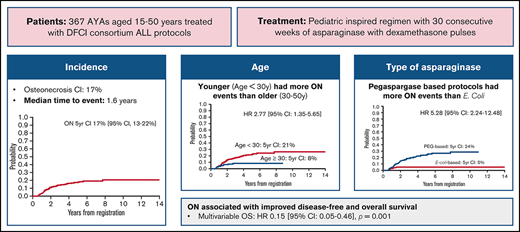
Key Points
Orthopedic toxicity was common in adolescent and young adult patients treated on DFCI Consortium ALL pediatric protocols.
Younger age and exposure to pegaspargase were associated with higher risk of ON, and patients with ON had superior OS.
Abstract
Adolescent and young adult patients with acute lymphoblastic leukemia (ALL) have superior outcomes when treated on pediatric regimens. Pediatric ALL regimens rely heavily on corticosteroids and asparaginase and are known to increase the risk of osteonecrosis (ON) and fractures in children, particularly adolescents. Orthopedic toxicity among young adults treated on pediatric-inspired regimens is not well described. Here, we report the symptomatic orthopedic toxicities of patients aged 15 to 50 years treated on sequential Dana-Farber Cancer Institute ALL Consortium protocols. Among 367 patients with a median age of 23 years (range, 15-50 years; 68% aged <30 years), 60 patients were diagnosed with ON (5-year cumulative incidence, 17%; 95% confidence interval [CI], 13-22), and 40 patients experienced fracture (5-year cumulative incidence, 12%; 95% CI, 8-15). Patients aged <30 years were significantly more likely to be diagnosed with ON (5-year cumulative incidence, 21% vs 8%; P = .004). Patients treated more recently on pegaspargase-based protocols were significantly more likely to be diagnosed with ON compared with those treated on earlier trials with native Escherichia coli asparaginase (5-year cumulative incidence, 24% vs 5%; P < .001). Of the 54 ON events for which adequate information was available, surgery was performed in 25 (46%). Patients with ON had superior overall survival (OS) compared with those without (multivariable OS hazard ratio, 0.15; 95% CI, 0.05-0.46; P = .001; ON included as a time-varying exposure). Increased rates of orthopedic toxicity in late-generation protocols may be driven by the pharmacokinetic drug interaction between pegaspargase and dexamethasone, leading to higher dexamethasone exposure.
Introduction
Adolescents and young adults (AYAs) diagnosed with acute lymphoblastic leukemia (ALL) are less likely to be cured of their disease than younger children.1 Although adverse disease biology is more commonly present in this population, it is now recognized that the therapeutic approach also has a major impact on outcome. A seminal 2008 analysis observed that patients aged 16 to 20 years treated on Children’s Cancer Group (CCG) trials from 1998 to 2001 had superior outcomes compared with same-aged patients enrolled in adult Cancer and Leukemia Group B (CALBG) trials.2 Subsequently, several retrospective studies were conducted worldwide that confirmed the observation that AYAs treated on pediatric protocols had improved outcomes compared with those treated on adult protocols.3-5 This prompted multiple groups of investigators in the United States and Europe to prospectively study pediatric or pediatric-inspired protocols in adults up to aged 50 years reporting safety and favorable outcomes compared with historical cohorts.6-8 These efforts have established pediatric-inspired regimens as a standard of care for AYA patients with ALL, making it essential that the unique toxicities of pediatric regimens, which rely heavily on corticosteroids and asparaginase, also be carefully studied in this population.
Orthopedic toxicity, including osteonecrosis (ON) and fracture, is a known complication of pediatric ALL treatment regimens and has been attributed primarily to corticosteroid exposure, with many, although not all, studies showing that dexamethasone (compared with prednisone) increases the risk of ON.9-11 The incidence of symptomatic ON reportedly ranges between 6% and 9.3% in pediatric cohorts, with adolescents at higher risk than younger children.10,12,13 Orthopedic toxicity in adults treated for ALL has been studied less, but younger adults have been reported to be more at risk than older adults whether treated on traditional adult14 or pediatric-inspired11 protocols. Some but not all studies have shown a higher incidence of ON among female patients,12,13 whereas recent investigations have found that African ancestry might be protective against ON and fracture.15 Given the long-term impact of orthopedic toxicity on ALL survivors, it is important to understand the frequency of, risk factors for, and impact of orthopedic events among AYAs treated on pediatric-inspired regimens.
Here, we present our experience with orthopedic toxicities in a large cohort of AYAs treated on sequential Dana-Farber Cancer Institute (DFCI) ALL Consortium protocols or off-study according to these protocol regimens. We aimed to characterize the incidence and risk factors for developing orthopedic toxicity in this treatment context.
