Key Points
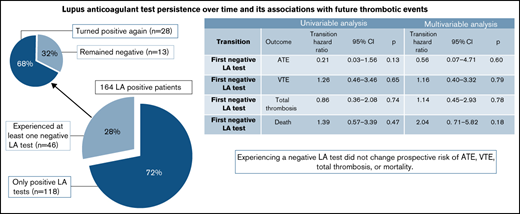
Twenty-eight percent of patients persistently positive for LA had a negative LA test during observation; most, subsequently, tested positive.
A negative LA test had no impact on a patient’s prospective risk of thrombosis or mortality.
Abstract
Data on lupus anticoagulant (LA) test stability in patients persistently positive for LA are limited, and its implications on clinical outcomes are lacking. We investigated the rate and predictors of a negative LA test and whether experiencing a negative test affected a patient’s risk of future thrombotic events or death in a prospective observational study of persistently LA+ patients. We followed 164 patients (84% women) for a median of 9.2 years and a total of 1438 follow-up visits. During the observation period, 50 thrombotic events (23 arterial and 27 venous events) occurred, and 24 patients died. Forty-six of the patients had at least 1 negative LA test during the observation period, corresponding to a 10-year cumulative incidence of a negative LA test of 28% (95% confidence interval, 20-35). The majority of patients with available follow-up after a negative LA test (n = 41) had at least 1 subsequent positive test for LA (n = 28/41, 68%). Vitamin K antagonist (VKA) treatment at baseline was associated with a negative LA test during follow-up. Using a multistate time-to-event model with multivariable adjustment, a negative LA test had no impact on a patient’s prospective risk of thrombosis or mortality. We conclude that a negative LA test during observation cannot be used clinically to stratify a patient’s risk for future events.
Introduction
Antiphospholipid antibodies (aPL) are a heterogenous group of antibodies directed against anionic phospholipids and phospholipid-binding plasma proteins. The association between lupus anticoagulant (LA), the first aPL identified, and thrombosis, both arterial and venous, was described in the 1960s.1 Additional antibodies, most notably anticardiolipin antibodies (aCL) and anti–glycoprotein-I antibodies (aβ2GPI), were later identified and associated with thrombosis and recurrent spontaneous abortions.2-4 The presence of 1 or more of these aPL for 12 or more weeks constitutes the laboratory criteria for antiphospholipid syndrome (APS), an autoimmune disorder defined clinically by arterial and venous thrombotic events and/or pregnancy complications.5
Patients with persistent aPL with or without APS are at increased risk of future thrombotic events, LA positivity having the strongest association with these events.3 Although it is known that persistence, defined currently by 12 weeks, is associated with thrombotic risk, little is known about the stability of these antibodies beyond this time, predictors of persistence, and whether subsequent fluctuations in antibody status affect future risk of thrombosis.6 These trends and predictors may be helpful in understanding or characterizing the clinical heterogeneity observed in patients with aPL with and without APS. Notably, most prior efforts to risk-stratify patients for future thrombotic events have used the patients’ aPL status from the first measurement or early in the observation period and have not analyzed changes over time.3,7,8
Recent work from Gkrouzman et al found that ∼80% of their patients with clinically meaningful aPL at baseline had stable antibodies, defined by a meaningful antibody profile in two-thirds of testing, over time.9 In a second retrospective recent study looking at stability of aPL positivity in 55 patients with systemic lupus erythematosus (SLE) and secondary APS, ∼70% of patients remained persistently positive. Treatment with immunosuppression was predictive of development of seronegativity, whereas triple positivity and diagnosis of APS prior to SLE were predictive of aPL stability over time.10 Overall, limited prospective data on the variability of aPL over time and no data on the clinical implications of this variability, specifically if it is predictive of future thrombotic events or mortality, are available
We conducted a prospective observational cohort study on patients persistently positive for LA with and without a history of thrombosis or pregnancy complications: the Vienna Lupus Anticoagulant and Thrombosis Study (LATS). The aim of this present analysis of LATS is to evaluate the development of a negative LA test over time in these patients, to explore variables that are associated with a negative LA test, and to determine the effect of a negative LA test on the risk of future thrombotic events and mortality.
Methods
Study design
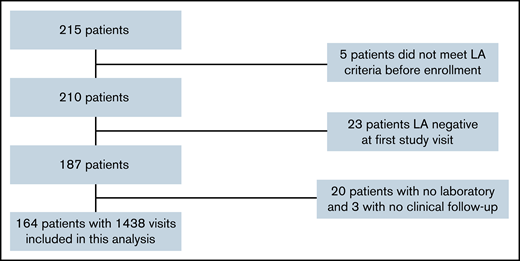
LATS is an ongoing, single-center, biobank-based prospective observational cohort study that enrolls adult patients who repeatedly test positive for LA (defined by 2 positive tests at least 6 or 12 weeks apart according to guidelines at the time of inclusion).5,11 Patients are enrolled with or without history of thrombosis or pregnancy complications and asked to return for clinical and laboratory follow-up every 6 months for the first 5 years and annually thereafter. Additional details of the study design have been previously reported.12 All patients provided informed consent and the study was approved by the ethics committee at the Medical University of Vienna. (Ethics application 068/2001 and 1268/2014). For this analysis, we considered all n = 215 adult patients enrolled in LATS as of October 2020. We excluded 5 patients because they did not meet laboratory criteria before enrollment or at least once during the study. An additional 23 patients were excluded because they tested negative for LA at their first study visit. Finally, 20 patients were excluded because they had no laboratory follow-up and 3 patients because they had no clinical follow-up (Figure 1).
At each follow-up visit, an updated patient history including detailed questions regarding interval thrombotic events and anticoagulation status was obtained. Arterial thromboembolism (ATE) included acute coronary syndrome, peripheral artery occlusion, transient ischemic attack, and ischemic stroke. Venous thromboembolism (VTE) included pulmonary embolism, deep venous, hepatic vein, retinal vein, and adrenal vein thrombosis. Thrombotic events were verified with appropriate diagnostic imaging tests and evaluated by an adjudication committee of designated experts in the field. SLE and lupus-like disease (LLD) were defined as previously described.12 Additionally, in October 2020, the electronic medical record was reviewed for interval clinical events and both the electronic medical record and the national death registry of Austria were reviewed for deaths.
