Key Points
Watchful waiting describes maintaining a warfarin dose after a slightly out-of-range INR and retesting within the following 2 weeks.
Although a warfarin dose change is more effective in producing a therapeutic INR, our study supports ACCP guidelines for watchful waiting.
Abstract
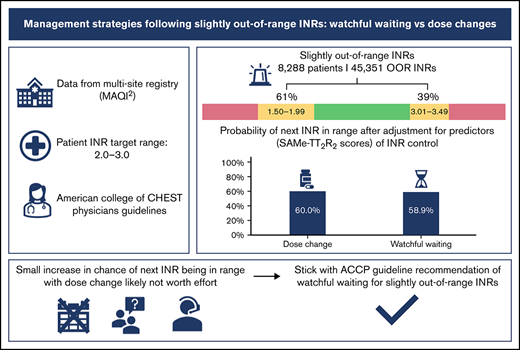
Patients’ international normalized ratios (INRs) often fall slightly out of range. In these cases, the American College of Chest Physicians (ACCP) guidelines suggest maintaining the current warfarin dose and retesting the INR within the following 2 weeks (watchful waiting). We sought to determine whether watchful waiting or dose changes for slightly out-of-range INRs is more effective in obtaining in-range INRs at follow-up. INRs and management strategies of warfarin-treated patients within the Michigan Anticoagulation Quality Improvement Initiative registry were analyzed. Management strategies included watchful waiting or dose changes. INRs slightly out of range (target range 2.0-3.0) and their associated management were identified. Multilevel mixed-effects logistic regression was used to estimate the probability of the next INR being in range, adjusted for clustering due to multiple out-of-range INRs per patient. A total of 45 351 slightly out-of-range INRs (ranging 1.50-1.99 and 3.01-3.49) from 8288 patients were identified. The next INR was slightly less likely to be in range with watchful waiting than with a dose change (predicted probabilities 58.9% vs 60.0%, P = 0.024). Although a significant statistical difference was detected in the probabilities of the next INR being back in range when managed by a dose change compared with watchful waiting following a slightly out-of-range INR, the magnitude of the difference was small and unlikely to represent clinical importance. Our study supports the current guideline recommendations for watchful waiting in cases of slightly out-of-range INRs values.
Introduction
Higher time in therapeutic range is a predictor of positive outcomes such as decreased risk of death, stroke, and major bleeding events.1-3 However, time in therapeutic range has been shown to be affected by a multitude of factors including adherence to regimen, diet, patient demographics, and comorbidities.4-8 Most of these factors lie outside the realm of adjustment, and clinicians must rely primarily on dose adjustments to maintain therapeutic international normalized ratios (INRs).
The American College of Chest Physicians (ACCP) guidelines recommend a therapeutic INR range of 2.0 to 3.0 for most indications for warfarin, including atrial fibrillation, venous thromboembolic disease, and low-risk mechanical aortic valve replacement.9 At any given time, 50% of patients receiving care within an anticoagulation management clinic may be outside of the recommended range, with 30% only slightly out of range (1.75-1.99 or 2.01-2.25).10 When an INR is found to be slightly out of range, clinicians must select a management strategy to reestablish a therapeutic INR. Warfarin management strategies include a weekly dose change and/or a single-dose change or no dose change. In cases where the INR is slightly out of range, 0.5 above or below the therapeutic range, ACCP guidelines suggest maintaining the patient’s current warfarin dosing and retesting the INR within the following 2 weeks.9 This management strategy is also known as watchful waiting.
The recommendation of the ACCP guidelines was based upon 2 studies, which sought to determine whether any clinical differences exist between the implementation of watchful waiting and any dose change for slightly out-of-range INRs. A retrospective study conducted by Banet et al11 found there was no appreciable difference between watchful waiting and a dose change ≤20% of the weekly warfarin regimen. The findings of the study were based on 231 patients, but few exclusion criteria were used.11 Schulman et al12 conducted a similar retrospective study in addition to a prospective study, which found there was little to no difference between watchful waiting and single-dose change in stable patients. In their sample of INRs (n = 364), they included multiple therapeutic INR ranges, 2 to 3 and 2.5 to 3.5, and did not include INRs with weekly dose changes.12 Although both studies appear to support the use of watchful waiting, there is an opportunity to validate the findings in a larger sample of patients using methods that address all possible management strategies and account for patient factors that may influence decisions.
The present study sought to determine if the ACCP guidelines are supported by data from a large, multicenter clinical registry. We aimed to see if dose changes had meaningful impact on INR control or should watchful waiting remain the preferred strategy.