Methods
Patients
Patients aged 1 to 50 years were treated on 4 sequential multicenter DFCI ALL Consortium protocols between 2000 and 2018. We identified all trial participants aged ≥15 years at time of diagnosis for the current analysis. We also identified patients aged ≥15 years not enrolled on these studies but treated per the same protocols at DFCI/Brigham and Women’s Hospital, Massachusetts General Hospital, and Boston Children’s Hospital using chart review. Earlier patients were enrolled on parallel pediatric 00-001 (2000-2004) and adult 01-175 (2002-2008) trials, while later patients were enrolled on parallel pediatric 05-001 (2005-2011) and adult 06-254 (2007-2011) trials. This research was approved by the Dana-Farber Cancer Institute Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
Treatment
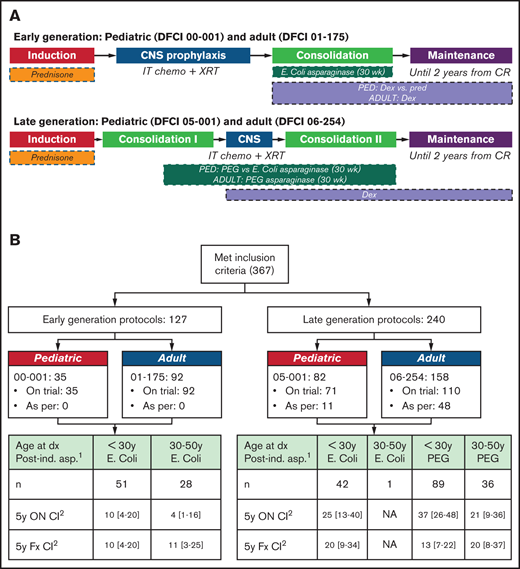
The treatment protocols of 00-001, 05-001, and 01-175 have been previously published.7,10,16 The 06-254 trial was based on the very high risk arm of 05-001.17 All trials used prednisone during induction. Protocol 00-001 randomized patients to receive either dexamethasone (18 mg/m2 per day for days 1-5 of each 3-week cycle) or prednisone (120 mg/m2 per day for days 1-5 of each 3-week cycle) during postinduction treatment phases, whereas the other 3 trials used dexamethasone (18 mg/m2 per day for days 1-5 of each 3-week cycle). Both 00-001 and 01-175 used native Escherichia coli(E. coli) asparaginase. Protocol 05-001 randomized patients to receive either pegaspargase or native E. coli asparaginase, whereas protocol 06-254 used pegaspargase for all patients. All patients were intended to receive 30 consecutive weeks of asparaginase during postinduction therapy (Figure 1A).
Overview of DFCI AYA ALL Treatment protocols. (A) Earlier patients were enrolled on parallel pediatric 00-001 (2000-2004) and adult 01-175 (2002-2008) trials while later patients were enrolled on parallel pediatric 05-001 (2005-2011) and adult 06-254 (2007-2011) trials. (B) Breakdown of patients according to treatment protocol, age, and asparaginase formulation. 1Intention-to-treat postinduction asparaginase formulation. Patients who did not receive postinduction asparaginase are excluded from this table. 2Cumulative incidence (death included as competing risk). CNS , central nervous system; Dx, diagnosis; Fx, fracture.
Overview of DFCI AYA ALL Treatment protocols. (A) Earlier patients were enrolled on parallel pediatric 00-001 (2000-2004) and adult 01-175 (2002-2008) trials while later patients were enrolled on parallel pediatric 05-001 (2005-2011) and adult 06-254 (2007-2011) trials. (B) Breakdown of patients according to treatment protocol, age, and asparaginase formulation. 1Intention-to-treat postinduction asparaginase formulation. Patients who did not receive postinduction asparaginase are excluded from this table. 2Cumulative incidence (death included as competing risk). CNS , central nervous system; Dx, diagnosis; Fx, fracture.
Orthopedic toxicities
Cases of symptomatic ON and fracture were extracted from case report forms for trial patients and by chart review for those not on trial. Toxicity events were submitted in case report forms by treating providers, and the selection of imaging modality for verification was according to provider discretion. Events identified through chart review were verified by magnetic resonance imaging, radiographs, or computed tomography imaging. Patients were not classified as having a symptomatic fracture if the fracture resulted specifically from a procedure for treatment of ON. Individual patients may have had more than one ON and/or more than one fracture during the treatment period. Additional chart review was performed on all patients treated at DFCI/ Brigham and Women’s Hospital, Massachusetts General Hospital, and Boston Children’s Hospital (Boston cohort) to identify surgical events.
Statistical analysis
All patients were combined in the modeling based on the intended treatment protocol. The 5-year cumulative incidences of ON and fracture were estimated and compared by using the Gray test. Univariate and multivariable competing risk regression models were constructed with death as a competing risk. Leukemic relapse and second malignant neoplasm were not included as competing risk, as we hypothesized that all surviving patients were at risk of developing ON from the initial exposure to the ALL regimen. Multivariable models included age (<30 years vs ≥30 years), sex, body mass index (BMI; underweight or normal vs overweight vs obese/morbidly obese), and treatment regimen backbone. Covariates were chosen based on previously published literature.
In subset analysis excluding patients on protocol 00-001 (due to lack of available data elements), the association of treatment-related dyslipidemia (grade 4 toxicity, including hypercholesterolemia and hypertriglyceridemia) with ON and fracture was explored. Similar analyses were also performed for patients who either received pegaspargase or native E. coli asparaginase postinduction. Median follow-up was calculated by using the reverse indicator for the Kaplan-Meier method. Overall survival (OS) was calculated from the time of study registration to the time of death censored at the time last known alive. A time-varying covariate was included for ON, and fracture was included in a Cox regression model to assess impact on OS. P values are two-sided and considered significant if <.05.
Results
Patient characteristics
A total of 367 patients were identified and included in the analysis (Table 1; Figure 1B). Early-generation protocols (00-001 and 01-175) accounted for 32% (n = 117) of patients, with the remaining patients (68% [n = 260]) treated on or as per late-generation protocols (05-001 and 06-254). Fifty-nine (16%) patients were treated according to these protocols, most commonly because protocol enrollment was closed at the time of presentation. The majority (61% [n = 225]) of patients were male. The median age at time of diagnosis was 23 years, with 68% (n = 249) younger than 30 years. The median BMI at diagnosis was 24.5 kg/m2, with 46% classified as overweight or obese. Seventy-seven percent (n = 281) of patients had B-lineage ALL, with 77% (n = 281) classified as central nervous system 1 status (negative). The median follow-up time for patients remaining alive on early-generation protocols was 4.9 years (range, 0.08-14.1 years), and on late-generation protocols it was 5.1 years (range, 0.01-11.8 years) and did not significantly differ between protocol generations (Wilcoxon rank-sum test, P = .15).
