Key Points
Ofatumumab with glucocorticoid therapy for cGVHD resulted in 62.5% ORR at 6 months and 53% FFS at 12 months.
Safety was observed with ofatumumab plus glucocorticoid for initial therapy.
Abstract
Standard initial therapy of chronic graft vs. host disease (cGVHD) with glucocorticoids results in suboptimal response. Safety and feasibility of therapy with ofatumumab (1000 mg IV on days 0 and 14) and prednisone (1 mg/kg/day) was previously established in our phase I trial (n = 12). We now report the mature results of the phase II expansion of the trial (n = 38). The overall NIH severity of cGVHD was moderate (63%) or severe (37%) with 74% of all patients affected by the overlap subtype of cGVHD and 82% by prior acute cGVHD. The observed 6 month clinician-reported and 2014 NIH-defined overall response rates (ORR = complete + partial response [CR/PR]) of 62.5% (1-sided lower 90% confidence interval=51.5%) were not superior to pre-specified historic benchmark of 60%. Post-hoc comparison of 6 month NIH response suggested benefit compared to more contemporaneous NIH-based benchmark of 48.6% with frontline sirolimus/prednisone (CTN 0801 trial). Baseline cGVHD features (organ involvement, severity, initial immune suppression agents) were not significantly associated with 6-month ORR. The median time to initiation of second-line therapy was 5.4 months (range 0.9-15.1 months). Failure-free survival (FFS) was 64.2% (95% CI 46.5-77.4%) at 6 months and 53.1% (95% CI 35.8-67.7%) at 12 months, whereas FFS with CR/PR at 12 months of 33.5% exceeded a benchmark of 15% in post-hoc analysis, and was associated with greater success in steroid discontinuation by 24 months (odds ratio 8 (95% CI 1.21-52.7). This single-arm phase II trial demonstrated acceptable safety and potential efficacy of the upfront use of ofatumumab in combination with prednisone in cGVHD. This trial was registered at www.clinicaltrials.gov as #NCT01680965.
Introduction
Chronic graft-versus-host disease (cGVHD) is a common immune-mediated disorder after allogeneic hematopoietic cell transplantation (alloHCT), affecting approximately 40% to 50% of HCT recipients.1,2 It is a leading cause of late HCT-associated morbidity, mortality, impaired quality of life, disability, and prolonged duration of immunosuppressive therapy (IST).3-8 Thus, it has a significant adverse impact on the life and well-being of alloHCT recipients.
Standard initial therapy for cGVHD includes high-dose glucocorticoids (commonly 1 mg/kg per day of prednisone) with or without calcineurin or mammalian target of rapamycin inhibitors. Although current combination therapies have not demonstrated superiority to glucocorticoids, trials of concurrent therapies with glucocorticoids are critical for limiting cumulative glucocorticoid exposure and related toxicities. Unfortunately, only approximately one-half of all initially treated patients achieve complete response (CR) or partial response (PR), whereas glucocorticoid-refractory nonresponders succumb to substantially higher risk of nonrelapse mortality (NRM). Additionally, failure of initial therapy is a poor prognostic factor because it necessitates subsequent IST with resulting adverse outcomes and treatment-related complications.9,10
Given these challenges, multiple prior large, well-designed trials have examined combined initial therapy approaches (corticosteroids plus other systemic IST), yet none has proven superiority over corticosteroids alone.11-14 More recently, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0801 trial tested the approach of sirolimus/prednisone vs calcineurin inhibitor/sirolimus/prednisone.15 The primary endpoint of CR/PR according to National Institutes of Health (NIH) criteria without death/relapse/secondary therapy at 6 months was 48.6% for 2-drug and 50% for 3-drug regimens, with comparable failure-free survival (FFS) to previously published estimates.16 Despite similar efficacy, the 2-drug regimen was associated with less renal impairment and an improvement in quality of life. Although the BMT CTN 0801 trial adhered to the NIH consensus criteria for cGVHD and set the current standard for the field, new therapies and approaches are still needed to optimize frontline therapy for cGVHD.