Methods
Study population
Patients were selected from the Michigan Anticoagulation Quality Improvement Initiative (MAQI2) database. MAQI2 is a Blue Cross Blue Shield and Blue Care Network–sponsored collaborative of 6 anticoagulation management services in the state of Michigan and has been previously described.13 Trained and audited MAQI2 abstractors collected patient information from the electronic medical record, including INR values and warfarin management strategies, from 10/12/2009 to 10/30/2019. Both active patients and nonactive patients were included. Patients were included if they had a target INR range of 2.0 to 3.0 and at least 1 slightly out-of-range INR defined as up to 0.5 above or below the therapeutic range. Patients with any indication for warfarin were included.
Protocol
Out-of-range INRs from patients were excluded if there was a known contributory factor (eg, illness or change in diet) or if a dietary vitamin K recommendation was also made. Lastly, to establish patient stability, out-of-range INRs were excluded if the previous INR was also out of range or if the INR was obtained within the first month of starting or restarting warfarin. For each patient, every eligible out-of-range INR value was included as an individual data point.
The management strategies selected by the anticoagulation management services staff member to respond to the out-of-range INRs were categorized into either watchful waiting or dose change. A dose change refers to any change in the warfarin regimen regardless of the magnitude of change recommended. The dose change group was further categorized into subgroups of weekly dose changes, 1-time dose changes, or both. SAMe-TT2R2 (sex, age, medical history, treatment, tobacco, and race)4 scores were assessed at the time of enrollment into the anticoagulation clinic for each patient. A SAMe-TT2R2 of ≥2 has been shown to predict poor INR control and the need for additional clinical interventions among patients on warfarin.4,14 A score of ≥2 was used in our regression model. Our primary outcome was the subsequent INR returning to the therapeutic range (2.0-3.0). The institutional review board of the University of Michigan (MAQI2 coordinating center) approved the registry, and a waiver of informed consent was implemented at each of the participating centers (1 site collects informed consent on all participating patients). The study was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
Multilevel mixed-effects logistic regression was used to account for clustering of multiple out-of-range INRs from the same patient. The INR-level covariates consisted of management strategy and number of days between the indexed out-of-range INR and the next INR check. The patient-level covariates were anticoagulation clinic that managed patient treatment and SAMe-TT2R2 score ≥2. The results are presented in predicted probabilities and marginal effects. A P value <.05 is considered statistically significant. The analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and STATA 16.1 (StataCorp LLC, College Station, TX).
Results
A total of 8288 patients with 45 351 INRs met criteria, and the majority of out-of-range INRs (61.2%) were slightly below the therapeutic range (1.50-1.99), whereas 38.8% were slightly above (3.01-3.49). As described by the univariable analysis, a dose change resulted in a therapeutic INR at follow-up more often than watchful waiting (60.3% vs 59.3%, respectively). Dose changes resulted in more therapeutic INRs at follow-up compared with watchful waiting (62.4% vs 58.8%) in the supra-therapeutic group but had no difference in the subtherapeutic group (59.1% vs 59.6%) (Table 1). Of those with next INRs out of range, dose changes were more likely to overcorrect (from below 2.0 to above 3.0 or vice versa) than watchful waiting (overall, 26.2% vs 20.3%; subtherapeutic group, 21.3% vs 18.1%; supra-therapeutic group, 35.7% vs 23.1%). A slight majority of patients (56.6%) had a SAMe-TT2R2 score of ≥2, indicating higher risk for poor INR control. Breaking down patients’ characteristics that compose the SAMe-TT2R2 score, 3952 (47.7%) patients were female, 5458 (65.9%) had hypertension, 2517 (30.4%) had a medical history of coronary artery disease and/or myocardial infarction, 2115 (25.5%) were diabetic, and 4319 (52.1%) had 2 or more adverse comorbidities (Table 2).