Patient demographic and disease characteristics
| Characteristic . | Value* . |
|---|---|
| Total, eligible patients | 367 |
| Protocol/treatment regimen | |
| 00-001 Pediatric early | 35 (10) |
| 05-001 (n = 11 treated as per) Pediatric late | 82 (22) |
| 01-175 Adult early | 92 (25) |
| 06-254 (n = 48 treated as per) Adult late | 158 (43) |
| Age, median (range), years | 23 (15, 50) |
| 15-19 | 138 (38) |
| 20-29 | 110 (30) |
| 30-39 | 62 (17) |
| 40-50 | 57 (16) |
| WBC, median (range), ×103/μL | 12.2 (0.1, 708.8) |
| <30 | 249 (68) |
| ≥30 | 117 (32) |
| Unknown | 1 (<1) |
| Blast %, median (range) | 23 (0, 98) |
| Sex | |
| Female | 142 (39) |
| Male | 225 (61) |
| Immunophenotype† | |
| B cell | 281 (77) |
| T cell | 86 (23) |
| CNS status | |
| CNS 1 | 281 (77) |
| CNS 2 | 38 (10) |
| CNS 3 | 11 (3) |
| Traumatic tap with blasts | 7 (2) |
| Traumatic tap without blasts | 18 (5) |
| Not performed | 12 (3) |
| Mediastinal mass | |
| Yes | 62 (17) |
| No | 299 (82) |
| Not evaluated | 6 (1) |
| Philadelphia chromosome positive | 44 (12) |
| KMT2A rearrangement (n = 330) | 20 (6) |
| High hyperdiploid (51-67 chromosomes) (n = 325) | 42 (13) |
| Complex karyotype ≥3 abnormalities (n = 228) | 45 (20) |
| BMI at diagnosis, median (range), kg/m2‡ | 24.5 (13.9, 57.0) |
| Underweight, <18.5 | 16 (4) |
| Normal, 18.5-24.9 | 182 (50) |
| Overweight, 25-29.9 | 94 (26) |
| Obese, 30-39.9 | 59 (16) |
| Morbidly obese, ≥40 | 16 (4) |
| Characteristic . | Value* . |
|---|---|
| Total, eligible patients | 367 |
| Protocol/treatment regimen | |
| 00-001 Pediatric early | 35 (10) |
| 05-001 (n = 11 treated as per) Pediatric late | 82 (22) |
| 01-175 Adult early | 92 (25) |
| 06-254 (n = 48 treated as per) Adult late | 158 (43) |
| Age, median (range), years | 23 (15, 50) |
| 15-19 | 138 (38) |
| 20-29 | 110 (30) |
| 30-39 | 62 (17) |
| 40-50 | 57 (16) |
| WBC, median (range), ×103/μL | 12.2 (0.1, 708.8) |
| <30 | 249 (68) |
| ≥30 | 117 (32) |
| Unknown | 1 (<1) |
| Blast %, median (range) | 23 (0, 98) |
| Sex | |
| Female | 142 (39) |
| Male | 225 (61) |
| Immunophenotype† | |
| B cell | 281 (77) |
| T cell | 86 (23) |
| CNS status | |
| CNS 1 | 281 (77) |
| CNS 2 | 38 (10) |
| CNS 3 | 11 (3) |
| Traumatic tap with blasts | 7 (2) |
| Traumatic tap without blasts | 18 (5) |
| Not performed | 12 (3) |
| Mediastinal mass | |
| Yes | 62 (17) |
| No | 299 (82) |
| Not evaluated | 6 (1) |
| Philadelphia chromosome positive | 44 (12) |
| KMT2A rearrangement (n = 330) | 20 (6) |
| High hyperdiploid (51-67 chromosomes) (n = 325) | 42 (13) |
| Complex karyotype ≥3 abnormalities (n = 228) | 45 (20) |
| BMI at diagnosis, median (range), kg/m2‡ | 24.5 (13.9, 57.0) |
| Underweight, <18.5 | 16 (4) |
| Normal, 18.5-24.9 | 182 (50) |
| Overweight, 25-29.9 | 94 (26) |
| Obese, 30-39.9 | 59 (16) |
| Morbidly obese, ≥40 | 16 (4) |
CNS, central nervous system; WBC, white blood cell.
Data are presented as N (%) unless otherwise indicated.
Two B-cell patients and 1 T-cell patient had myeloid coexpression.
BMI = weight (kg)/[height (m)]2.
Orthopedic events
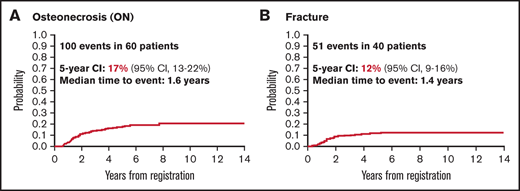
In total, there were 100 ON events in 60 patients (5-year cumulative incidence, 17%; 95% confidence interval [CI], 13-22), and 51 fractures in 40 patients (5-year cumulative incidence, 12%; 95% CI, 8-15). The median time from diagnosis to ON event was 1.6 years (range, 0.5-7.7 years). The median time from diagnosis to fracture event was 1.4 years (range, 0.2-5.2 years) (Figure 2). Nine of 40 first fractures (22.5%) and 22 of 60 first ON events (36.7%) occurred ≥2 years from diagnosis. Table 2 shows the 5-year cumulative incidence of orthopedic events for patient subgroups. The majority of the 100 ON events documented occurred in the hip (n = 48), with the remainder occurring in the knee (n = 20) and other locations (n = 32). Of the 51 fractures, 13 were vertebral, 10 affected the foot, and the rest were in other locations (supplemental Table 3). Among 54 ON events for which adequate follow-up information was available (Boston cohort), surgery was performed in 25 (46%), among whom 18 (33%) patients received total joint replacements. Of the 9 fracture events with available follow-up, 3 (33%) required surgery.
