TO THE EDITOR:
Fanconi anemia (FA) is the most common inherited bone marrow failure syndrome and is related to defects in DNA repair pathways that predispose affected individuals to malignancies.1 Monomorphic posttransplant lymphoproliferative disorder (PTLD) is a rare complication of hematopoietic cell transplantation (HCT) and behaves like an aggressive malignancy, often treated with alkylator-based chemotherapy. The underlying DNA repair defect in FA increases susceptibility to significant HCT treatment-related morbidity and mortality and makes standard monomorphic PTLD therapy challenging. We hypothesized that the novel approach of blinatumomab immunotherapy would have efficacy against PTLD that expresses CD19, with minimal toxicity.
A 6-year-old male with FA, FANCA subtype, received an HLA-C 1-allele mismatched unrelated donor bone marrow HCT for progressive pancytopenia without evidence of myelodysplasia that was unresponsive to androgen therapy. On pretransplant evaluation, he was immunoglobulin G (IgG)–negative for Epstein-Barr virus (EBV). The donor’s EBV serologic status was unknown. He was enrolled in a phase 2 clinical trial (registered on ClinicalTrials.gov as #NCT03924401) and, per protocol, received a preparative regimen of thymoglobulin (12 mg/kg), fludarabine (150 mg/m2), and cyclophosphamide (30 mg/kg). Graft-versus-host disease (GVHD) prophylaxis included tacrolimus (serum target level, 8-12 ng/dL), mycophenolate mofetil through day 30, and the study drug abatacept on days −1, +5, +14, +28, +56, +87, +112, and +150. The patient had an uneventful early transplant course, with neutrophil engraftment on day +18. He was discharged on day +30 without evidence of GVHD and was on our institutional standard infectious prophylactic agents: acyclovir, Bactrim, and voriconazole at discharge.
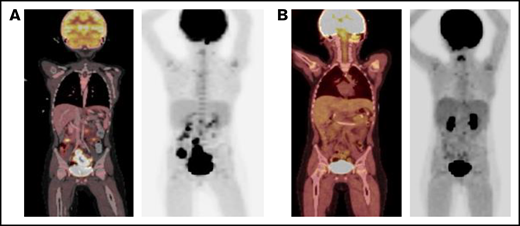
Routine plasma EBV polymerase chain reaction surveillance first showed a viral presence (1783 IU/mL) on day +63. The patient was admitted on day +85 with fever, abdominal pain, and marked EBV viremia (29 585 IU/mL). Weekly rituximab therapy was initiated, the serum tacrolimus prophylaxis goal was decreased to 5-8 ng/mL, and abatacept was discontinued. A positron emission tomography (PET) scan showed multifocal lymphadenopathy in the abdomen and pelvis, concerning for PTLD (Figure 1A). A core biopsy of the abdominal mass on day +100 confirmed monomorphic PTLD, diffuse large B-cell lymphoma subtype, EBV positive, with immunohistochemical stains expressing PAX5, CD30, CD45, CD79a, BCL6, MUM1, and EBER, but not CD3, CD10, CD138, TDT, HHV8, or ALK1. CD20 was positive in a small subset of cells. After 3 doses of rituximab, a second PET scan demonstrated increasing size of the abdominopelvic lymph nodes and new liver lesions.
PET scan response before and after blinatumomab treatment. (A) PET scan on day +92 showing concern for PTLD with multifocal, fluorodeoxyglucose-avid uptake and lymphadenopathy in the abdomen and pelvis. (B) PET scan on day +179 showing significant metabolic and structural improvement of abdominal and pelvic lesions 1 day after completion of blinatumomab. PET, positron emission technology; PTLD, posttransplant lymphoproliferative disease.
PET scan response before and after blinatumomab treatment. (A) PET scan on day +92 showing concern for PTLD with multifocal, fluorodeoxyglucose-avid uptake and lymphadenopathy in the abdomen and pelvis. (B) PET scan on day +179 showing significant metabolic and structural improvement of abdominal and pelvic lesions 1 day after completion of blinatumomab. PET, positron emission technology; PTLD, posttransplant lymphoproliferative disease.
Given this disease progression and the desire to avoid cytotoxic chemotherapy in a patient with FA, we administered EBV active, HLA-A24–restricted match (Michael D. Keller, Jeffrey Modell Diagnostic and Research Center for Primary Immunodeficiency Disorders at Children's National Health System, e-mail, 3 January 2021 and 2 January 2022 ), third-party, EBV-specific, cytotoxic T lymphocytes (CTLs) on day +116 (clinicaltrials.gov NCT03475212). EBV titers continued to increase 4 weeks after CTL infusion, with a peak at 90 872 IU/mL. A PET scan again showed disease progression, including new and worsening liver lesions and concern for bowel and sacral involvement with no central nervous system lesions. A biopsy of a liver lesion demonstrated ongoing PTLD, with similar immunohistochemical staining, as well as diffuse CD19+ staining. The cerebrospinal fluid and bone marrow aspiration were negative for disease. Given the improving immune reconstitution with a total CD3 count of 1342 cells per microliter, blinatumomab (15 μg/m2 per day) was administered on day +150 as a 28-day continuous IV infusion and was well tolerated. One day after completion of the infusion, a PET scan demonstrated significant metabolic and structural improvement of the liver, bowel, and sacral lesions with resolution of the abdominopelvic adenopathy (Figure 1B). EBV viremia decreased from 74 186 IU/mL at the time of blinatumomab initiation to undetectable over 39 days. At the time of this report, the patient is >1 year after HCT and 7 months after completion of blinatumomab and is clinically well without detectable EBV viremia, other viral reactivation, or clinical signs of GVHD. His clinical course is summarized in Figure 2.