Success of warfarin management strategies following slightly out-of-range INR values
| . | No. of INRs . | Dose change (weekly +/− 1 time) . | Watchful waiting . | ||
|---|---|---|---|---|---|
| n (%) . | n (%) next INR in range . | n (%) . | n (%) next INR in range . | ||
| Overall | 45 351 | 27 475 (60.6) | 16 558 (60.3) | 17 876 (39.4) | 10 594 (59.3) |
| 1.50-1.99 | 27 749 | 17 647 (63.6) | 10 422 (59.1) | 10 102 (36.4) | 6 024 (59.6) |
| 3.01-3.49 | 17 602 | 9 828 (55.8) | 6 136 (62.4) | 7 774 (44.2) | 4 570 (58.8) |
| . | No. of INRs . | Dose change (weekly +/− 1 time) . | Watchful waiting . | ||
|---|---|---|---|---|---|
| n (%) . | n (%) next INR in range . | n (%) . | n (%) next INR in range . | ||
| Overall | 45 351 | 27 475 (60.6) | 16 558 (60.3) | 17 876 (39.4) | 10 594 (59.3) |
| 1.50-1.99 | 27 749 | 17 647 (63.6) | 10 422 (59.1) | 10 102 (36.4) | 6 024 (59.6) |
| 3.01-3.49 | 17 602 | 9 828 (55.8) | 6 136 (62.4) | 7 774 (44.2) | 4 570 (58.8) |
Patient demographics and comorbidities
| Enrollment . | Patients (n = 8288) . |
|---|---|
| SAMe-TT2R2 ≥2 | 4693 (56.6) |
| Female, n (%) | 3952 (47.7) |
| Age <60 y, n (%) | 2515 (30.3) |
| Non-White,* n (%) | 1429/7869 (18.2) |
| Medical history (≥2), n (%) | 4319 (52.1) |
| Hypertension | 5458 (65.9) |
| CAD/MI† | 2517 (30.4) |
| Diabetes mellitus | 2115 (25.5) |
| CHF‡ | 1622 (19.6) |
| CRI§ | 1153 (13.9) |
| Stroke | 1005 (12.1) |
| PAD∥ | 514 (6.2) |
| Chronic liver disease | 179 (2.2) |
| Treatment,¶ n (%) | 1233 (14.9) |
| Current tobacco use, n (%) | 674 (8.1) |
| Enrollment . | Patients (n = 8288) . |
|---|---|
| SAMe-TT2R2 ≥2 | 4693 (56.6) |
| Female, n (%) | 3952 (47.7) |
| Age <60 y, n (%) | 2515 (30.3) |
| Non-White,* n (%) | 1429/7869 (18.2) |
| Medical history (≥2), n (%) | 4319 (52.1) |
| Hypertension | 5458 (65.9) |
| CAD/MI† | 2517 (30.4) |
| Diabetes mellitus | 2115 (25.5) |
| CHF‡ | 1622 (19.6) |
| CRI§ | 1153 (13.9) |
| Stroke | 1005 (12.1) |
| PAD∥ | 514 (6.2) |
| Chronic liver disease | 179 (2.2) |
| Treatment,¶ n (%) | 1233 (14.9) |
| Current tobacco use, n (%) | 674 (8.1) |
CAD/MI, XXX; CRI, XXX; CHF, XXX; PAD, XXX.
Race was self-reported based on medical charts.
Coronary artery disease/myocardial infarction.
Congestive heart failure.
Chronic renal insufficiency.
Peripheral artery disease.
Concurrent use of interacting medication: fluconazole, amiodarone, Bactrim, Flagyl, carbamazepine, phenobarbital, primidone, phenytoin, and rifampin.
The multilevel logistic regression showed that for slightly subtherapeutic out-of-range INRs, the predicted probabilities of next INR back in range were 58.8% after dose change and 59.3% after watchful waiting, with a marginal effect −0.5% (−1.7% to 0.8%) and P = .46. For slightly supra-therapeutic out-of-range INRs, predicted probabilities were 61.8% after dose change vs 58.2% after watchful waiting, with a marginal effect 3.6% (2.1% to .1%) and P < .001. Overall, the analysis found that watchful waiting was slightly less effective in producing a therapeutic follow-up INR compared with a dose change, 58.9% vs 60.0%, with a marginal effect of 1.1% (0.1% to 2.1%), P = .024 (Table 3).