Incidence over time of ON and fracture in 367 AYA patients with ALL. Median time to event was 1.6 years for ON (A) and 1.4 years for fracture (B).
Incidence over time of ON and fracture in 367 AYA patients with ALL. Median time to event was 1.6 years for ON (A) and 1.4 years for fracture (B).
Cumulative incidence of ON and fracture
| Variable . | 5-y Cumulative Incidence: ON, % [95% CI] . | P* . | 5-y Cumulative Incidence: Fracture, % [95% CI] . | P* . |
|---|---|---|---|---|
| Overall | 17 [13-22] | 12 [8-15] | ||
| Treatment regimens | ||||
| Early generation | 5 [2-10] | <.001 | 9 [5-16] | .74 |
| Late generation | 24 [18-30] | 13 [9-18] | ||
| Age | ||||
| <20 y | 18 [12-25] | .003 | 14 [9-21] | .44 |
| 20-29 y | 26 [17-36] | 10 [5-18] | ||
| 30-39 y | 12 [5-23] | 14 [6-26] | ||
| ≥40 y | 4 [1-11] | 7 [2-17] | ||
| Age | ||||
| <30 y | 21 [16-27] | .004 | 12 [8-17] | .54 |
| ≥30 y | 8 [4-14] | 11 [6-18] | ||
| BMI at diagnosis, kg/m2 | ||||
| Underweight/normal | 19 [14-26] | .53 | 14 [9-20] | .39 |
| Overweight | 14 [8-23] | 9 [4-16] | ||
| Morbidly obese/obese | 15 [8-26] | 10 [4-18] | ||
| Sex | ||||
| Male | 16 [11-22] | .50 | 9 [6-13] | .051 |
| Female | 19 [13-27] | 17 [10-24] | ||
| Lipidemia (grade 4) (N = 332)† | ||||
| Yes | 32 [18-46] | .019 | 5 [1-16] | .11 |
| No | 16 [12-21] | 13 [9-17] |
| Variable . | 5-y Cumulative Incidence: ON, % [95% CI] . | P* . | 5-y Cumulative Incidence: Fracture, % [95% CI] . | P* . |
|---|---|---|---|---|
| Overall | 17 [13-22] | 12 [8-15] | ||
| Treatment regimens | ||||
| Early generation | 5 [2-10] | <.001 | 9 [5-16] | .74 |
| Late generation | 24 [18-30] | 13 [9-18] | ||
| Age | ||||
| <20 y | 18 [12-25] | .003 | 14 [9-21] | .44 |
| 20-29 y | 26 [17-36] | 10 [5-18] | ||
| 30-39 y | 12 [5-23] | 14 [6-26] | ||
| ≥40 y | 4 [1-11] | 7 [2-17] | ||
| Age | ||||
| <30 y | 21 [16-27] | .004 | 12 [8-17] | .54 |
| ≥30 y | 8 [4-14] | 11 [6-18] | ||
| BMI at diagnosis, kg/m2 | ||||
| Underweight/normal | 19 [14-26] | .53 | 14 [9-20] | .39 |
| Overweight | 14 [8-23] | 9 [4-16] | ||
| Morbidly obese/obese | 15 [8-26] | 10 [4-18] | ||
| Sex | ||||
| Male | 16 [11-22] | .50 | 9 [6-13] | .051 |
| Female | 19 [13-27] | 17 [10-24] | ||
| Lipidemia (grade 4) (N = 332)† | ||||
| Yes | 32 [18-46] | .019 | 5 [1-16] | .11 |
| No | 16 [12-21] | 13 [9-17] |
The Gray test.
Excludes 00-001 protocol. Includes hypertriglyceridemia and hypercholesterolemia. No grade 5 events were observed.
Risk factors for orthopedic toxicity
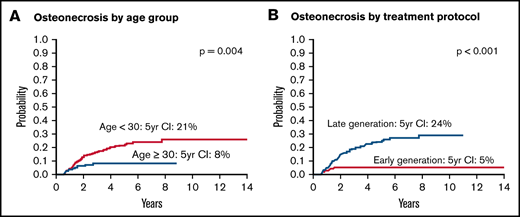
To understand factors associated with increased risk for development of orthopedic toxicities, we performed univariate and multivariate competing risk regression analyses for ON and fracture. Patients aged <30 years were significantly more likely to be diagnosed with ON (5-year cumulative incidence, 21%; 95% CI, 16-27) compared with those aged 30 to 50 years (5-year cumulative incidence, 8% [95% CI, 4-14]; univariate hazard ratio [HR], 2.77 [95% CI, 1.35- 5.65]; P = .005) (Figure 3A). Of note, patients aged 30 to 50 years were just as likely to have received ≥26 weeks of asparaginase than those younger than 30 years (41% vs 47%; P = .39), a threshold previously identified as being associated with improved outcomes.18 Patients treated on late-generation pegaspargase-based protocols were significantly more likely to be diagnosed with ON (5-year cumulative incidence, 24%; 95% CI, 18-30) compared with those treated on early-generation native E. coli asparaginase-based protocols (5-year cumulative incidence, 5% [95% CI, 2-10]; HR, 5.28 [95% CI, 2.24-12.48]; P < .001) (Figure 3B). Patients aged <30 years on late-generation protocols had a 5-year cumulative incidence of 29% (95% CI, 22-37). The association between age <30 years and treatment on late-generation protocols and risk for ON remained statistically significant in multivariate analysis, including treatment backbone, age, BMI, and sex. On univariate analysis, BMI and sex were not associated with ON. There were no significant associations of age, protocol generation, or any other variable and risk for facture, although there were fewer fractures in male patients, which was of marginal statistical significance (HR, 0.55; 95% CI, 0.29-1.02; P = .057) (Table 3). Only 20 patients in this cohort treated on an early-generation protocol received postinduction prednisone, and thus the impact of steroid formulation could not be reliably investigated.
