, cholangitis, abdominal abscess; abdominal surgery in the previous 3 months; estrogen hormonal therapy; paroxysmal nocturnal hemoglobinuria) and the presence of known gastroesophageal varices; and information on concomitant medications, including the use of prophylaxis of variceal bleeding (eg, beta-blockers).
Laboratory tests included complete blood count, PT/INR, aPTT, renal function test, and liver function test. The decision on the need for thrombophilia testing and the timing of the tests was entirely left to the discretion of the attending physician. If available, data were collected for the following thrombophilia types: factor V Leiden and prothrombin G20210A gene mutations; antithrombin, protein C, and protein S levels; antiphospholipid antibodies; and homocysteinemia. The same applied for the presence of the JAK2 V617F mutation.
Study treatment
All enrolled patients received rivaroxaban 15 mg twice daily for 3 weeks, followed by rivaroxaban 20 mg once daily for a total of 3 months. If any of the efficacy and/or safety outcomes were identified, the study drug was discontinued and appropriate clinical management was initiated.
Follow-up
All enrolled patients were followed for a total of 6 months. During the 3-month study treatment phase, patients were scheduled for in-person visits at 3 weeks, 2 months, and 3 months.
The following evaluations occurred at all study visits: surveillance for adverse events, clinical manifestations of VTE and bleeding, review of concomitant medications, and assessment of compliance to the study drug.
A blood sample was taken for measuring complete blood count, renal function test, and liver function test during the 3-week, 2-month, and 3-month visits.
At the end of the treatment period, abdominal ultrasound or CT scan was requested to assess recanalization. These tests were also performed in case of symptoms of recurrence during the study. Patients were instructed to contact the study team if events occurred between visits or phone calls. Interpretation of baseline and follow-up imaging tests was performed locally. Adjudication of these tests by the independent adjudication committee was based on local reports.
After the end of the treatment period, the decision to continue anticoagulant treatment was at the discretion of the attending physician.
Three months after the end of the treatment period, a final visit (6-month follow-up) either in person or by phone call was scheduled to collect information on mortality, duration, and type of anticoagulant treatment after the end of the study period, recurrent SVT or VTE in other sites, and bleeding.
Source documents were requested in case clinical events occurred during this period. All events that occurred between the end of treatment period and the 6-month follow-up visit were reported but not counted as study outcomes.
Study outcomes
The primary outcome of the study was major bleeding during the 3 months of active treatment and up to 2 days after the end of study treatment. Major bleeding events were classified using a modified ISTH definition,7 the European Medicines Agency definition. According to this definition, bleeding was defined as major if it was associated with death; if it was overt and associated with a decrease in hemoglobin concentration by at least 2.0 g/L or with the need for transfusion of 2 or more units of whole blood or red cells; if it occurred in a critical area or organ such as retroperitoneal, intracranial, intraocular, intraspinal, intraarticular or pericardial, or intramuscular with compartment syndrome; or if it resulted in surgical intervention.
Secondary outcomes included all-cause and SVT-related mortality; clinically relevant, nonmajor bleeding occurred during the 3 months of active treatment and up to 2 days after the end of study treatment; detection of alanine aminotransferase levels of 3 times the upper limit of the normal range or higher with or without bilirubin levels of 2 times the upper limit of the normal range or higher during follow-up; patency of the portal vein trunk and at least 1 of its main right or left branches and patency of the superior and inferior mesenteric veins and of the splenic veins; symptomatic recurrent SVT; symptomatic VTE in other sites; and mesenteric infarction.
In patients who died during follow-up, death certificates and, if available, autopsy findings were reviewed to establish the cause of death.
Clinically relevant nonmajor bleeding was defined as any overt bleeding requiring a medical intervention, unscheduled contact with a physician, or temporary cessation of oral anticoagulants or causing discomfort for the patient (such as pain) or impairment of activities of daily living and not meeting any of the criteria for major bleeding.8
Major bleeding and clinically relevant nonmajor bleeding were confirmed with investigational reports, including clinic notes, hemoglobin results, and imaging. Diagnostic tests were performed as per standard of care.
Patency of SVT was defined as the normal appearance of a previously obstructed segment as opposed to “obstruction,” defined as the presence of solid material in the vascular lumen. In case of reduction of thrombus extension as compared with the baseline test or patency of at least 1 previously occluded multiple vessels, patients with obstruction were subclassified as having partial recanalization.
Recurrent symptomatic SVT was defined as thrombus extension or occurrence in a previously patent splanchnic vein segment; symptomatic VTE in other sites, which included all VTE events occurring outside of the splanchnic veins, was diagnosed by appropriate imaging tests according to the site of thrombosis. Adjudication of mesenteric infarction was based on evidence from a pathology specimen. All efficacy outcomes were assessed until the end of the study treatment period.
Sample size
In the absence of adequate information from previous studies on the incidence of the primary outcome, a formal sample size calculation was considered not feasible. For this reason, we decided to carry out a pilot study with the aim of enrolling an initial cohort of 100 consecutive patients with PVT, mesenteric vein thrombosis, or splenic vein thrombosis. An interim analysis was planned and performed after the enrollment of the first 50 patients.