Insights into cGVHD pathogenesis continue to accumulate,17 and this knowledge, together with multiple newer targeted therapies, provides opportunities for therapeutic advances. Amid this complexity, B lymphocyte–targeting agents have shown promise in prevention of and therapy for cGVHD.18-26 We previously reported in detail the rationale for use of ofatumumab compared with rituximab phase I results from a phase I-II trial testing prednisone and ofatumumab for initial cGVHD therapy (NCT01680965). In contrast to chimeric rituximab, ofatumumab is a fully humanized third generation of anti-CD20 antibody with distinct binding site and unique mode of cytotoxicity, as reviewed previously.27 The phase I portion of this trial identified ofatumumab at 1000 mg (given at baseline and 14 days later combined with prednisone) as the safe recommended phase II dose for further testing. Here, we report the mature results of the phase II portion of the trial.
Methods
Overview of trial design
This multicenter phase II trial examined the safety and efficacy of prednisone (1 mg/kg per day) and ofatumumab (1000 mg on day 0 and 14) for the initial therapy of cGVHD. Study patients were followed for a total of 24 months (baseline, study therapy day 0 and day 14, and months 1, 3, 6, 12, 18, 24 following therapy). The primary efficacy endpoint was the 6-month clinician-reported overall response rate (ORR; composite of CR and PR). It was selected as the primary outcome measure because the initial development of this trial predated currently accepted NIH response metrics, which allowed benchmarking against expected response rates in prior initial therapy trials. Additional endpoints included use of second-line systemic IST and additional clinical and patient-reported outcomes. The 6-month ORR per 2014 NIH response criteria was computed using the NIH organ scores routinely captured on trial.
Trial participants
Adults ≥18 years with cGVHD requiring systemic glucocorticoid therapy were included in the trial. Chronic GVHD diagnosis and severity scoring adhered to the NIH Consensus Criteria on Diagnosis and Staging of Chronic GVHD.28 Patients had to begin ofatumumab therapy within 14 days of initiation of 1 mg/kg per day of prednisone therapy for cGVHD. The following conditions were study exclusion criteria: relapsed malignancy after HCT; previous systemic glucocorticoid therapy at ≥1 mg/kg per day of prednisone or equivalent for cGVHD; current hepatic/biliary disease (with exception of that due to cGVHD); treatment with experimental non–US Food and Drug Administration–approved therapy within 5 terminal half-lives or 4 weeks before enrollment; other solid tumor within past 5 years (except completely resected nonmelanoma skin cancer); prior treatment with any anti-CD20 monoclonal antibody or alemtuzumab within 3 months; uncontrolled infectious complications; significant cerebrovascular disease in past 6 months; HIV positivity; uncontrolled significant cardiac or other medical conditions; clinically active hepatitis B defined as positive HBsAg or positive HBcAb with detectable HBV DNA viral load; active hepatitis C confirmed by RNA viral load; pregnancy or lactation; women and men unable or unwilling to use adequate contraceptive methods through 1 year after completion of protocol therapy; absolute neutrophil count <1.0 × 109/L; creatinine >2 × upper limit of normal (ULN); and total bilirubin >1.5 × ULN, ALT >2 × ULN or alkaline phosphatase >2.5 × ULN, except for that due to cGVHD.
Treatment plan
Prednisone was started at 1 mg/kg actual body weight per day. The duration of prednisone therapy and tapering schedule were not mandated by the protocol and were coordinated by the treating physicians according to their institutional guidelines. If cGVHD activity increased upon taper, prednisone could be increased to an upper limit of 2 mg/kg per day (or equivalent), but doses higher than that were considered a separate line of additional systemic IST. Taper of other systemic agents and use of topical agents (eg, ocular drops, mouth rinses, topical steroid creams) were similarly not regulated by the trial or considered treatment failure. Ofatumumab was administered by IV infusion; preparation and infusion conditions were per standard procedures. Subjects in this phase II portion of the trial were uniformly dosed at 1000 mg of ofatumumab (delivered once on day 0 and again on day 14 of therapy). Premedication was uniformly delivered within 30 minutes before each infusion: acetaminophen 1000 mg, diphenhydramine 50 mg, and methylprednisolone IV 50 mg. Vital signs were monitored every 30 (± 5) minutes during infusion or more frequently as needed. The initial infusion rate, timing of sequential infusion rate escalation, and rules surrounding response to observed infusion reactions were enforced. If grade 4 infusion reactions occurred, no further ofatumumab therapy was to be given; lesser grade infusion reactions were managed with supportive care, including IV fluids, antihistamines, and steroids. Supportive antimicrobial prophylaxis followed institutional standards. IV immunoglobulin replacement was delivered per discretion of the treating physician.