Probability of ON according to risk group. Probability of ON by age group (A) and by treatment protocol (B). Early-generation protocols 00-001 and 01-175 used E. coli asparaginase, whereas late-generation protocols 05-001 and 06-254 mostly used pegaspargase.
Probability of ON according to risk group. Probability of ON by age group (A) and by treatment protocol (B). Early-generation protocols 00-001 and 01-175 used E. coli asparaginase, whereas late-generation protocols 05-001 and 06-254 mostly used pegaspargase.
Competing risk regression models of ON and fracture
| Variable . | Univariate, HR [95% CI] . | P . | Multivariable, HR [95% CI] . | P . |
|---|---|---|---|---|
| ON | ||||
| Treatment regimen, late vs early | 5.28 [2.24-12.48] | <.001 | 5.08 [2.12-12.16] | <.001 |
| Age | ||||
| <30 vs 30-50 y | 2.77 [1.35-5.65] | .005 | 2.58 [1.26-5.30] | .010 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 0.81 [0.41-1.60] | .55 | 0.92 [0.46-1.84] | .82 |
| Overweight vs underweight/normal | 0.70 [0.36-1.34] | .28 | 0.97 [0.51-1.86] | .94 |
| Sex | ||||
| Male vs female | 0.85 [0.51-1.41] | .52 | 0.84 [0.50-1.40] | .51 |
| Fracture | ||||
| Treatment regimen, late vs early | 1.12 [0.57-2.17] | .75 | 1.12 [0.57-2.20] | .74 |
| Age | ||||
| <30 vs 30-50 y | 1.25 [0.63-2.50] | .52 | 1.18 [0.54-2.57] | .68 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 0.72 [0.31-1.67] | .45 | 0.78 [0.32-1.87] | .58 |
| Overweight vs underweight/normal | 0.58 [0.25-1.32] | .19 | 0.66 [0.27-1.64] | .37 |
| Sex | ||||
| Male vs female | 0.55 [0.29-1.02] | .057 | 0.56 [0.29-1.08] | .084 |
| Variable . | Univariate, HR [95% CI] . | P . | Multivariable, HR [95% CI] . | P . |
|---|---|---|---|---|
| ON | ||||
| Treatment regimen, late vs early | 5.28 [2.24-12.48] | <.001 | 5.08 [2.12-12.16] | <.001 |
| Age | ||||
| <30 vs 30-50 y | 2.77 [1.35-5.65] | .005 | 2.58 [1.26-5.30] | .010 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 0.81 [0.41-1.60] | .55 | 0.92 [0.46-1.84] | .82 |
| Overweight vs underweight/normal | 0.70 [0.36-1.34] | .28 | 0.97 [0.51-1.86] | .94 |
| Sex | ||||
| Male vs female | 0.85 [0.51-1.41] | .52 | 0.84 [0.50-1.40] | .51 |
| Fracture | ||||
| Treatment regimen, late vs early | 1.12 [0.57-2.17] | .75 | 1.12 [0.57-2.20] | .74 |
| Age | ||||
| <30 vs 30-50 y | 1.25 [0.63-2.50] | .52 | 1.18 [0.54-2.57] | .68 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 0.72 [0.31-1.67] | .45 | 0.78 [0.32-1.87] | .58 |
| Overweight vs underweight/normal | 0.58 [0.25-1.32] | .19 | 0.66 [0.27-1.64] | .37 |
| Sex | ||||
| Male vs female | 0.55 [0.29-1.02] | .057 | 0.56 [0.29-1.08] | .084 |
To further investigate the role of asparaginase formulation, we performed a subset analysis among the 247 patients (67%) who received postinduction asparaginase (defined as receiving one or more doses of asparaginase after completion of induction) and classified patients according to their intention-to-treat type of asparaginase (E. coli vs pegaspargase). Reasons for not proceeding to postinduction asparaginase included early death, severe early asparaginase toxicity, or treatment decision to be consolidated with allogeneic stem cell transplant. In this analysis, patients who were assigned to receive postinduction pegaspargase were more likely to develop ON than those assigned to receive native E. coli asparaginase (univariate HR, 2.53; 95% CI, 1.42-4.49; P = .002) (supplemental Table 1). This result remained statistically significant in a multivariable analysis. Of note, in this group, duration of asparaginase was not statistically associated with ON, whether considered as a continuous variable (P = .36) or as a categorical variable (<26 or ≥26 weeks; P = .89).
To assess the contribution of treatment-emergent dyslipidemia (defined as grade 4 hypercholesterolemia or hypertriglyceridemia) to orthopedic toxicity, we performed a separate analysis excluding patients from protocol 00-001, as dyslipidemia was not reported consistently for those patients (supplemental Table 2). In this analysis, dyslipidemia was associated with a higher ON incidence in a univariate analysis (univariate HR, 2.04; 95% CI, 1.15-3.64; P = .016). This association was not statistically significant in a multivariable analysis. Of note, no patient in this analysis received postinduction prednisone, and the associations between late-generation protocols and ON as well as between age and ON remained statistically significant.