Statistical analysis
Continuous variables are expressed as mean plus or minus the standard deviation (SD) or as median with interquartile range (IQR) when data do not have a normal distribution, according to the Wilk-Shapiro test; categorical variables as absolute values and relative frequencies. According to the distribution and the number of detected events, the Student’s t test or Wilcoxon rank-sum (Mann-Whitney U test) test and the χ2 or Fisher’s exact tests were used to compare groups of quantitative and qualitative variables, respectively. Independent predictive factors for bleeding events (including major and clinically relevant, nonmajor bleeding) were assessed with Cox model regression. Follow-up was considered from the beginning of rivaroxaban treatment (day 0). The occurrence of outcomes during the 3-month follow-up was expressed as cumulative incidence (with 95% Wilson confidence interval [CI]). Overall survival rates were assessed by the Kaplan-Meier method. The software STATA/BE version 17 (StataCorp LP, College Station, TX) was used for statistical analysis, with P < .05 considered statistically significant.
Results
Patients
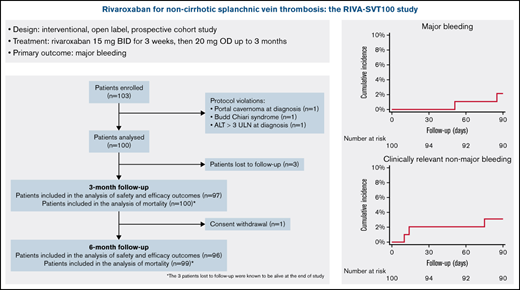
A total of 103 patients with objectively diagnosed SVT were enrolled between June 2015 and March 2021. Three patients had major protocol violations, thus leaving 100 patients for the analysis. The flowchart of the enrolled population is shown in Figure 1. The reasons for exclusion of the 3 patients from the analysis were portal cavernoma at diagnosis, Budd-Chiari syndrome, and alanine aminotransferase levels >3-fold the upper limit of the normal range due to acute Epstein Barr virus infection. All 3 patients were started on rivaroxaban according to the study protocol. The first 2 patients completed the first 3 months of treatment and were still on rivaroxaban at the 6-month follow-up visit with no events. The third patient was switched to LMWH after 10 days of treatment because of further increase of liver enzymes and died 14 days after enrollment due to septic shock with multiorgan failure.
The baseline characteristics of the study population are depicted in Table 1. More than half of patients had multiple site thrombosis; the most common site of thrombosis was the portal vein. In particular, 30 patients had concomitant involvement of the portal, splenic, and mesenteric veins; 23 patients had 2 vessels involved (13 portal and mesenteric veins, 6 portal and splenic veins, and 4 splenic and mesenteric veins); and 47 patients had a single vessel involved. In 14 patients, venous obstruction at the time of diagnosis was defined as partial. Known esophageal varices and ascites were present in 7 (7%) and 15 patients (15%), respectively. The median delay between symptoms onset and diagnosis was 7 days.
Baseline characteristics of the population
| . | No. of patients (n = 100) . |
|---|---|
| Demographics | |
| Age (y), mean (SD) range (y) | 54.4 (15.5) 23-89 |
| Male sex, n (%) | 64 (64.0) |
| Caucasians, n (%) | 95 (95.0) |
| Personal history of VTE, n (%) | 14 (14.0) |
| Family history of VTE, n (%) | 15 (15.0) |
| SVT details | |
| Diagnostic imaging | |
| Ultrasound, n (%) | 13 (13.0) |
| CT, n (%) | 82 (82.0) |
| Magnetic resonance imaging, n (%) | 5 (5.0) |
| SVT location* | |
| Portal vein, n (%) | 74 (74.0) |
| Mesenteric veins, n (%) | 61 (61.0) |
| Splenic vein, n (%) | 48 (48.0) |
| Multiple vein thrombosis, n (%) | 53 (53.0) |
| Other site of VTE, n (%) | 4 (4.0) |
| Symptoms at onset* | |
| Abdominal pain, n (%) | 90 (90.0) |
| Nausea, n (%) | 27 (27.0) |
| Anorexia, n (%) | 8 (8.0) |
| Diarrhea, n (%) | 12 (12.0) |
| GI bleed, n (%) | 2 (2.0) |
| Fever, n (%) | 23 (23.0) |
| Jaundice, n (%) | 2 (2.0) |
| Dyspnea, n (%) | 3 (3.0) |
| Delay symptoms-diagnosis (d), median (IQR) | 7 (3-15) |
| Esophageal varices, n (%) | 7 (7.0) |
| Ascites, n (%) | 15 (15.0) |
| Laboratory tests at diagnosis | |
| Hb (g/dL), mean (SD) | 13.0 (2.0) |
| PLT (×109), median (IQR) | 254 (199-358) |
| Body weight (kg), median (IQR) | 76 (68.5-84) |
| Creatinine clearance (mL/min), median (IQR) | 105.0 (75.8-135.9) |
| . | No. of patients (n = 100) . |
|---|---|
| Demographics | |
| Age (y), mean (SD) range (y) | 54.4 (15.5) 23-89 |
| Male sex, n (%) | 64 (64.0) |
| Caucasians, n (%) | 95 (95.0) |
| Personal history of VTE, n (%) | 14 (14.0) |
| Family history of VTE, n (%) | 15 (15.0) |
| SVT details | |