Determination of LA and LA-associated autoantibodies
Blood samples were drawn with a 21-gauge butterfly needle (Greiner Bio-One, Kremsmünster, Austria) into vacuette tubes (Greiner Bio-One) containing trisodium citrate (9 parts of whole blood, 1 of trisodium citrate 3.8%) for the determination of LA and containing Z Serum Sep Clot Activator for the determination of aCL and aβ2GPI antibodies. All samples were mixed adequately and were processed within 3 hours after venipuncture. The determination of aPL was performed as previously described.12 LA positivity was determined following the Scientific and Standardization Committee (SSC)/International Society on Thrombosis and Hemostasis (ISTH) recommendations at the time of testing.11,13 A lupus-sensitive activated partial thromboplastin time (PTT-LA, Diagnostica Stago, Asniere-sur-Seine, France) and a dilute Russell’s viper venom time (dRVVT) were used as screening tests. Only the PTT-LA was used for screening in patients receiving vitamin K antagonism (VKA) therapy. In the case of prolongation of 1 or both screening tests, mixing and confirmatory tests were completed. The StaClot LA (Diagnostica Stago) and the dilute Russell’s viper venom time LA confirm (Life Diagnostics, Clarkston, GA, USA) were used as the confirmatory assays.
As there are no clearly defined criteria for LA positivity in patients on VKA treatment, and as it is not possible to interrupt VKA in patients with history of thrombosis just for study purposes, we used the Rosner Index in addition to the predefined LA criteria.14 In this study, for patients receiving VKA therapy, a LA test was positive if the confirmatory assay was positive and the mixing study had a Rosner Index ≥15.13-15 The Rosner Index was calculated as 100× (clotting times of the 1:1 mixture, normal plasma)/patient’s plasma. For patients not receiving anticoagulation or on unfractionated heparin or low molecular weight heparin (LMWH) with an anti-Xa level ≤1.0 internal unit (IU) per mL, a LA test was positive if either the confirmatory test was positive or the mixing study was positive (again defined by the Rosner Index). Mixing and confirmatory tests were not completed when patients receiving LMWH had an anti-Xa level >1.0 internal unit per mL, and these LA tests were excluded from this analysis. LA tests completed while patients were receiving direct oral anticoagulants were also excluded.
Immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies against cardiolipin and β2-GPI were determined using commercially available indirect solid-phase enzyme immunoassays. Between 2001 and September 2005, IgG and IgM aCL were determined using the Varelisa Cardiolipin test (Pharmacia [Phadia AB], Uppsala, Sweden) performed semiautomatically on the Tecan Genesis liquid handling system (Tecan Group Ltd., Maennedorf, Switzerland). From October 2005, the Orgentec Cardiolipin, and from October 2006, the Orgentec aβ2GPI tests (both Orgentec, Mainz, Germany), were performed on a fully automated BEP2000 Advanced System (Siemens Healthcare Diagnostics, Marbury, Germany). Triple antibody positivity was defined by the following cutoffs: aCL were regarded as positive if >40 IgG phospholipid units (GPL)/IgM phospholipid units (MPL) U/mL (VarelisaCardiolipin and Orgentec Cardiolipin test), according to the recommendations made in 2006, and positivity for aβ2GPI IgG and IgM was defined as results >8 GPL/MPL U/mL, corresponding to the 99th percentile of healthy controls (Orgentec aβ2GPI test).5 IgG and IgM antibodies against prothrombin were determined using a commercially available enzyme-linked immunoassay CoaChrom Anti-Prothrombin (HYPHEN BioMed, Neuville-sur-Oise, France). Positivity for antiprothrombin IgM and IgG antibodies was defined as >90th percentile of the levels of 33 healthy volunteers without a history of thrombosis.16
Statistical methods
All statistical analyses were performed with Stata 15.1 (Stata Corp., Houston, TX, USA). Continuous variables were reported as medians (25th-75th percentile) and count data as absolute frequencies (percent). Median follow-up was estimated with the reverse Kaplan-Meier estimator.17 Cumulative incidences of ATE, VTE, and total thrombosis were estimated with competing risk cumulative incidence estimators, and risks of death were estimated with 1-Kaplan-Meier estimators.18 Similarly, competing risk cumulative incidence estimators were implemented to compute the risks of a change in LA test status. Thrombosis, LA test status change, and mortality risk curves were compared with Gray’s tests and log-rank tests as appropriate.19 Uni- and multivariable modeling of the risk of change in LA status was performed with Fine & Gray competing risk regression models. The association between changes in LA test status and LA-related biomarkers (lupus-sensitive aPTT [aPTT-LA], aCL, aβ2GPI) was quantified with linear mixed effects model with a random intercept for the dependent variables.20 To gauge the impact of changes in LA test status on 4 clinical outcomes (ATE, VTE, total thrombosis, and death-from-any-cause), we used unidirectional “illness-death” multistate models21 and visualized these associations with landmark analysis (landmark set empirically at 1.5 years after study inclusion, Mantel-Byar test).22 Missing data were reported in Table 1 and supplemental Figure 1, and a complete case analysis was performed. The full analysis code is available on reasonable request from F.P.