Association of orthopedic toxicity and survival
The association of orthopedic toxicity and survival was explored by constructing multivariable models of OS with ON or fracture as the time-varying exposure of interest. After adjusting for covariates, including age, presenting leukocyte count, immunophenotype, BMI and Philadelphia chromosome status, patients experiencing an ON or fracture event were significantly less likely to die than those who did not experience an event (multivariable HR for ON, 0.15 [95% CI, 0.05-0.46; P = .001]; HR for fracture, 0.40 [95% CI, 0.16-0.99; P = .048]) (Table 4). Of note, when postinduction steroid formulation (dexamethasone vs prednisone) was included in the model, it was not associated with OS in a statistically significant fashion (data not shown).
Univariate and multivariable Cox modeling for OS
| Variable . | OS Univariate, HR [95% CI] . | P . | OS Multivariable, HR [95% CI] . | P . |
|---|---|---|---|---|
| ON* | 0.11 [0.04-0.35] | .0002 | 0.15 [0.05-0.46] | .001 |
| Age 30-50 y vs < 30 y | 1.88 [1.27-2.78] | .002 | 1.42 [0.93-2.16] | .11 |
| Sex, male vs female | 1.08 [0.72-1.61] | .72 | ||
| WBC , ≥50 x103/μL vs <50 | 2.10 [1.42-3.10] | .0002 | 2.17 [1.45-3.23] | .0002 |
| B cell vs T cell | 2.31 [1.29-4.14] | .005 | 2.31 [1.27-4.18] | .006 |
| CNS blasts vs no blasts | 1.41 [0.84-2.35] | .19 | ||
| CNS Unknown vs no blasts | 0.89 [0.45-1.79] | .75 | ||
| Philadelphia chromosome positive, yes vs no | 1.89 [1.12-3.19] | .017 | 1.03 [0.59-1.79] | .92 |
| Philadelphia chromosome Unknown vs no | 1.81 [0.25-12.98] | .56 | 2.25 [0.30-17.01] | .43 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 2.25 [1.39-3.64] | .001 | 1.91 [1.16-3.15] | .011 |
| Overweight vs underweight/normal | 2.27 [1.43-3.59] | .0005 | 2.19 [1.36-3.53] | .001 |
| Treatment regimen, late vs early | 0.69 [0.47-1.02] | .065 | ||
| Fracture* | 0.31 [0.13-0.77] | .012 | 0.40 [0.16-0.99] | .048 |
| Age 30-50 vs <30 y | 1.88 [1.27-2.78] | .002 | 1.62 [1.06-2.48] | .026 |
| Sex male vs female | 1.08 [0.72-1.61] | .72 | ||
| WBC ≥50 x103/μL vs <50 | 2.10 [1.42-3.10] | .0002 | 2.30 [1.54-3.44] | <.001 |
| B cell vs T cell | 2.31 [1.29-4.14] | .005 | 2.31 [1.27-4.18] | .006 |
| CNS blasts vs no blasts | 1.41 [0.84-2.35] | .19 | ||
| CNS Unknown vs no blasts | 0.89 [0.45-1.79] | .75 | ||
| Philadelphia chromosome positive, yes vs no | 1.89 [1.12-3.19] | .017 | 1.06 [0.61-1.85] | .84 |
| Philadelphia chromosome unknown vs no | 1.81 [0.25-12.98] | .56 | 1.68 [0.22-12.70] | .61 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 2.25 [1.39-3.64] | .001 | 1.89 [1.14-3.13] | .013 |
| Overweight vs underweight/normal | 2.27 [1.43-3.59] | .0005 | 2.31 [1.27-4.18] | .006 |
| Treatment regimen, late vs early | 0.69 [0.47-1.02] | .065 |
| Variable . | OS Univariate, HR [95% CI] . | P . | OS Multivariable, HR [95% CI] . | P . |
|---|---|---|---|---|
| ON* | 0.11 [0.04-0.35] | .0002 | 0.15 [0.05-0.46] | .001 |
| Age 30-50 y vs < 30 y | 1.88 [1.27-2.78] | .002 | 1.42 [0.93-2.16] | .11 |
| Sex, male vs female | 1.08 [0.72-1.61] | .72 | ||
| WBC , ≥50 x103/μL vs <50 | 2.10 [1.42-3.10] | .0002 | 2.17 [1.45-3.23] | .0002 |
| B cell vs T cell | 2.31 [1.29-4.14] | .005 | 2.31 [1.27-4.18] | .006 |
| CNS blasts vs no blasts | 1.41 [0.84-2.35] | .19 | ||
| CNS Unknown vs no blasts | 0.89 [0.45-1.79] | .75 | ||
| Philadelphia chromosome positive, yes vs no | 1.89 [1.12-3.19] | .017 | 1.03 [0.59-1.79] | .92 |
| Philadelphia chromosome Unknown vs no | 1.81 [0.25-12.98] | .56 | 2.25 [0.30-17.01] | .43 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 2.25 [1.39-3.64] | .001 | 1.91 [1.16-3.15] | .011 |
| Overweight vs underweight/normal | 2.27 [1.43-3.59] | .0005 | 2.19 [1.36-3.53] | .001 |
| Treatment regimen, late vs early | 0.69 [0.47-1.02] | .065 | ||
| Fracture* | 0.31 [0.13-0.77] | .012 | 0.40 [0.16-0.99] | .048 |
| Age 30-50 vs <30 y | 1.88 [1.27-2.78] | .002 | 1.62 [1.06-2.48] | .026 |
| Sex male vs female | 1.08 [0.72-1.61] | .72 | ||
| WBC ≥50 x103/μL vs <50 | 2.10 [1.42-3.10] | .0002 | 2.30 [1.54-3.44] | <.001 |
| B cell vs T cell | 2.31 [1.29-4.14] | .005 | 2.31 [1.27-4.18] | .006 |
| CNS blasts vs no blasts | 1.41 [0.84-2.35] | .19 | ||
| CNS Unknown vs no blasts | 0.89 [0.45-1.79] | .75 | ||
| Philadelphia chromosome positive, yes vs no | 1.89 [1.12-3.19] | .017 | 1.06 [0.61-1.85] | .84 |
| Philadelphia chromosome unknown vs no | 1.81 [0.25-12.98] | .56 | 1.68 [0.22-12.70] | .61 |
| BMI | ||||
| Morbidly obese/obese vs underweight/normal | 2.25 [1.39-3.64] | .001 | 1.89 [1.14-3.13] | .013 |
| Overweight vs underweight/normal | 2.27 [1.43-3.59] | .0005 | 2.31 [1.27-4.18] | .006 |
| Treatment regimen, late vs early | 0.69 [0.47-1.02] | .065 |
CNS, central nervous system; WBC, white blood cell.