| Diagnostic imaging | |
| Ultrasound, n (%) | 13 (13.0) |
| CT, n (%) | 82 (82.0) |
| Magnetic resonance imaging, n (%) | 5 (5.0) |
| SVT location* | |
| Portal vein, n (%) | 74 (74.0) |
| Mesenteric veins, n (%) | 61 (61.0) |
| Splenic vein, n (%) | 48 (48.0) |
| Multiple vein thrombosis, n (%) | 53 (53.0) |
| Other site of VTE, n (%) | 4 (4.0) |
| Symptoms at onset* | |
| Abdominal pain, n (%) | 90 (90.0) |
| Nausea, n (%) | 27 (27.0) |
| Anorexia, n (%) | 8 (8.0) |
| Diarrhea, n (%) | 12 (12.0) |
| GI bleed, n (%) | 2 (2.0) |
| Fever, n (%) | 23 (23.0) |
| Jaundice, n (%) | 2 (2.0) |
| Dyspnea, n (%) | 3 (3.0) |
| Delay symptoms-diagnosis (d), median (IQR) | 7 (3-15) |
| Esophageal varices, n (%) | 7 (7.0) |
| Ascites, n (%) | 15 (15.0) |
| Laboratory tests at diagnosis | |
| Hb (g/dL), mean (SD) | 13.0 (2.0) |
| PLT (×109), median (IQR) | 254 (199-358) |
| Body weight (kg), median (IQR) | 76 (68.5-84) |
| Creatinine clearance (mL/min), median (IQR) | 105.0 (75.8-135.9) |
GI, gastrointestinal; Hb, hemoglobin; PLT, platelets; SD, standard deviation.
More than 1 answer is possible.
Risk factors associated with SVT are described in Table 2. The event was unprovoked in 43 patients (43%), 28 patients (28%) had abdominal inflammation or infection (2 of whom had inflammatory bowel disease), 9 (9%) patients had solid cancer, and 9 patients (9%) had overt myeloproliferative neoplasm. Among the tested patients, the most represented thrombophilic abnormalities were JAK2 V617F mutation (26%), prothrombin G20210A mutation (23%), and hyperhomocysteinemia (16%). No cases of paroxysmal nocturnal hemoglobinuria were detected.
Risk factors for SVT
| . | No. of patients (n = 100) . |
|---|---|
| Risk factors | |
| Unprovoked, n (%) | 43 (43.0) |
| Multiple risk factors, n (%) | 5 (5.0) |
| Abdominal inflammation/infection, n (%) | 28 (28.0) |
| Solid cancer, n (%) | 9 (9.0) |
| Overt myeloproliferative neoplasm, n (%) | 9 (9.0) |
| Recent abdominal surgery, n (%) | 7 (7.0) |
| Estrogen hormonal therapy, n (%) | 9 (9.0) |
| Thrombophilia and JAK2 V617F mutation testing | |
| Factor V Leiden mutation, n/N tested (%) | 3/42 (7.1) |
| Prothrombin G20210A mutation, n/N tested (%) | 9/39 (23.1) |
| Protein C deficiency, n/N tested (%) | 2/27 (7.4) |
| Protein S deficiency, n/N tested (%) | 1/27 (3.7) |
| Antithrombin deficiency, n/N tested (%) | 1/26 (3.9) |
| Hyperhomocysteinemia, n/N tested (%) | 4/25 (16.0) |
| Lupus anticoagulant, n/N tested (%) | 2/29 (6.9) |
| Anti-cardiolipin antibodies, n/N tested (%) | 1/34 (2.9) |
| Anti-β-2-glycoprotein I, n/N tested (%) | 0/33 (0) |
| JAK2 V617F mutation, n/N tested (%) | 13/50 (26.0) |
| . | No. of patients (n = 100) . |
|---|---|
| Risk factors | |
| Unprovoked, n (%) | 43 (43.0) |
| Multiple risk factors, n (%) | 5 (5.0) |
| Abdominal inflammation/infection, n (%) | 28 (28.0) |
| Solid cancer, n (%) | 9 (9.0) |
| Overt myeloproliferative neoplasm, n (%) | 9 (9.0) |
| Recent abdominal surgery, n (%) | 7 (7.0) |
| Estrogen hormonal therapy, n (%) | 9 (9.0) |
| Thrombophilia and JAK2 V617F mutation testing | |
| Factor V Leiden mutation, n/N tested (%) | 3/42 (7.1) |
| Prothrombin G20210A mutation, n/N tested (%) | 9/39 (23.1) |
| Protein C deficiency, n/N tested (%) | 2/27 (7.4) |
| Protein S deficiency, n/N tested (%) | 1/27 (3.7) |
| Antithrombin deficiency, n/N tested (%) | 1/26 (3.9) |
| Hyperhomocysteinemia, n/N tested (%) | 4/25 (16.0) |
| Lupus anticoagulant, n/N tested (%) | 2/29 (6.9) |
| Anti-cardiolipin antibodies, n/N tested (%) | 1/34 (2.9) |
| Anti-β-2-glycoprotein I, n/N tested (%) | 0/33 (0) |
| JAK2 V617F mutation, n/N tested (%) | 13/50 (26.0) |
Twenty-one patients were immediately started on rivaroxaban, with a median time between diagnosis and the first dose of rivaroxaban of 2 days (IQR 0-10 days). Parenteral therapy prior to the start of the study treatment was administered to the remaining 79 patients (79%), of whom 75 received LMWH (39 at therapeutic dose), 3 received UFH, and 3 received fondaparinux. Two of these patients received >1 parenteral agent. The median time between diagnosis and first dose of parenteral agents was 0 days (IQR 0-1 days). The median duration of parenteral treatment was 5 days (IQR, 3-6 days). Five patients (5%) received concomitant antiplatelet therapy, of whom 4 were on aspirin and 1 on clopidogrel. Proton pump inhibitors were prescribed to 39 patients (39%), whereas 12 (12%) were on beta-blockers.