Baseline characteristics of the study population (n=164)
| Variables . | n (% miss.) . | Overall . |
|---|---|---|
| Demographic characteristics | ||
| Age at entry, years (IQR) | 164 (0%) | 41 (31-58) |
| Female Gender, n (%) | 164 (0%) | 137 (84%) |
| Clinical history, n (%) | ||
| Prior thrombosis | 164 (0%) | 98 (60%) |
| Arterial | 164 (0%) | 26 (16%) |
| Venous | 164 (0%) | 78 (48%) |
| Both | 164 (0%) | 6 (4%) |
| Prior pregnancy complications* | 102 (0%) | 50 (49%) |
| Established APS | 164 (0%) | 118 (72%) |
| Comorbidities at baseline, n (%) | ||
| Autoimmune rheumatic diseases† | 164 (0%) | 45 (27%) |
| SLE | 164 (0%) | 26 (16%) |
| LLD | 164 (0%) | 18 (11%) |
| Active smoker at baseline | 163 (1%) | 55 (34%) |
| Hypertension | 163 (1%) | 48 (29%) |
| Diabetes | 164 (0%) | 13 (8%) |
| Anticoagulation at baseline, n (%) | ||
| VKA | 164 (0%) | 49 (30%) |
| LMWH | 163 (1%) | 23 (14%) |
| LDA | 163 (1%) | 50 (31%) |
| None | 163 (1%) | 67 (41%) |
| Disease-related autoantibodies, n (%) | ||
| LA+ only | 164 (0%) | 39 (24%) |
| IgM aCL–positive‡ | 164 (0%) | 24 (15%) |
| IgG aCL–positive‡ | 164 (0%) | 53 (32%) |
| aCL+ (IgM and/or IgG)‡ | 164 (0%) | 67 (41%) |
| IgM aβ2GPI–positive‡ | 145 (12%) | 63 (43%) |
| IgG aβ2GPI–positive‡ | 162 (1%) | 87 (54%) |
| aβ2GPI+ (IgM and/or IgG)‡ | 153 (7%) | 113 (74%) |
| “Triple positive”§ | 152 (7%) | 64 (42%) |
| Antiprothrombin IgM–positiveǁ | 119 (27%) | 26 (22%) |
| Antiprothrombin IgG–positive-positiveǁ | 119 (27%) | 30 (25%) |
| Antiprothrombin+ (IgM and/or IgG)ǁ | 119 (27%) | 49 (41%) |
| Variables . | n (% miss.) . | Overall . |
|---|---|---|
| Demographic characteristics | ||
| Age at entry, years (IQR) | 164 (0%) | 41 (31-58) |
| Female Gender, n (%) | 164 (0%) | 137 (84%) |
| Clinical history, n (%) | ||
| Prior thrombosis | 164 (0%) | 98 (60%) |
| Arterial | 164 (0%) | 26 (16%) |
| Venous | 164 (0%) | 78 (48%) |
| Both | 164 (0%) | 6 (4%) |
| Prior pregnancy complications* | 102 (0%) | 50 (49%) |
| Established APS | 164 (0%) | 118 (72%) |
| Comorbidities at baseline, n (%) | ||
| Autoimmune rheumatic diseases† | 164 (0%) | 45 (27%) |
| SLE | 164 (0%) | 26 (16%) |
| LLD | 164 (0%) | 18 (11%) |
| Active smoker at baseline | 163 (1%) | 55 (34%) |
| Hypertension | 163 (1%) | 48 (29%) |
| Diabetes | 164 (0%) | 13 (8%) |
| Anticoagulation at baseline, n (%) | ||
| VKA | 164 (0%) | 49 (30%) |
| LMWH | 163 (1%) | 23 (14%) |
| LDA | 163 (1%) | 50 (31%) |
| None | 163 (1%) | 67 (41%) |
| Disease-related autoantibodies, n (%) | ||
| LA+ only | 164 (0%) | 39 (24%) |
| IgM aCL–positive‡ | 164 (0%) | 24 (15%) |
| IgG aCL–positive‡ | 164 (0%) | 53 (32%) |
| aCL+ (IgM and/or IgG)‡ | 164 (0%) | 67 (41%) |
| IgM aβ2GPI–positive‡ | 145 (12%) | 63 (43%) |
| IgG aβ2GPI–positive‡ | 162 (1%) | 87 (54%) |
| aβ2GPI+ (IgM and/or IgG)‡ | 153 (7%) | 113 (74%) |
| “Triple positive”§ | 152 (7%) | 64 (42%) |
| Antiprothrombin IgM–positiveǁ | 119 (27%) | 26 (22%) |
| Antiprothrombin IgG–positive-positiveǁ | 119 (27%) | 30 (25%) |
| Antiprothrombin+ (IgM and/or IgG)ǁ | 119 (27%) | 49 (41%) |
n (% miss.)” reports the number of patients with fully observed data (% missing). IQR, interquartile range (median [25th-75th] percentile); LDA, low-dose aspirin.
Pregnancy complications were defined according to Sapporo criteria in the subgroup of 117 females who had at least 1 documented pregnancy.
Autoimmune rheumatic diseases were defined as a composite of systemic lupus erythematosus and lupus-like disease according to a local panel of rheumatology experts.
Cutoffs were defined as follows according to ISTH/Sapporo criteria cutoffs: aCL >40GPL/MPL U/mL, >8 GPL/MPL U/mL.
Triple-positivity was defined as being positive for LA and at least one class of immunoglobulins (IgM or IgG) for aCL and aβ2GPI.
Cutoffs were defined by the 90th percentile in 33 healthy volunteers without history of thrombosis, antiprothrombin IgM ≥11.54 U/mL, antiprothrombin IgG ≥8.68 U/mL.
Results
Study cohort and follow-up
We included 164 patients in the analysis (Figure 1), the characteristics of whom are presented in Table 1. One hundred thirty-seven patients (84%) were women, and the median age at time of enrollment was 41 years old (25th-75th percentile, 31-58). Sixty percent of patients had a history of any thrombosis, including arterial thrombosis (26 patients, 16%), venous thrombosis (78 patients, 48%), or both (6 patients, 4%). According to inclusion criteria, all patients were positive for LA, but patients had varying positivity for aCL, aβ2GPI, or both. The proportion of patients with “triple-positivity” at baseline was 42%. Over time, the 164 patients returned for a total of 1438 follow-up visits, corresponding to a mean number of 9 visits per patient [range, 2-25] and a median time of 182 days [range, 63-3084] between visits. Overall, missing data for key variables was minimal except for aβ2GPI (as this parameter was established after the inception of the cohort, supplemental Figure 1). During a median follow-up for thrombosis of 9.2 years (with 75% and 25% of the cohort being followed-up for at least 4.0 and 14.9 years, respectively), we observed 23 prospective ATEs, 27 prospective VTEs, and 24 patients died. This corresponded to 10-year prospective ATE, VTE, and overall thrombosis incidences of 14% (95%CI: 9-21), 20% (13-28), and 34% (26-43), respectively, and a 10-year mortality of 14% (9-22) (supplemental Figure 2).