Included as a time-varying covariate.
Discussion
The current study represents the largest investigation to date of orthopedic toxicity associated with ALL treatment in AYA patients treated on pediatric-inspired regimens. We found that orthopedic toxicities are common and that age between 15 and 30 years at treatment initiation, compared with age ≥30 years, is associated with a higher risk of developing ON. Our cumulative incidence was higher than in other recently published studies,19 which might be related to longer follow-up, higher treatment completion rates, or differences in the doses and schedule of asparaginase and steroid exposure between DFCI and other protocols. The finding that younger adults are more at risk for orthopedic toxicity compared with older adults is consistent with reports of the recent adult Eastern Cooperative Oncology Group 2993 trial and the Nordic Society of Pediatric Hematology and Oncology NOPHO ALL2008 trial (which applied a pediatric regimen to patients aged 1-45 years).11,14 Because it has been previously established that adolescents aged >10 years are at higher risk of ON than younger children,20 our study helps confirm a “vulnerable window” of late adolescence and young adulthood during which ON risk is highest for patients receiving ALL therapy. A definitive mechanistic explanation for why adolescents and young adults are particularly vulnerable to ON has not been established. The procoagulant effects of increasing sex hormone concentrations, the timing of epiphyseal closure, and the peak of growth hormone/insulin-like growth factor-1 during puberty leading to increased bone metabolic activity and increased vulnerability to hypoxia have all been identified as possible contributors.19 Our study did not confirm previous associations between ON and female sex, high BMI, and race/ethnicity.12,13,15,21 With regard to sex, this may be due to the fact that the increased risk for ON among female subjects is more prominent in younger adolescents (aged 10-15 years). In terms of race/ethnicity, our cohort was predominantly white and did not reliably capture ethnicity data.
A key finding of our study is the demonstration that asparaginase formulation influences risk for ON, with the cumulative incidence of ON being 24% in those on pegaspargase-based protocols compared with 5% in those treated on E. coli–based protocols. One of the contributing studies to our analysis did not show this difference: DFCI 05-001, in which 7% of patients were aged >15 years at time of diagnosis, reported statistically similar ON rates between patients receiving E. coli vs pegaspargase.16,22 Because AYAs are at significantly higher risk for ON than younger children, we hypothesize that DFCI 05-001 was not adequately powered to detect differences between these formulations.
We also hypothesize that pegaspargase increases the risk of ON through more sustained asparaginase exposure with the pegylated formulation. ON may occur due to more toxicity from asparaginase itself and/or the interaction of asparaginase with steroid clearance. Asparaginase is believed to directly influence risk for orthopedic toxicity via its influence on coagulation parameters and lipid metabolism. Asparaginase induces a hypercoagulable state through suppression of antithrombin and elevation of von Willebrand factor/factor VIII complex, which may contribute to ON.23 It also causes alterations in lipid metabolism that could lead to lipid droplet formation in bony vascular beds and subsequent damage to the vascular endothelium.24 Recent experiments in mice have shown that reduction of serum triglycerides with fenofibrate can reduce the incidence of ON.25 Our study also found an association (although not statistically significant on multivariable analysis) between dyslipidemia and orthopedic toxicity that was previously reported in patients treated on the NOPHO ALL2008 protocol.26 Asparaginase may also indirectly affect risk for orthopedic toxicity via its pharmacokinetic interaction with steroids. One prior analysis showed that patients who were exposed to lower amounts of asparaginase as a result of developing an asparaginase allergy had higher dexamethasone clearance, leading to lower overall dexamethasone exposure.27 Hence, asparaginase seems to reduce steroid clearance when administered concurrently, which may lead to increased corticosteroid exposure and thus increase the risk for ON. This hypothesis can be tested in the future with formal monitoring of both asparaginase activity and corticosteroid levels in prospective studies.
Because pegaspargase has a longer half-life than native E. coli asparaginase,28 we propose that pegaspargase may lead to longer, more sustained exposure to both asparaginase and dexamethasone, thus leading to higher ON risk. It is important to note that pediatric and young adult regimens are now predominantly based on pegylated asparaginase formulations, including pegaspargase and calaspargase, a formulation with an even longer half-life.29 Although these formulations may offer important oncologic benefits, they may also increase orthopedic toxicity, especially in vulnerable AYAs.