Three-month follow-up
Three patients (3%) were lost to follow-up at 3 months. After reviewing administrative databases, survival of these patients at 6 months could be confirmed. Of the 97 evaluable patients at 3 months, 90 completed study treatment with rivaroxaban according to the protocol.
Of the remaining 7 patients, 6 were switched to parenteral therapy and 1 permanently stopped anticoagulation because of deterioration of clinical conditions. Mean duration of rivaroxaban treatment before interruption was 1.4 months (SD, 0.8 months). Reasons for switching to parenteral therapy included newly started chemotherapy (n = 1), decision of the treating physician (n = 1), need for surgical procedure (n = 1), recurrent thrombosis (n = 2), and bleeding (n = 1). Four of these 6 patients were subsequently restarted on rivaroxaban after a minimum of 32 and a maximum of 53 days.
Three-month outcomes
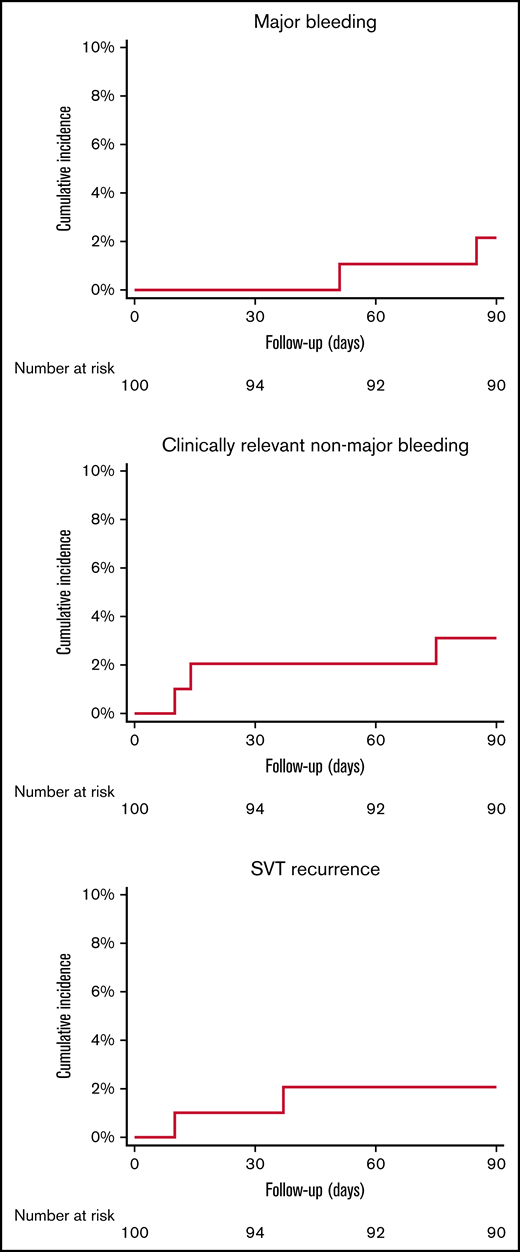
There were 2 major bleeding events (2.1%; 95% CI, 0.6% to 7.2%) and 4 clinically relevant nonmajor bleeding events in 3 patients (3.1%; 95% CI, 1.1% to 8.7%) during the treatment period (Figure 2). Four of 5 patients with major or clinically relevant nonmajor bleeding events were women, 2 had solid cancer, 1 had an unprovoked event, 1 had an underlying inflammatory disease, and 1 had SVT secondary to hormonal therapy. One patient was receiving concomitant antiplatelet treatment (supplemental Appendix 1). Due to the low number of events, the prespecified multivariable Cox regression analysis aimed to identify predictive factors for bleeding events could not be performed.
Cumulative incidence of major bleeding, clinically relevant non-major bleeding, and SVT recurrent events.
Cumulative incidence of major bleeding, clinically relevant non-major bleeding, and SVT recurrent events.
Symptomatic SVT recurrence occurred in 2 patients (2.1%; 95% CI, 0.6% to 7.2%) (Figure 2). One patient died (1.0%; 95% CI, 0.2% to 5.4%). Death was adjudicated as non–SVT related. A detailed description of bleeding events, recurrences, and mortality is provided in Table 3.