Multilevel logistic regression model for slightly out-of-range INRs
| . | Predicted probability of next INR in range . | |||
|---|---|---|---|---|
| Dose change (weekly +/− 1 time) . | Watchful waiting . | Marginal effect . | P . | |
| Overall | 60.0 (59.3-60.6) | 58.9 (58.1-59.7) | 1.1 (0.1-2.1) | .024 |
| 1.50-1.99 | 58.8 (58.0-59.6) | 59.3 (58.3-60.3) | −0.5 (−1.7-0.8) | .46 |
| 3.01-3.49 | 61.8 (60.8-62.8) | 58.2 (57.1-59.4) | 3.6 (2.1-5.1) | <.001 |
| . | Predicted probability of next INR in range . | |||
|---|---|---|---|---|
| Dose change (weekly +/− 1 time) . | Watchful waiting . | Marginal effect . | P . | |
| Overall | 60.0 (59.3-60.6) | 58.9 (58.1-59.7) | 1.1 (0.1-2.1) | .024 |
| 1.50-1.99 | 58.8 (58.0-59.6) | 59.3 (58.3-60.3) | −0.5 (−1.7-0.8) | .46 |
| 3.01-3.49 | 61.8 (60.8-62.8) | 58.2 (57.1-59.4) | 3.6 (2.1-5.1) | <.001 |
Discussion
We opined that there would be no appreciable difference in watchful waiting and dose changes as a management strategy for slightly out-of-range INRs, and confirmation would reinforce ACCP guidelines, which suggest watchful waiting. The results would also build upon prior research, which found no appreciable difference between watchful waiting and any dose change. Our study found that dose change after adjustment for the SAMe-TT2R2 score had a small, statistically significant improvement in the next INR being in range. However, the magnitude of 1.1% for all patients with slight out-of-range INRs and 3.6% for slightly supra-therapeutic (3.01-3.49) INRs likely does not justify the added burden placed on patients and clinicians when implementing dose changes. We feel the magnitude of improvement is not clinically meaningful.
Prior studies have shown there to be no appreciable difference between watchful waiting and dose change when obtaining a follow-up therapeutic INR in previously stable patients with an out-of-range INR.11,12 The difference in outcomes between our study and previous studies may be attributed to the fact that we accounted for patient risk factors by using SAMe-TT2R2 scores. By adjusting SAMe-TT2R2 scores, underlying patient risk factors were better accounted for and help to explain differences in management strategy selection by clinicians. In addition, A large sample size of INR values made it likely that we would find a statistically significant difference with small absolute differences.
Dose changes have been shown to be a barrier for adherence in patients who have been prescribed warfarin.6,15-17 In order to improve and maintain adherence among patients, clinicians may want to limit the number of unnecessary dose changes prescribed to a patient. Watchful waiting decreases the time required for patient care, making it more efficient. If watchful waiting is used, follow-up testing at 2 weeks should still be implemented to ensure that the management strategy chosen produces therapeutic INRs, concurrent with previous studies and the ACCP guidelines.9,11,12
Limitations
As with all retrospective studies, we were not able to control for unknown confounding variables and, causation cannot be assumed. Additionally, we did not have data for changes in diet, exercise, or adherence. Our outcome of interest was the next INR, and we do not report bleeding or thromboembolic events. Strengths of our study include data from 6 anticoagulation management services, albeit all from 1 geographic area, and adjustment utilizing the SAMe-TT2R2 score.
Conclusion
Across all slightly out-of-range INRs, watchful waiting was less likely to result in the follow-up INRs being in range compared with a warfarin dose adjustment. For slightly elevated INRs, we had similar finding. But we didn’t find difference in the likelihood of the follow-up INRs being in range between the 2 strategies. The magnitude of these differences was small and is unlikely to represent clinical importance. Our study supports current guidelines that suggest watchful waiting in cases of slightly out-of-range INRs.
Authorship
Contribution: H.B.R. drafted the paper and contributed to the conception and design of the work; X.G. performed analysis of the data; B.H. contributed to the conception and design of the work; S.K. contributed to the conception and design of the work; and all authors made substantial contributions to the acquisition and interpretation of data and paper revisions, and gave approval of the final version.
Conflict-of-interest disclosure: G.D.B. reports the following disclosures: consulting (Pfizer/Bristol-Myers Squibb, Janssen, Acelis Connected Health, AMAG Pharmaceuticals) and board of directors (Anticoagulation Forum, National Certification Board of Anticoagulation Providers). J.B.F. reports the following disclosures: consultant/advisory role (Janssen Pharm, Merck, Boehringer-Ingelheim, Pfizer, Novartis). S.K. reports the following disclosures: research funding to institution (BMS, Osmosis Research, Janssen), consultant (Janssen, BMS, Pfizer, Portola/Alexion, Novartis, CSL Behring, Gilead), and nonprofit board of directors (Anticoagulation Forum, Scientific Advisory Board for the National Blood Clot Alliance). The remaining authors declare no competing financial interests.
Correspondence: Hallie Remer, Michigan Cardiovascular Outcomes Research and Reporting Program (MCORRP), Domino's Farms, 24 Frank Lloyd Wright Drive, Lobby A/3201, Ann Arbor, MI 48106-0384; e-mail: remerh@umich.edu.
References
Author notes
Requests for data sharing may be submitted to Hallie Remer (remerh@umich.edu).
The full-text version of this article contains a data supplevment.