LA stability over time
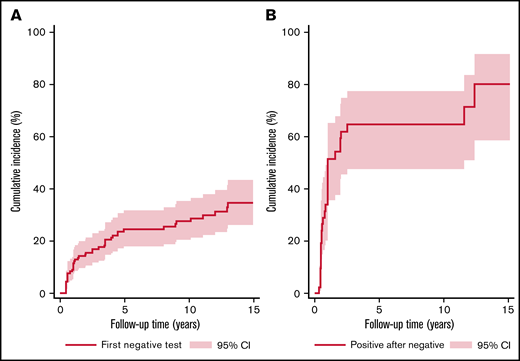
During the observation period, 118 patients (72%) had stable LA status, defined by having only positive LA tests. The other quarter of the study cohort (n = 46, 28%) had at least 1 negative LA test. In detail, among these 46 patients, we observed a median number of 2 within-patient changes in LA status (25th-75th percentile, 1-2; range, 1-6), and a median number of 0.3 within-patient changes in LA status per year of observation time (25th-75th percentile, 0.2-0.5; range, 0.05-1.7). Overall, this corresponded to a 10-year cumulative incidence of a negative LA test of 28% (95% confidence interval [CI], 20-35, Figure 2A).
Change in LA test status over time. (A) Cumulative incidence of first negative LA test over time. Line shows the risk of developing at first negative LA test in the study cohort (n = 164), with the x-axis representing the time in years from study inclusion. (B) Cumulative incidence of next positive LA test after first negative LA test over time. Line reports the risk of a positive LA test after the first negative LA test in the n = 42 patients who experienced a first LA negative test and had follow-up. The x-axis represents the time elapsed since the first negative LA test. Curves were estimated with competing risk estimators. Gray shaded area represents 95% confidence interval.
Change in LA test status over time. (A) Cumulative incidence of first negative LA test over time. Line shows the risk of developing at first negative LA test in the study cohort (n = 164), with the x-axis representing the time in years from study inclusion. (B) Cumulative incidence of next positive LA test after first negative LA test over time. Line reports the risk of a positive LA test after the first negative LA test in the n = 42 patients who experienced a first LA negative test and had follow-up. The x-axis represents the time elapsed since the first negative LA test. Curves were estimated with competing risk estimators. Gray shaded area represents 95% confidence interval.
Among those 46 patients who turned LA−, 5 patients did not have further follow-up, and 28 (68%) of the remaining 41 patients turned LA+ again, corresponding to a 10-year cumulative incidence of turning positive again of 64% (95% CI, 48-77, Figure 2B). Median time to a first subsequent positive LA test for those who turned LA negative was 1.0 years (95% CI, 0.8-2.5).
Predictors of LA test stability
In the study population, VKA treatment at baseline was a strong predictor of experiencing at least 1 negative LA test. The 10-year probability of this outcome was 21% in those without VKA at baseline and 37% in those with VKA at baseline (Gray’s test, P = .034). Additionally, history of any thrombosis, history of venous thrombosis, and presence and level of aβ2GPI IgG emerged as univariable predictors of an increased probability of experiencing at least 1 negative LA test (Table 2). Conversely, patients who were not treated with any antithrombotic therapy or had higher titer antiprothrombin IgM antibodies were significantly less likely to have a negative test for LA. The associations between antithrombotic treatment or thrombotic events and a negative LA test are consistent with the fact that LA testing is perturbed by anticoagulant treatment.23,24 Higher aCL IgG levels and presence of aβ2GPI IgG and/or IgM or aCL IgG and/or IgM were associated borderline statistically with a higher risk of experiencing a negative LA test. Importantly, after adjusting for VKA treatment, only a history of thrombosis and positivity for aβ2GPI IgG remained independently associated with at least 1 negative LA test, whereas an increased aPTT-LA and higher levels of antiprothrombin IgM or IgG antibodies independently predicted a lower probability of developing at least 1 negative LA test.
Predictors of experiencing a negative LA test (n = 164)
| Variables . | Univariable . | Adjusted for VKA at baseline . |
|---|---|---|
| Demographic characteristics | Subdistribution hazard ratio (95% CI, P) | |
| Age at study inclusion (per 5 y increase) | 0.95 (0.88-1.03, P = .244) | 0.97 (0.90-1.06, P = .540) |
| Female gender | 0.73 (0.35-1.52, P = .399) | 0.77 (0.37-1.61, P = .486) |
| Clinical history | ||
| Prior history of thrombosis | 3.15 (1.54-6.44, P = .002) | 2.82 (1.27-9.25, P = .011) |
| Arterial | 1.62 (0.77-4.30, P = .200) | 1.52 (0.71-3.27, P = .290) |
| Venous | 2.39 (1.28-4.45, P = .006) | 2.02 (0.98-4.17, P = .057) |
| Both | 2.20 (0.73-6.60, P = .178) | 1.61 (0.45-5.77, P = .460) |
| History of pregnancy complications* | 1.48 (0.68-3.24, P = .330) | 1.34 (0.61-2.94, P = .470) |
| Comorbidities at baseline | ||
| Autoimmune rheumatic diseases† | 1.23 (0.67-2.26, P = .507) | 1.23 (0.68-2.24, P = .496) |
| SLE | 1.60 (0.79-3.23, P = .191) | 1.52 (0.76-3.01, P = .235) |
| LLD | 0.63 (0.25-1.62, P = .342) | 0.67 (0.26-1.71, P = .403) |
| Active smoker at baseline | 0.52 (0.25-1.07, P = .074) | 0.55 (0.27-1.14, P = .109) |
| Hypertension | 0.98 (0.52-1.87, P = .962) | 0.94 (0.49-1.80, P = .851) |
| Diabetes | 0.81 (0.25-2.61, P = .730) | 0.77 (0.24-2.48, P = .660) |
| Anticoagulation at baseline | ||
| VKA | 2.03 (1.13-3.64, P = .018) | N/A |
| LMWH | 1.19 (0.52-2.74, P = .681) | 1.31 (0.58-2.94, P = .520) |