Details of events occurred during the treatment period (up to 90 ± 3 days)
| Sex . | Age (y) . | Type of event . | Event time (d) . | Event details . |
|---|---|---|---|---|
| F | 64 | CRNMB | 10 | Hematuria, rivaroxaban dose reduced |
| F | 55 | SVT recurrence* | 10 | Symptomatic thrombosis of superior mesenteric vein and left renal vein in metastatic adenocarcinoma of the biliary tract; switched to LMWH |
| F | 54 | CRNMB† | 14 | Rectorrhagia in adenocarcinoma of the rectum with intestinal stoma; rivaroxaban withheld for 2 d |
| F | 55 | Death (non–SVT related)* | 27 | Pneumonia and septic shock in progression of metastatic adenocarcinoma of the biliary tract |
| M | 52 | SVT recurrence | 37 | Symptomatic unprovoked thrombosis of distal portal vein branches; switched to LMWH |
| F | 76 | MB | 51 | Melena with severe anemia (Hb drop ≥2 g/dL) in squamous cell carcinoma of the distal esophagus; required hospitalization and red blood cells transfusion; anticoagulation was stopped |
| F | 54 | CRNMB† | 57 | Another episode of rectorrhagia in adenocarcinoma of the rectum with intestinal stoma; rivaroxaban withheld for 2 d |
| F | 23 | CRNMB | 75 | Menorrhagia with patient discomfort; rivaroxaban was continued |
| M | 61 | MB | 85 | Melena with severe anemia (Hb drop ≥2 g/dL) in reflux esophagitis grade IV and suspected esophageal varices; required hospitalization; anticoagulation was stopped for a few days and then patient was switched to fondaparinux |
| Sex . | Age (y) . | Type of event . | Event time (d) . | Event details . |
|---|---|---|---|---|
| F | 64 | CRNMB | 10 | Hematuria, rivaroxaban dose reduced |
| F | 55 | SVT recurrence* | 10 | Symptomatic thrombosis of superior mesenteric vein and left renal vein in metastatic adenocarcinoma of the biliary tract; switched to LMWH |
| F | 54 | CRNMB† | 14 | Rectorrhagia in adenocarcinoma of the rectum with intestinal stoma; rivaroxaban withheld for 2 d |
| F | 55 | Death (non–SVT related)* | 27 | Pneumonia and septic shock in progression of metastatic adenocarcinoma of the biliary tract |
| M | 52 | SVT recurrence | 37 | Symptomatic unprovoked thrombosis of distal portal vein branches; switched to LMWH |
| F | 76 | MB | 51 | Melena with severe anemia (Hb drop ≥2 g/dL) in squamous cell carcinoma of the distal esophagus; required hospitalization and red blood cells transfusion; anticoagulation was stopped |
| F | 54 | CRNMB† | 57 | Another episode of rectorrhagia in adenocarcinoma of the rectum with intestinal stoma; rivaroxaban withheld for 2 d |
| F | 23 | CRNMB | 75 | Menorrhagia with patient discomfort; rivaroxaban was continued |
| M | 61 | MB | 85 | Melena with severe anemia (Hb drop ≥2 g/dL) in reflux esophagitis grade IV and suspected esophageal varices; required hospitalization; anticoagulation was stopped for a few days and then patient was switched to fondaparinux |
Day 0 was considered as the day when rivaroxaban was started.
Two patients had 2 events each (identified with * and †).
CRNMB, clinically relevant nonmajor bleeding; MB, major bleeding.
Neither increased levels of liver enzymes nor episodes of symptomatic VTE in other sites or mesenteric infarction were reported during the study period.
Imaging tests were performed in 91 patients at the end of study treatment after a median of 99 days (IQR, 88-118). Complete recanalization was documented in 43 patients (47.3%; 95% CI, 37.3% to 57.4%). Of the 47 patients with residual obstruction, partial recanalization was documented in 33 (36.3%; 95% CI, 27.1% to 46.5%), and the remaining 14 showed persistent full obstruction. Finally, 1 patient underwent imaging test on day 98 after a new hospitalization associated with hepatocarcinoma and after the end of the treatment period. The CT scan revealed symptomatic progression of thrombosis (event described below in section on 6-month outcomes). The comparison between patients with partial/complete recanalization vs patients with no sign of recanalization is reported in supplemental Appendix 2.
Six-month follow-up
One patient withdrew consent for further contacts after the 3-month visit and was lost to the 6-month follow-up, thus leaving a total of 96 patients. Of those patients, 69 (71.9%) were still receiving rivaroxaban, 11 (11.4%) had stopped anticoagulation, 4 (4.2%) had been switched to antiplatelet treatment, 3 (3.1%) switched to LMWH, and 3 (3.1%) switched to VKAs. Of the 15 patients who were no longer taking anticoagulant drugs, 4 had unprovoked SVT, 2 had solid cancer, and the remaining ones had transient risk factors.
Events during six-month follow-up
Between 3 and 6 months of follow-up, there were 1 major bleeding and 2 clinically relevant nonmajor bleeding events (both in patients still on rivaroxaban treatment), 1 symptomatic SVT recurrence in a patient also on rivaroxaban treatment, and 2 deaths, both non–SVT related. Thus, no thrombotic or bleeding events occurred in the 15 patients who stopped anticoagulant treatment after the first 3 months. A detailed description of bleeding events, recurrences, and mortality is provided in Table 4.