Trial endpoints
The primary endpoint was ORR at 6 months after initiation of trial therapy based on clinician-assessed response. This primary endpoint was selected during the initial design of the trial, which predated the establishment of the 2014 NIH Response Criteria.29 CR was defined as resolution of all reversible manifestations in each organ or site of cGVHD involvement. PR was defined as improvement in at least 1 organ or site without progression in any other organ or site. The key secondary endpoint of the trial was assessment of ORR at 6 months based on the 2014 NIH Consensus Response Criteria.29 Here, organ-specific and overall responses were calculated per the established response criteria objectively. Additional secondary endpoints included (1) CR at 6 months (assessed both as reported by clinician-assessed response and calculated 2014 NIH response); (2) NRM (cumulative mortality in the absence of primary malignancy relapse); (3) relapse (defined by usual definitions in clinical practice, including but not limited to, morphologic, radiologic, immunophenotypic, or molecular methods and assessed from time of study enrollment); (4) overall survival (OS; assessed from time of study entry with death as an event, while surviving patients were censored at time of last follow-up); (5) use of second-line systemic IST (estimated from time of study entry and included all IST beyond prednisone/ofatumumab). Topical agents or adjustment in dose of existing IST to target therapeutic levels was not considered failure. FFS was estimated from time of study entry with composite events, including death from any cause, relapse, and addition of secondary IST.16 FFS with CR/PR at 12 months was used as an additional post hoc endpoint as previously described. Functional measures included grip strength and 2-minute walk test (assessed at baseline and 3, 6, and 12 months).30
Statistical methods
The phase II trial was powered for the primary outcome of 6-month clinician-reported ORR (CR+PR) with associated 1-sided 90% CI. A Simon’s 2-stage optimum design was used. The single-arm phase II trial considered a historical benchmark of 6-month ORR of 60% based on previously published data.12-14 With this historical benchmark ORR, postulated 6-month ORR of 80% following prednisone and ofatumumab therapy, power of 90%, and 1-side α of 0.1, a total of 38 subjects were needed for analysis of efficacy. A total of 6 patients treated at the maximal tolerated dose/recommended phase II dose from the phase I portion of the trial were carried forward into the phase II analysis. Early termination would occur if ≤6 of 11 subjects in the first stage demonstrated ORR at 6 months. In the second stage of the trial, the null hypothesis would not be rejected if ≤26 subjects demonstrated ORR at 6 months. All treated subjects were studied for efficacy and safety endpoints, including all those who received at least 1 dose of ofatumumab. A total of 38 subjects were enrolled in the study, but 6 of them were not evaluable for the primary endpoint (2 relapses, 3 deaths, and 1 heart failure leading to disenrollment); thus, 32 evaluable subjects were included to assess the efficacy of the combination regimen. The 3 nonrelapse deaths occurring before the 6-month assessment point were caused by respiratory failure in 2 cases (1 pneumonia, 1 pneumonia and pulmonary hemorrhage), and gastrointestinal (GI) bleeding in 1 case. The etiology of the heart failure was unknown, and the patient recovered (heart failure event resolved).