| LDA | 1.04 (0.56-1.95, P = .894) | 1.13 (0.61-2.09, P = .691) |
| None | 0.44 (0.22-0.91, P = .028) | 0.58 (0.25-1.35, P = .207) |
| Disease-related biomarkers | ||
| aPTT-LA (per 10 s increase) | 0.93 (0.85-1.02, P = .110) | 0.90 (0.82-0.99, P = .029) |
| LA positivity only | 0.50 (0.21-1.16, P = .106) | 0.52 (0.22-1.23, P = .138) |
| IgM aCL–positive‡ | 1.41 (0.65-3.04, P = .390) | 2.00 (0.85-4.71, P = .110) |
| IgG aCL–positive‡ | 1.61 (0.88-2.95, P = .120) | 1.41 (0.76-2.61, P = .280) |
| aCL IgM (per doubling) | 0.99 (0.83-1.18, P = .921) | 1.04 (0.85-1.28, P = .673) |
| aCL IgG (per doubling) | 1.12 (1.00-1.26, P = .055) | 1.08 (0.96-1.22, P = .176) |
| aCL+ (IgM and/or IgG)‡ | 1.68 (0.93-3.05, P = .086) | 1.58 (0.87-2.87, P = .133) |
| IgM aβ2GPI–positive‡ | 0.99 (0.53-1.87, P = .980) | 1.06 (0.57-1.99, P = .860) |
| IgG aβ2GPI–positive‡ | 2.83 (1.42-5.61, P = .003) | 2.55 (1.27-5.09, P = .008) |
| aβ2GPI IgM (per doubling) | 0.98 (0.83-1.17, P = .860) | 1.03 (0.86-1.24, P = .732) |
| aβ2GPI IgG (per doubling) | 1.14 (1.03-1.26, P = .009) | 1.09 (0.98-1.22, P = .098) |
| aβ2GPI+ (IgM and/or IgG)‡ | 2.26 (0.96-5.30, P = .061) | 2.12 (0.90-5.02, P = .086) |
| “Triple positivity”‡ | 1.55 (0.84-2.86, P = .158) | 1.48 (0.86-2.73, P = .206) |
| Antiprothrombin IgM (per doubling) | 0.72 (0.56-0.93, P = .011) | 0.75 (0.79-0.99, P = .040) |
| Antiprothrombin IgG (per doubling) | 0.91 (0.78-1.05, P = .199) | 0.87 (0.76-0.99, P = .037) |
| Antiprothrombin IgM–positive§ | 0.73 (0.33-1.63, P = .438) | 0.86 (0.37-2.00, P = .730) |
| Antiprothrombin+ IgG§ | 0.89 (0.44-1.78, P = .738) | 0.65 (0.31-1.35, P = .251) |
| Antiprothrombin+ (IgM and/or IgG)§ | 0.68 (0.35-1.31, P = .247) | 0.60 (0.31-1.16, P = .131) |
| Variables . | Univariable . | Adjusted for VKA at baseline . |
|---|---|---|
| Demographic characteristics | Subdistribution hazard ratio (95% CI, P) | |
| Age at study inclusion (per 5 y increase) | 0.95 (0.88-1.03, P = .244) | 0.97 (0.90-1.06, P = .540) |
| Female gender | 0.73 (0.35-1.52, P = .399) | 0.77 (0.37-1.61, P = .486) |
| Clinical history | ||
| Prior history of thrombosis | 3.15 (1.54-6.44, P = .002) | 2.82 (1.27-9.25, P = .011) |
| Arterial | 1.62 (0.77-4.30, P = .200) | 1.52 (0.71-3.27, P = .290) |
| Venous | 2.39 (1.28-4.45, P = .006) | 2.02 (0.98-4.17, P = .057) |
| Both | 2.20 (0.73-6.60, P = .178) | 1.61 (0.45-5.77, P = .460) |
| History of pregnancy complications* | 1.48 (0.68-3.24, P = .330) | 1.34 (0.61-2.94, P = .470) |
| Comorbidities at baseline | ||
| Autoimmune rheumatic diseases† | 1.23 (0.67-2.26, P = .507) | 1.23 (0.68-2.24, P = .496) |
| SLE | 1.60 (0.79-3.23, P = .191) | 1.52 (0.76-3.01, P = .235) |
| LLD | 0.63 (0.25-1.62, P = .342) | 0.67 (0.26-1.71, P = .403) |
| Active smoker at baseline | 0.52 (0.25-1.07, P = .074) | 0.55 (0.27-1.14, P = .109) |
| Hypertension | 0.98 (0.52-1.87, P = .962) | 0.94 (0.49-1.80, P = .851) |
| Diabetes | 0.81 (0.25-2.61, P = .730) | 0.77 (0.24-2.48, P = .660) |
| Anticoagulation at baseline | ||
| VKA | 2.03 (1.13-3.64, P = .018) | N/A |
| LMWH | 1.19 (0.52-2.74, P = .681) | 1.31 (0.58-2.94, P = .520) |
| LDA | 1.04 (0.56-1.95, P = .894) | 1.13 (0.61-2.09, P = .691) |
| None | 0.44 (0.22-0.91, P = .028) | 0.58 (0.25-1.35, P = .207) |
| Disease-related biomarkers | ||
| aPTT-LA (per 10 s increase) | 0.93 (0.85-1.02, P = .110) | 0.90 (0.82-0.99, P = .029) |
| LA positivity only | 0.50 (0.21-1.16, P = .106) | 0.52 (0.22-1.23, P = .138) |
| IgM aCL–positive‡ | 1.41 (0.65-3.04, P = .390) | 2.00 (0.85-4.71, P = .110) |
| IgG aCL–positive‡ | 1.61 (0.88-2.95, P = .120) | 1.41 (0.76-2.61, P = .280) |
| aCL IgM (per doubling) | 0.99 (0.83-1.18, P = .921) | 1.04 (0.85-1.28, P = .673) |
| aCL IgG (per doubling) | 1.12 (1.00-1.26, P = .055) | 1.08 (0.96-1.22, P = .176) |
| aCL+ (IgM and/or IgG)‡ | 1.68 (0.93-3.05, P = .086) | 1.58 (0.87-2.87, P = .133) |
| IgM aβ2GPI–positive‡ | 0.99 (0.53-1.87, P = .980) | 1.06 (0.57-1.99, P = .860) |
| IgG aβ2GPI–positive‡ | 2.83 (1.42-5.61, P = .003) | 2.55 (1.27-5.09, P = .008) |
| aβ2GPI IgM (per doubling) | 0.98 (0.83-1.17, P = .860) | 1.03 (0.86-1.24, P = .732) |
| aβ2GPI IgG (per doubling) | 1.14 (1.03-1.26, P = .009) | 1.09 (0.98-1.22, P = .098) |
| aβ2GPI+ (IgM and/or IgG)‡ | 2.26 (0.96-5.30, P = .061) | 2.12 (0.90-5.02, P = .086) |
| “Triple positivity”‡ | 1.55 (0.84-2.86, P = .158) | 1.48 (0.86-2.73, P = .206) |
| Antiprothrombin IgM (per doubling) | 0.72 (0.56-0.93, P = .011) | 0.75 (0.79-0.99, P = .040) |
| Antiprothrombin IgG (per doubling) | 0.91 (0.78-1.05, P = .199) | 0.87 (0.76-0.99, P = .037) |
| Antiprothrombin IgM–positive§ | 0.73 (0.33-1.63, P = .438) | 0.86 (0.37-2.00, P = .730) |
| Antiprothrombin+ IgG§ | 0.89 (0.44-1.78, P = .738) | 0.65 (0.31-1.35, P = .251) |
| Antiprothrombin+ (IgM and/or IgG)§ | 0.68 (0.35-1.31, P = .247) | 0.60 (0.31-1.16, P = .131) |
Reported results are subdistribution hazard ratios for time to first negative LA test. Subdistribution hazard ratios >1 indicate a higher risk of a change and ratios <1 a lower risk. Presented data are from univariable models (left column) and from multivariable models adjusted for VKA use at baseline (right column). Results “per doubling” were obtained by using a log2(+1)-transformation of the underlying variable. LDA, low-dose aspirin.