Details of events occurred from 3 and 6 months of follow-up
| Sex . | Age (y) . | Type of event . | Event time (d) . | Event details . |
|---|---|---|---|---|
| M | 69 | SVT recurrence | 96 | Symptomatic portal vein thrombosis in infiltrative hepatocellular carcinoma during treatment with rivaroxaban 20 mg once daily; switched to LMWH |
| F | 76 | Death (non–SVT related)* | 98 | Death for clinical deterioration in esophageal cancer progression |
| F | 70 | CRNMB | 102 | Profuse epistaxis during treatment with rivaroxaban 20 mg once daily; finding of varices of the nasal septum treated with nasal packing; anticoagulation was temporarily stopped and then restarted on reduced dose of rivaroxaban |
| F | 44 | CRNMB | 117 | Menometrorrhagia with mild anemia during treatment with rivaroxaban 20 mg once daily; treated with tranexamic acid; patient was then switched to VKA |
| F | 73 | Death (non–SVT related) | 136 | Death for clinical deterioration in pancreatic cancer progression |
| F | 55 | MB† | 187 | Rectorrhagia in adenocarcinoma of the rectum with intestinal stoma; during treatment with rivaroxaban 20 mg once daily, severe anemia (Hb drop ≥2 g/dL) required hospitalization and red blood cells transfusion; anticoagulation was temporarily stopped; patient was then switched to LMWH |
| Sex . | Age (y) . | Type of event . | Event time (d) . | Event details . |
|---|---|---|---|---|
| M | 69 | SVT recurrence | 96 | Symptomatic portal vein thrombosis in infiltrative hepatocellular carcinoma during treatment with rivaroxaban 20 mg once daily; switched to LMWH |
| F | 76 | Death (non–SVT related)* | 98 | Death for clinical deterioration in esophageal cancer progression |
| F | 70 | CRNMB | 102 | Profuse epistaxis during treatment with rivaroxaban 20 mg once daily; finding of varices of the nasal septum treated with nasal packing; anticoagulation was temporarily stopped and then restarted on reduced dose of rivaroxaban |
| F | 44 | CRNMB | 117 | Menometrorrhagia with mild anemia during treatment with rivaroxaban 20 mg once daily; treated with tranexamic acid; patient was then switched to VKA |
| F | 73 | Death (non–SVT related) | 136 | Death for clinical deterioration in pancreatic cancer progression |
| F | 55 | MB† | 187 | Rectorrhagia in adenocarcinoma of the rectum with intestinal stoma; during treatment with rivaroxaban 20 mg once daily, severe anemia (Hb drop ≥2 g/dL) required hospitalization and red blood cells transfusion; anticoagulation was temporarily stopped; patient was then switched to LMWH |
Same patient with previously 1 MB.
Same patient with previously 2 CRNMB.
One patient had alanine aminotransferase levels >3-fold the upper limit of the normal range at day 98, with normal bilirubin levels. Further investigation revealed hepatocarcinoma and thrombosis progression (symptomatic event reported above).
Discussion
In this study, 100 patients with objectively diagnosed acute SVT were treated with oral rivaroxaban for an intended duration of 3 months. During the treatment period, there were 2 major gastrointestinal bleeding events, neither of which was life threatening and both of which occurred in patients with underlying local predisposing factors. There were 2 recurrent thrombotic events, 1 unprovoked and 1 in a patient with metastatic cancer, and 1 fatality that was not associated with thrombosis. More than 80% of the enrolled patients showed recanalization of the splanchnic veins at 3 months, of which 47% were complete.
To the best of our knowledge, this is the first interventional study that specifically assessed the safety and efficacy of DOAC for the acute treatment of SVT in patients without cirrhosis. A previous randomized clinical trial enrolled 80 patients with cirrhosis with acute SVT, but the dose of rivaroxaban prescribed (10 mg twice daily) is not approved for clinical use in the treatment of acute VTE.9 All other DOAC studies in this field have observational designs and reported heterogenous dosages and anticoagulation starting times.10
The management of patients with SVT is challenging because the risk of bleeding can be substantial and may sometimes be as high as the risk of recurrence. Anticoagulant therapy with heparins and VKAs still represents the established treatment of most patients, and the same therapeutic regimens and dose adjustments as for usual site VTE are generally suggested.11 In a large, prospective cohort study that included >600 patients with SVT, the annualized risk of recurrent thrombosis during standard anticoagulant treatment was 5.6% and the risk of major bleeding was 3.9%.12 These risks varied across patient subgroups, being highest in patients with cirrhosis and solid cancer and lowest in patients with transient risk factors.12 The cumulative incidence of major bleeding events at 3 months in was 1.7% (unpublished data). In a study on administrative data from Denmark, the incidence of major bleeding events in a cohort of 1915 patients with SVT at 30 days was 4.3% and was >8-fold higher than the 0.5% incidence documented in the cohort of 18 373 patients with PE or DVT.13 This risk remained >3-fold higher after up to 1 year follow-up. In a recent meta-analysis of studies on the treatment of SVT, the event rate of recurrent VTE in patients who received anticoagulant therapy was 11% and the rate of major bleeding was 9% after a median treatment duration of 8.4 months.3 Of interest, in the subgroup of patients with liver cirrhosis, these risks were 10% and 6%, respectively.14