Another major finding of our study is the association of an orthopedic event and improved oncologic outcomes in our cohort. We found that patients with ON had an OS HR at 5 years of 0.15 compared with those without ON, after adjusting for relevant covariates. A similar finding was reported in the CCG-1961 trial, in which an improved event free survival (HR, 0.32) was reported for patients aged >10 years who had an ON event compared with those who did not.13 We hypothesize that patients who develop ON are likely to have had higher and/or more sustained steroid and asparaginase exposure due to individual differences in drug metabolism. Thus, ON may be a proxy for chemotherapeutic exposure and may provide reassurance for oncologists choosing to omit corticosteroids in patients who have experienced an orthopedic event while still on treatment, as these patients have likely already had more intense exposure than patients who have not had an orthopedic event. For patients who have already completed treatment at the time of ON detection, focus will be on addressing the orthopedic issue and ensuring appropriate orthopedic care and bone health.
The retrospective nature of our analysis is a limitation as there may have been differences in adverse event ascertainment in earlier (E. coli based) vs later (pegaspargase based) treatment regimens. However, our findings are consistent with those from CCG-1961, in which a subset of patients exposed to pegaspargase had higher ON rates than patients exposed to E. coli asparaginase.13 It is important to note that our study only investigated clinical (symptomatic) ON and fractures. Although prospective radiographic screening in asymptomatic patients identifies more events,30 the clinical significance of asymptomatic lesions remains unclear and would benefit from further prospective study in patients followed up during survivorship.
We hope our study will inspire several areas of future research. First, the generalizability of our findings will need to be confirmed in other regimens. DFCI AYA protocols are unique in that consolidation is based on 30 weeks of continuous asparaginase with corticosteroid pulses. Deliberate prospective characterization of orthopedic toxicities is required in every ALL protocol used to treat children and young adults. This is especially important, as pediatric-inspired protocols are being used more frequently in the AYA population and incorporated into national guidelines.31 Second, careful documentation of surgical interventions, quality of life measures, and functional outcomes should be incorporated into future AYA protocol design to capture the full clinical impact of orthopedic toxicity beyond what is captured in routine Common Terminology Criteria for Adverse Events grading. The fact that more than one-third of the first ON events in our study occurred ≥2 years from diagnosis highlights the fact that ON events may be under-ascertained in clinical trials. This should be a focus of future survivorship studies recognizing that “loss to follow-up” can be a particular challenge in transitory AYA patients. Third, additional studies should focus on identifying reliable individual predictors and risk scores for clinically significant orthopedic complications. Candidate predictors include serum biomarkers of bone metabolism, specific radiographic indicators, additional asparaginase toxicities (ie, pancreatitis, thromboembolism), and combinations of clinical factors (eg, age, BMI, lipid profile). Of note, germline polymorphisms have been identified as risk factors for ON: genome-wide association studies have identified genes involved in adipogenesis and the glutamate receptor pathways to be associated with ON,20 whereas polymorphisms in the pro-apoptotic BCL2L11 gene have been implicated in ON risk through both retrospective clinical studies and in vitro assays.32 With better understanding of toxicity burden and at-risk populations, prospective interventions to mitigate the incidence and morbidity of ON in patients who are at high risk of ON based on demographic characteristics, genetics, and treatment regimen could be developed.
In summary, our study defines a vulnerable window of adolescence and young adulthood in which orthopedic toxicity is a particular risk when being treated on pediatric-inspired ALL regimens associated with prolonged steroid and asparaginase exposure. Pegylated formulations of asparaginase now in common use may potentiate risk for this toxicity. As survival improves in patients treated with intensive pediatric-inspired regimens, focus on toxicity and survivorship will be increasingly important. Progress in this realm will require cooperation between pediatricians, adult medical oncologists, endocrinologists, orthopedic surgeons, and survivorship experts to achieve the best outcomes for AYA patients with ALL.
Authorship
Contribution: Y.K.V. extracted the data from the charts, designed the data analysis, and drafted the manuscript; K.E.S. designed and performed the data analysis; A.E.P., L.B.S., L.M.V., G.G., and A.M.B. designed the analysis and identified patients for inclusion in the cohort; M.N. extracted pharmacy data; D.J.D. conceptualized the study and helped design the analysis; M.R.L. oversaw the creation of the cohort, the design of the analysis, and the drafting of the manuscript; and all authors revised the final manuscript.
Conflict-of-interest disclosure: L.B.S. was on the advisory board of Jazz Pharma, Takeda, Servier, and Syndax. A.M.B. received consultancy funding from Acceleron Pharma, Biogen, Celgene/BMS, Forty Seven, Jazz Pharma, Novartis, Takeda, and Xcenda; and research funding from Celgene/BMS, Novartis, Takeda, GSK, Janssen, and AstraZeneca. D.J.D. received consultancy funding from Amgen, Autolos, Agios, Blueprint Medicines Corporation, Forty Seven, Incyte Corporation, Jazz Pharma, Novartis, Pfizer, Shire, and Takeda; and research funding from Blueprint Medicines Corporation, Novartis, AbbVie, and Glycomimetics. The remaining authors declare no competing financial interests.
Correspondence: Marlise R. Luskin, 450 Brookline Ave, Dana 2056, Boston, MA 02215; e-mail: marlise_luskin@dfci.harvard.edu.
References
Author notes
Data presented here were presented as a virtual oral abstract at the American Society of Hematology 2020 conference on 7 December 2020.
Data are available by e-mailing the corresponding author (marlise_luskin@dfci.harvard.edu).
The full-text version of this article contains a data supplement.