Pregnancy complications were defined according to Sapporo criteria in the subgroup of 117 females who had at least 1 documented pregnancy.
Autoimmune rheumatic diseases were defined as a composite of systemic lupus erythematosus and lupus-like disease according to a local panel of rheumatology experts.
Cutoffs were defined as follows according to ISTH/Sapporo criteria cutoffs: aCL >40 GPL/MPL U/mL, aβ2GPI >8 GPL/MPL U/mL. Triple-positivity was defined as being positive for LA and at least one class of immunoglobulins (IgM or IgG) for aCL and aβ2GPI.
Cutoffs were defined by the 90th percentile in 33 healthy volunteers without history of thrombosis, antiprothrombin IgM ≥11.54 U/mL, antiprothrombin IgG ≥8.68 U/mL.
Time-dependent effect of changes in LA test and VKA treatment status on aPL-related biomarkers
We then sought to explore whether baseline VKA status or a change in LA status, VKA status, or both had an immediate or subsequent effect on aCL IgM, aCL IgG, aβ2GPI IgM, or aβ2GPI IgG titers or the length of aPTT. Using linear mixed models, we studied the relationship between time-dependent changes in LA test status and/or in VKA anticoagulation status and concurrent and subsequent changes in these 5 aPL-related biomarkers (aPTT-LA, aCL IgM and IgG, aβ2GPI IgM and IgG) over time. In these models, LA, aPL-related biomarkers, and VKA anticoagulation status were treated as time-dependent variables (ie, VKA anticoagulation status could change over time, etc.). The average aPTT-LA at baseline was 91 seconds and declined by 1.1 seconds per year (supplemental Table 1). VKA treatment at baseline was associated with a 17-second longer aPTT-LA (P < .001), and a change in VKA treatment status was associated with a subsequent shortening of the aPTT-LA over time (−0.6 seconds per year, P = .005). However, neither a negative LA test nor a simultaneous change in LA test and VKA status affected the subsequent decline in aPTT-LA (P = .269 and P = .469, respectively). VKA status at baseline was also associated or borderline associated with the baseline levels of aCL IgM, aCL IgG, aβ2GPI IgM, and aβ2GPI IgG (supplemental Tables 2 and 3). The levels of these antibodies decreased over time. Additionally, a change in VKA treatment (either initiation or cessation of VKA) was associated with a subsequent decrease in the IgG aβ2GPI level over time (−1.5 U/mL per year, P = .028). Neither a negative LA test nor a simultaneous change in LA test and VKA status affected the levels of IgM or IgG isotype aCL or aβ2GPI, nor their subsequent trajectory over time (supplemental Tables 2 and 3).
Effect of a negative LA test on prospective risk of thrombosis and mortality
We then used multistate time-to-event models to estimate the impact of a negative LA test on prospective risks of ATE, VTE, total thrombosis, and mortality (supplemental Figure 3). A negative LA test did not change the prospective risk of ATE, VTE, any thrombosis, or mortality. These results prevailed in multivariable adjustment (adjusted for age, diabetes, active smoking, VKA use at baseline, and PTT-LA, Table 3). Moreover, a negative LA test did not translate into a change in prospective thrombosis risk for patients with or without APS (not shown). To gauge whether the lack of association between a negative LA test and prospective risk of thrombotic events or death was due to VKA status, we fit 4 generalized linear models of outcomes (ATE, VTE, total thrombosis, and death) with LA positivity and VKA status as time-dependent variables. These models found that time-dependent VKA status did not explain the lack of association of LA status and thrombotic outcomes (supplemental Table 4).
Multistate model on the impact of LA stability on the risks of thrombosis and death
| . | . | Univariable analysis . | Multivariable analysis* . | ||||
|---|---|---|---|---|---|---|---|
| Transition . | Outcome . | Transition hazard ratio . | 95% CI . | P . | Transition hazard ratio . | 95% CI . | P . |
| First negative LA test | ATE | 0.21 | 0.03-1.56 | .127 | 0.56 | 0.07-4.71 | .596 |
| First negative LA test | VTE | 1.26 | 0.46-3.46 | .649 | 1.16 | 0.40-3.32 | .786 |
| First negative LA test | Total thrombosis | 0.86 | 0.36-2.08 | .744 | 1.14 | 0.45-2.93 | .783 |
| First negative LA test | Death | 1.39 | 0.57-3.39 | .470 | 2.04 | 0.71-5.82 | .183 |
| . | . | Univariable analysis . | Multivariable analysis* . | ||||
|---|---|---|---|---|---|---|---|
| Transition . | Outcome . | Transition hazard ratio . | 95% CI . | P . | Transition hazard ratio . | 95% CI . | P . |
| First negative LA test | ATE | 0.21 | 0.03-1.56 | .127 | 0.56 | 0.07-4.71 | .596 |
| First negative LA test | VTE | 1.26 | 0.46-3.46 | .649 | 1.16 | 0.40-3.32 | .786 |
| First negative LA test | Total thrombosis | 0.86 | 0.36-2.08 | .744 | 1.14 | 0.45-2.93 | .783 |
| First negative LA test | Death | 1.39 | 0.57-3.39 | .470 | 2.04 | 0.71-5.82 | .183 |
Results were obtained with a unidirectional “illness-death” model. P, Wald test P value.