The results of the present study support the hypothesis that rivaroxaban can be an important alternative to VKAs, with event rates of recurrent SVT of 2.1% and of major bleeding of 2.1% at 3 months, which compare well with rates reported in studies with heparins and VKAs. In particular, in a retrospective study of 375 patients treated with VKAs (84.4% of them without liver cirrhosis), the incidence rate of major bleeding at 6 months from starting treatment was 2.84%.15 In previous observational studies in which DOACs were prescribed to patients with SVT, major bleeding rates ranged from 2% to 9.1%, but duration of follow-up was rather heterogeneous.10,16-18 In the previously quoted meta-analysis of studies on anticoagulation for patients with SVT, event rates in patients on DOACs were similar to those reported on VKAs for recurrent VTE (8% in both groups) and major bleeding (7% on DOACs and 11% on VKAs, respectively).3
In the present study, patients with cirrhosis were excluded, but solid cancer and hematologic malignancies were present in nearly a quarter of patients, and nearly half of patients had unprovoked SVT, which is also associated with a nonnegligible risk of both recurrent thrombosis and bleeding.12 The 2 major bleeding events were gastrointestinal bleeds, which occurred in a patient with gastrointestinal cancer and a patient with severe esophagitis. Some trials comparing DOACs with LMWH in patients with cancer-associated thrombosis have reported higher rates of gastrointestinal bleeding in patients with gastrointestinal cancer receiving DOACs as compared with patients treated with LMWH.19,20 For this reason, international guidelines suggest caution with the use of the DOACs in patients with VTE and gastrointestinal cancer or patients at high risk of gastrointestinal bleeding or recommend LMWH over DOACs.21-23 Similarly, the guidance document from the ISTH on the treatment of SVT suggests LMWH over DOACs for patients with neoplasms at increased bleeding risk.6 The present study is not sufficiently powered to provide adequate evidence on the topic, but it confirms the need for a careful assessment of patients with known gastrointestinal lesions or other predisposing factors for bleeding. For example, anemia at baseline may identify patients with previous occult bleeding who are thus at increased risk for subsequent overt bleeding, as suggested by univariate analysis reported in supplemental Appendix 1.
One of the main goals of anticoagulant treatment in patients with SVT is obtaining a good recanalization of the affected vessels. Residual obstruction favors increased pressure and long-term bleeding risk, particularly in patients with PVT. In the recent meta-analysis on anticoagulant treatment of patients with SVT, the estimated rate of recanalization without anticoagulation was 22% and was increased to 57% in patients receiving anticoagulant therapy.3 However, the timing of assessment was highly heterogeneous across studies and did not allow a comparison with the RIVA-SVT100 study, in which thrombus improvement was achieved in 83% of the patients at 3 months and complete recanalization by as many as 47%. Of interest, when we compared the characteristics and management strategies between patients with and without residual vein obstruction, the only variable associated with reperfusion was the immediate treatment with rivaroxaban without any previous anticoagulant therapy, which reached borderline statistical significance (data in the supplemental Appendices 1-2).
Some limitations of this study need to be acknowledged. First, the slow enrollment of patients in this pilot study may suggest a limited generalizability of the results. Unfortunately, not all centers were able to keep an updated screening log during the entire duration of the study, so the total number of patients assessed for this study was not available. However, we collected this information from 8 centers, which enrolled a total of 58 patients (58%) in the study. In these centers, the total number of patients with SVT evaluated for inclusion in the study was 264; therefore, the number of excluded patients was 242 (80.7% of the screened population). The most common reasons for exclusion were parenteral treatment duration of >7 days in 38.0% of cases, known liver cirrhosis in 16.9%, ongoing treatment with VKAs in 11.6%, and low platelet count in 10.7%. These data suggest that for a substantial proportion of patients, the exclusion was due to a delay in the referral to participating centers and not to clinical exclusion criteria. Indeed, selection bias due to referral of selected patients to participating centers cannot be excluded, which may have influenced the prevalence of provoking factors in our population.
The second limitation is that the allowed 7-day window of full anticoagulant treatment with parenteral drugs prior to enrollment in the study is longer than the 48-hour window used in the pivotal phase 3 studies carried out in patients with DVT or PE. However, this longer window was chosen to reflect standard clinical practice and ensure a pragmatic study. On the one hand, this longer time window often allows a thorough assessment of these commonly fragile patients to better drive the therapeutic decision. On the other hand, delayed referrals to specialized centers are common, which is why a shorter enrollment window would have excluded even more patients from participation. In this study, 21% of patients were immediately started on rivaroxaban, and 79% received a parenteral agent for a median duration of 5 days. No difference in outcomes was detected between the 2 groups. In the XALIA study, a phase 4 study on rivaroxaban for the treatment of DVT and PE, a substantial proportion of patients was started on rivaroxaban after >48 hours of parenteral anticoagulation without any difference in clinical outcomes when compared with those who were immediately started on the oral agent.24 For this reason, we believe that our results also are generalizable to patients with SVT entirely treated with rivaroxaban.
Third, this was a single-arm study, and no control group was available. For this reason, we reflected our results against data from historical cohorts. Unfortunately, given the time taken to complete enrollment, an adequately powered randomized control study seems unlikely to be feasible and completed in a reasonable period.