Clinical course. Clinical course by EBV PCR titer, detailed with key events including timing of rituximab, CTLs, and blinatumomab. EBV, Epstein-Barr Virus; IR, interventional radiology; IU/mL, international units per milliliter; LP, lumbar puncture; PCR, polymerase chain reaction; PET, positron emission technology; PTLD, posttransplant lymphoproliferative disease.
Clinical course. Clinical course by EBV PCR titer, detailed with key events including timing of rituximab, CTLs, and blinatumomab. EBV, Epstein-Barr Virus; IR, interventional radiology; IU/mL, international units per milliliter; LP, lumbar puncture; PCR, polymerase chain reaction; PET, positron emission technology; PTLD, posttransplant lymphoproliferative disease.
This case highlights management challenges in the treatment of monomorphic PTLD after HCT in a patient with FA and describes the novel use of blinatumomab in the successful treatment of this life-threatening complication of HCT. The incidence of PTLD ranges from 0.5% to 17% of HCT recipients and typically develops during the first year after transplant.2,3 PTLD ranges from nondestructive PTLD (plasmacytic hyperplasia, infectious mononucleosis, and florid follicular hyperplasia) to polymorphic PTLD, to monomorphic PTLD (B- and T-cell neoplasms) and classic Hodgkin lymphoma.4 Impaired immune reconstitution with dysfunctional T-cell activity sets the stage for unchecked viral proliferation in EBV-driven PTLD. Identified risk factors for the development of EBV-driven PTLD after HCT include HLA mismatch and EBV recipient(−)/donor(+) status, reduced intensity conditioning, stages 2-4 acute GVHD, splenectomy, and mesenchymal stromal cell treatment.5 It has been shown that belatacept, a T-cell costimulatory blocking agent, has an associated risk of EBV-driven PTLD; however, a significant association between EBV-driven PTLD and abatacept, the shorter-acting T-cell costimulatory blocking agent used in our patient, has not been described, and trials are ongoing.6,7 PTLD treatments vary by subtype and patient characteristics. Given our patient’s underlying FA, we opted to use immune therapy up front to avoid the use of the traditional cytotoxic alkylating chemotherapy that is often used to treat monomorphic PTLD.
Monoclonal antibodies in addition to rituximab are used increasingly in PTLD. Daratumumab, an anti-CD38 monoclonal antibody, was used for the treatment of PTLD or PTLD-like disease after solid organ transplantation in 5 patients; all 5 patients had complete or significant hematologic response.8 Lenalidomide, an immunomodulator, has shown efficacy against PTLD; however, several reports of lenalidomide potentially inducing GVHD after allogeneic HCT prompted us to consider a more targeted treatment.9-11 Brentuximab vedotin, an anti-CD30 antibody drug conjugate, along with EBV-specific CTLs, sustained a complete response over 3.5 years in a patient with progressive EBV-driven PTLD after HCT.12
Immune therapy is another emerging treatment for PTLD. The efficacy of EBV-specific CTLs to treat EBV viremia and EBV-driven PTLD is under investigation. Given the lengthy process for generating donor-specific CTLs, third-party CTLs manufactured and banked in bulk can be used instead. In a phase 2 study of third-party EBV CTLs used to treat refractory EBV+ PTLD in 33 HCT recipients, a partial or complete response was achieved in 22 patients (68%) without significant toxicities13 ; GVHD is a rare complication despite the HLA mismatch.14 Blinatumomab is a bispecific T-cell antigen engager antibody (BiTE), engaging CD-3+ T-cells to CD19-expressing cells.15 In early relapses of pediatric B-cell acute lymphoblastic leukemia, blinatumomab resulted in complete response in 48% of patients, although cytokine release syndrome is a potential severe complication.15 Although there are no known reports of the use of blinatumomab in PTLD, given the diffuse CD19+ expression in affected tissue and known but uncommon side effects, we trialed a cycle of blinatumomab in our patient. Dexamethasone was given before infusion, and the patient did not experience cytokine release syndrome or any other side effects. He had a rapid clinical and laboratory improvement to treatment (Figures 1 and 2). Blinatumomab may be more accessible than CTLs and does not carry a risk of GVHD, making it an appealing alternative immune therapy approach.
In summary, we describe a challenging case of progressive monomorphic EBV-driven PTLD in a child with FA after HCT despite rituximab and EBV-specific CTL treatments, in which a complete response was achieved with blinatumomab. This case illustrates the utility of blinatumomab in the treatment of CD19+ PTLD. Moreover, this case highlights the importance of exploring novel target-specific immunotherapies for PTLD treatments, especially in patients intolerant of the toxicity of traditional chemotherapies.
Contribution: S.J. performed research and drafted the manuscript; M.S. and K.L. provided direct patient care, conceptualized the manuscript, and helped with writing the manuscript; K.L. was responsible for clinical decision making and followed and analyzed the results; S.A. and F.K. provided input, assisted with immune therapy, and edited the manuscript; E.H., B.W., K.W., S.C., S.P., and M.Q., provided direct patient care and edited the manuscript; A.A. interpreted all radiology scans and selected scans for the figures in the manuscript; and all authors reviewed and approved the final manuscript before submission and agreed to be accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kathryn Leung, Children’s Healthcare of Atlanta, 1760 Haygood Drive, W356 (HSRB Bridge), Atlanta, GA 30322; e-mail: Kathryn.Leung@emory.edu.
References
Author notes
M.S. and K.L. are joint senior authors.
Requests for data sharing may be submitted to Kathryn Leung (kathryn.leung@emory.edu).