Model adjusted for 3 variables previously included in a lupus anticoagulant thrombosis risk score (active smoking, lupus-sensitive aPTT, and diabetes), as well VKA use at baseline (the strongest determinant of LA stability) and age (strong epidemiologic determinant of [arterial] thrombosis and mortality).
In landmark analysis, 10-year risks of ATE, VTE, total thrombosis, and death were 12%, 18%, 31%, and 12% in those patients with stable LA within the first 1.5 years of follow-up, and 0%, 17%, 18%, and 17% in those with at least 1 negative LA test during the first 1.5 year of follow-up, respectively (Figure 3).
Landmark analyses of LA stability and prospective risk of thrombosis and mortality. The blue dashed line represents the landmark date, which was set at 1.5 years after study inclusion. This landmark date was chosen because more than half of the first negative LA tests had occurred by that time. Solid lines represent thrombosis risks after the landmark date in those patients that had exclusively positive LA tests within the first 1.5 years of follow-up, and dashed lines represent thrombosis risks after the landmark date in those patients that had at least 1 negative LA test within the first 1.5 years of follow-up, respectively. (A) Risk of arterial thrombosis. (B) Risk of venous thromboembolism. (C) Risk of overall thrombosis (arterial plus or minus venous). (D) Risk of mortality.
Landmark analyses of LA stability and prospective risk of thrombosis and mortality. The blue dashed line represents the landmark date, which was set at 1.5 years after study inclusion. This landmark date was chosen because more than half of the first negative LA tests had occurred by that time. Solid lines represent thrombosis risks after the landmark date in those patients that had exclusively positive LA tests within the first 1.5 years of follow-up, and dashed lines represent thrombosis risks after the landmark date in those patients that had at least 1 negative LA test within the first 1.5 years of follow-up, respectively. (A) Risk of arterial thrombosis. (B) Risk of venous thromboembolism. (C) Risk of overall thrombosis (arterial plus or minus venous). (D) Risk of mortality.
Sensitivity analysis
Using an alternative definition of LA positivity that is only based on the LA test and the Rosner Index and applied to patients regardless of VKA status (described in supplemental Paragraph 1), we found that n = 63 patients (38%) developed at least 1 negative LA test during follow-up, corresponding to a 10-year cumulative incidence of 39% (95% CI, 31-47). However, again with this alternative classification, we did not observe evidence for an association between turning LA− and subsequent thrombotic outcomes (supplemental Table 5).
Discussion
In our cohort of patients persistently positive for LA, the majority of patients remained positive throughout the observation period. However, over one-quarter of patients had at least 1 negative test for LA during the observation period of almost 10 years. There was a clear association between VKA treatment and experiencing a negative LA test. Most patients had just a transiently negative LA test as 68% of those who had a negative test result and had followed up turned positive again. Most importantly, a negative test for LA did not impact a patient’s future risk of thrombotic events (ATE, VTE, or TE), and time-dependent VKA status did not explain the lack of association between LA status and thrombotic outcomes.
VKA use at baseline and factors likely associated with VKA exposure over time including history of thrombosis were the strongest predictors of experiencing a negative LA test. Interpretation of LA tests for patients on VKA remains controversial, and VKA use is associated with false negative and false positive LA results.25,26 We hypothesize some of the negative LA tests for patients on VKAs may have been false negatives. Nevertheless, when an alternative definition of LA positivity was applied to all LA tests independent of VKA status, again, no association experiencing a negative LA test and subsequent thrombotic outcomes was found. The most recent guidance from the SSC of the ISTH recommends testing patients for LA when they have stopped anticoagulation treatment whenever possible.6,23 Given the regularity in which patients were tested for LA during this study, it was infeasible to transition each patient to LMWH before each evaluation, and stopping anticoagulation would have led to an unacceptable risk-to-benefit profile.
Our strict criteria for interpreting a LA test as positive in patients on VKA, specifically positive when the confirmatory test was positive and the mixing study had a Rosner index >15, may have also increased the number of negative LA tests for patients on VKA. Although the recent SSC guidance recommends using a mixing test–specific cutoff (normalized ratio) to interpret mixing studies because it may be more sensitive that the Rosner index,6,27 we were unable to interpret our mixing studies using a local cutoff as local values at the time of testing were not available for most of the observation period. The association between VKA exposure and factors associated with VKA exposure and a negative LA test could also be partially explained by transient consumption of antiphospholipid antibodies during and after a thrombotic event, as suggested by Alarcon-Segovia.28 Khawaja et al also found a high incidence of transient loss of aPL following thrombosis in SLE patients persistently positive for aPL.29 However, this is unlikely to explain our findings as only 37% (17/46) of the patients that experienced a negative test had a thrombotic event during observation. Additionally, the lupus cofactor phenomenon, which is the lowering of prothrombin levels during VKA treatment and, thus, the loss of the cofactor (prothrombin) necessary for the antiprothrombin antibodies underlying LA to bind, might be a possible biologic explanation for our findings.30,31 However, in the subset of patients in our cohort who had antiprothrombin antibodies measured and were positive for antiprothrombin antibodies at baseline, there was no association between the antiprothrombin antibody positivity and a negative LA test. Although, higher titer antiprothrombin antibodies at baseline were associated with exclusively positive LA tests. Additionally, in only 1 of the 63 negative tests in patients who were antiprothrombin antibody positive was there prolongation of the aPTT with the 1:1 mix of patient plasma and normal plasma. Therefore, we did not find evidence that loss of prothrombin during VKA treatment explains the association between LA negativity and VKA treatment in our cohort.