Lastly, these findings cannot be applied to patients with liver cirrhosis, Budd-Chiari syndrome, portal vein cavernoma, or platelet count <100 000 mm3 because these were all exclusion criteria. However, information on other specific subgroups such as patients with myeloproliferative neoplasms or solid abdominal cancer needs to be taken cautiously given the small number of patients in each of these groups. In addition, these findings may not apply to non-White patients because only 5% of our study population was non-White. The prevalence and outcomes of SVT are largely influenced by the proportion of underlying provoking factors, which may substantially vary among different ethnic groups and geographical regions.
In conclusion, rivaroxaban represents an important alternative to current standard of treatment with LMWH and VKAs for the acute management of patients without cirrhosis with SVT. Careful selection of patients based on their individual bleeding risk profile is warranted. Additional data from interventional and observational studies are needed to confirm our findings and provide information on the long-term risks (eg, after 3 months) of anticoagulation.
Acknowledgments
The authors wish to thank Dr. Giovanna Colombo and Dr. Lorenza Bertù for their active role as data managers for this study. Gualtiero Palareti, Paolo Prandoni, and Franco Piovella are the central independent adjudication committee for clinical outcomes.
This research was sponsored by the University of Insubria, Varese, Italy, and partially financed by Bayer AG, Germany and CanVECTOR. The CanVECTOR Network holds grant funding from the Canadian Institutes of Health Research (CDT-142654) and from the Fonds de recherche du Québec – Santé (File # 309911).
M.C., A.D., and A.L.-L. are members of the CanVECTOR Network. W.A., N.R., J.B.W., M.D.N., V.D.S., and M.S. are members of the steering committee.
The following list of investigators were involved in patient enrollment: Samuela Pegoraro, Lucia Maria Caiano, and Giulia Conte, University of Insubria, Varese, Italy; Luise Tittl, Sandra Marten, and Christiane Naue, Dresden University Hospital, Dresden, Germany; Roberto Santi, Luca Albertin, and Patrizia Sciancalepore, “A.O. SS. Antonio e Biagio” Hospital, Alessandria, Italy; Eugenio Bucherini, SS Aziendale di Angiologia Faenza AUSL Romagna, Ravenna, Italy; Alessandro Ruzzarin, Azienda Ospedaliera Universitaria di Padova, Padova, Italy; Donatella Colaizzo and Angelo Andriulli, IRCCS Casa Sollievo della Sofferenza, S. Giovanni Rotondo, Italy; Rita Santoro, Marzia Leotta, Antonella Ierardi, and Alessandra Strangio, Azienda Ospedaliera Pugliese-Ciaccio, Catanzaro, Italy; Nahya Awada, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON, Canada; Elena Rossi, Francesca Bartolomei, and Denise Soldati, Catholic University, Fondazione Policlinico Gemelli IRCCS, Rome, Italy; Fulvio Pomero, Michele and Pietro Ferrero Hospital, Verduno, Cuneo Italy; Ilaria Nichele, S. Bortolo Hospital, Vicenza, Italy; Pasquale Cianci, University of Perugia, Perugia, Italy; Maria Abbattista, IRCCS Fondazione Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy; Barbara Nardo, Busto Arsizio Hospital, Busto Arsizio, Italy; Laurent Bertoletti, Service De Médecine Vasculaire Et Thérapeutique, Chu de Saint-Étienne, France; Marcello Di Nisio, University “G. d’Annunzio” of Chieti-Pescara, Chieti, Italy; Alejandro Lazo-Langner, Western University, London, ON, Canada; and Alessandro Schenone, Galliera Hospital, Genoa, Italy.
Authorship
Contribution: W.A. and N.R. designed and conducted the study, collected data, analyzed and interpreted data, and drafted the manuscript; J.B.W., M.D.N., V.D.S., and M.S. designed and conducted the study, collected data, interpreted data, and approved the manuscript; and L.C., E.B., M.T.S., E.G., R.S., M.C., A.D., F.P., M.P.D., A.T., C.B., I.M., B.N., L.B., A.L.-L., and A.S. collected and interpreted data and approved the manuscript.
Conflict-of-interest disclosure: W.A. received research support from Bayer and participated in advisory boards for Bayer, Portola, Aspen, Norgine, and Sanofi. J.B.W. reported personal fees from Bayer, Daiichi Sankyo, BMS-Pfizer, Portola/Alexion, and Sanofi outside the submitted work. M.D.N. reported personal fees from Bayer, Daiichi Sankyo, BMS-Pfizer, Leo Pharma, and Sanofi outside the submitted work. M.C. received research funding from BMS, Pfizer, and Leo Pharma and participated in advisory boards from Bayer, Sanofi, BMS, Pfizer, Servier, and Leo Pharma. A.D. reported grants from Leo Pharma and Pfizer and personal fees from BMS, Leo Pharma, Pfizer, and Servier. L.B. received research support from Bayer and MSD and participated in advisory boards for Aspen, Bayer, BMS/Pfizer, Leo-Pharma, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Walter Ageno, Department of Medicine and Surgery, Via Guicciardini 9, 21100 Varese, Italy; e-mail: walter.ageno@uninsubria.it.
References
Author notes
Preliminary results of this study were part of an oral presentation at the 63rd annual American Society of Hematology meeting, Atlanta, GA, 13 December 2021.
The study protocol is available upon request to the corresponding author: walter.ageno@uninsubria.it.
The full-text version of this article contains a data supplement.