In the study by Khawaja et al on the fluctuation of aPL after development of a thrombotic event, they found that although the majority prethrombosis LA+ patients became negative after a thrombotic event, 70% of the patients who became negative subsequently became positive again.29 This is quite comparable to our data as we found 68% of patients that experienced at least 1 negative LA test subsequently became positive. These changes may reflect fluctuations that occur spontaneously or due to immune-suppressive treatments but might also be due to diagnostic issues as there are no strict rules on how to investigate LA during anticoagulation. We assume that our patients on VKA had a higher baseline risk of thrombosis given they were more likely to have a history of thrombosis compared with patients on no anticoagulation. We hypothesize that transient loss of LA might at least in part be due to fluctuations in VKA treatment and problems in diagnosing LA during VKA treatment in our cohort.
The presence of aβ2GPI IgG at baseline was associated with a negative LA test. Patients positive for aβ2GPI IgG antibodies were more likely to have a history of thrombosis and, therefore, this finding again could be partially explained by VKA exposure during the observation period as we only controlled for VKA exposure at initial testing and not for exposure over time. Notably, a negative LA test did not affect the levels of aβ2GPI IgM or IgG at the time of the negative test nor going forward.
Conversely, after controlling for initial VKA use, a longer aPTT-LA and higher titer IgM or IgG antiprothrombin antibodies at baseline predicted LA stability. Patients with a longer aPTT-LA at baseline may have an antibody that more strongly interacts with the phospholipids in the assays and, thus, were more likely to remain persistently positive throughout observation and also during anticoagulation treatment. Similarly, because antiprothrombin antibodies may be the antibodies responsible for LA positivity,30,32,33 patients with higher titer antiprothrombin antibodies may be more strongly LA positive and, thus, more likely to remain positive throughout the observation period.
In our multivariable analysis, neither the presence of aCL nor triple positivity predicted LA test results over time. These results differ from the recent reported by Gkrouzman et al, who found that patients with an unstable, clinically meaningful aPL profile were more likely to be isolated LA+ or isolated positive for aβ2GPI IgM and/or IgG antibodies, whereas patients who were positive for LA, positive for aCL IgG, or triple positive were more likely to have a stable, clinically meaningful aPL profile.9 In this study, treatment with VKA did not differ between patients with or without a stable, clinically meaningful aPL profile. However, in their subgroup of patients who were isolated LA+ at baseline, patients not receiving anticoagulation were more likely to have a stable aPL profile compared with patients receiving anticoagulation. Explanations for these disparities include differences in factors considered in the multivariable analysis and in definitions of stability. Gkrouzman et al defined a stable, clinically meaningful profile of antibodies by the presence of LA and/or aCL or aβ2GPI IgG or IgM ≥40 U/mL in at least two-thirds of follow-up samples. Therefore, triple positive patients who transiently or permanently lost their LA but continued to test positive for either aCL or aβ2GPI IgG or IgM antibodies in two-thirds of samples would be considered stable. We showed that a negative LA test did not predict loss of aCL or aβ2GPI IgM or IgG and, therefore, our triple-positive patients that experienced a negative LA test may have been defined as having stable, clinically meaningful profile using the Gkrouzman et al definition.
Our primary objective was to assess whether a change in LA status was a predictor of future events, such that a negative test could inform clinicians and the patient about the patient’s future risk of thrombosis. We found that in patients persistently positive for LA, having a negative LA test did not affect the patient’s prospective risk of future thrombotic events or mortality. Thus, a negative LA test cannot be used to risk-stratify patients or individualize therapy. Neither Gkrouzman et al nor Khawaja et al analyzed the risk of prospective events.
Our study has several limitations. First, our findings cannot be generalized to all APS patients or aPL+ patients as we included only patients who were persistently positive for LA. However, this can also be seen as a strength as LA is considered the strongest aPL risk factor for thrombotic events.3,34,35 Second, given the regularity in which patients underwent antibody testing, we were unable to complete all testing for LA while patients were off VKA. Although this accurately represents routine LA testing in clinical practice, LA interpretation under VKA is controversial and challenging. However, the proportion of patients in our cohort who remained persistently LA+ (72%) was similar to the rates in other studies that used different definitions of LA stability and positivity (78% in the study by Gkrouzman et al, 71% in the study by Zen et al, and 87% in study by Erkan et al.).9,10,36 Finally, we only analyzed the effect of the first negative test on future events. We did not compare patients who became transiently LA negative to patients who became persistently negative, as the number of those latter patients was very low (only 14/42 patients remained persistently negative). There have been a number of reports of safe discontinuation of anticoagulation in patients with APS who became persistently negative for aPL, and our analysis cannot be applied to these patients.10,37 However, these studies also included patients with only aCL or aβ2GPI, which are regarded as weaker risk factor for thrombosis.35,38-40 Similar to findings by Khawaja et al and Zen et al.,10,29 we found that the majority of patients who had a negative LA test subsequently had at least 1 additional positive test.
In our cohort of LA+ patients rigorously followed for a long observation time, we found that most patients remain positive for LA over an extended period or only transiently test negative for LA, VKA use is associated with negative LA test, probably due to methodological problems, and, importantly, a negative LA test did not attenuate the risk of thromboembolism. We conclude that the decision for duration of anticoagulation cannot be based on a negative LA test if a patient has previously been persistently positive. Additionally, based on our findings, there is no evidence for the clinical utility of routine monitoring of LA status in persistently positive patients.
Acknowledgments
The authors thank Florian Moik and Stephan Nopp for analytical support.
This work was supported by the Austrian Science Fund (Special Research Program [SFB] 54) and by the Intramural Research Program of the NIH.
Authorship
Contribution: M.E.C., C.A., I.P., F.P., and J.G designed the study; J.G., S.K., C.A., and I.P. contributed patients; S.K. and P.Q. performed laboratory analyses; S.K. and F.P. supported data storage; M.E.C., S.K., I.P, J.G., and F.P. collected data; F.P. and D.K. analyzed the data; M.E.C., I.P., F.P., D.K., and J.G. interpreted the data; M.E.C., I.P., J.G., and F.P. wrote the manuscript; and all authors had access to the study data, proofread the manuscript, agreed with the content, and approved its submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johanna Gebhart, Clinical Division of Haematology and Haemostaseology, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: johanna.gebhart@meduniwien.ac.at.
References
Author notes
F.P. and J.G. are joint senior authors.
Requests for data sharing may be submitted to Johanna Gebhart (johanna.gebhart@meduniwien.ac.at).
The full-text version of this article contains a data supplement.