Association between clinical variables (cGVHD severity, organ involvement, patient sociodemographic data, disease, and transplantation variables) and response were examined. Cumulative incidences of relapse and NRM were estimated, accounting for competing risk events. OS and other time-to-event secondary endpoints (eg, FFS) were estimated by the Kaplan-Meier method from the date of therapy initiation. All P values were 2-sided except the primary endpoint, and 2-sided 95% CIs were presented for all endpoints except the primary endpoint. Post hoc analyses compared primary and secondary trial endpoints to more contemporaneous benchmarks of first-line cGVHD therapy such as (1) 6-month ORR of 48.6% from BMT CTN 080115 ; (2) 12-month FFS of 54%16 ; and (3) 12-month FFS with CR/PR of 15%.31 The updated benchmark ORR of 48.6% based on 32 evaluable subjects was used to compute the 1-sided P value and 1-sided 90% CI by using Atkinson and Brown method for post hoc assessment.32 Two-sided P values for both standard FFS and FFS with CR/PR were computed by normal approximation at 12 months with standard error estimated by Greenwood’s approach.33 The correlation between clinician-reported and NIH-calculated response was evaluated by the Kendall τ correlation coefficient.
Data and safety monitoring
A comprehensive data and safety monitoring plan was implemented consistent with standard procedures of the H. Lee Moffitt Cancer Center & Research Institute. The trial was conducted with the approval of the University of South Florida Institutional Review Board and in accordance with the Declaration of Helsinki. The study principal investigator and study team members monitored toxicity and reported adverse events (AEs) and serious adverse events to the Institutional Review Board, the Moffitt Protocol Monitoring Committee, and US Food and Drug Administration. All AEs (grade 1-5) were collected within 24 hours of start of ofatumumab infusion, grade 3 to 5 AEs were collected within 60 days, and only AEs deemed related to the investigational therapy were collected beyond day 61. Additional monitoring was employed for hepatic function, screening for hepatitis B reactivation, development of progressive multifocal leukoencephalopathy (PML), and pregnancy. An early stopping rule for toxicity was based on excess grade 4 or higher AE attributable to the study therapy.
Results
Patient characteristics
A total of 38 subjects were treated with prednisone and ofatumumab as initial cGVHD therapy at 3 study sites (Moffitt Cancer Center, n = 24; Fred Hutchinson Cancer Research Center, n = 9; and University of Minnesota, n = 5) between 2014 and 2018. Baseline patient, disease, and HCT variables are presented in Table 1. Most had received peripheral blood stem cell transplants from unrelated donors, with most common HCT indications being acute leukemia (n = 13) or myelodysplastic syndrome (n = 4). Chronic GVHD characteristics at the time of enrollment are presented in Table 2. Baseline thrombocytopenia, elevated bilirubin, or Karnofsky performance status <80% were uncommon. Most patients had experienced prior acute GVHD (81.6%). Overlap subtype (73.7%) was predominant as compared with classic cGVHD (26.3%). Overall NIH severity score was moderate in 63% and severe in 37%. In keeping with expected organ involvement, skin, mouth, and eye were the most commonly affected organs (Table 2; supplemental Figure 1). There was moderate representation of GI, liver, and joint/fascia cGVHD whereas lung and genital involvement was less common. Among patients with skin involvement, 17 had cutaneous sclerosis and 18 had erythematous rash (9 had erythema and concurrent cutaneous sclerosis). Among subjects with oral involvement, 20 had lichenoid changes, 17 had erythema, 6 had ulceration, and 4 had mucoceles at enrollment. Among patients with GI involvement, 10 had upper GI involvement, 5 had esophageal involvement, and 3 had lower GI cGVHD. Baseline systemic immune suppression at time of study entry was most commonly tacrolimus and sirolimus combined or either one individually. Three total subjects were on lower dose prednisone (median 0.18 mg/kg per day dose, range 0.14-0.5 mg/kg per day) before start of the study intervention. The use of topical agents at baseline and subsequently on trial was tracked but not counted as treatment failure.
Baseline patient, disease, and HCT variables 13
| Variables . | Moffitt (n = 24) . | FHCRC (n = 9) . | UMN (n = 5) . | Total (n = 38) . | P value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, median year (range) | 54.5 (26-73) | 69 (38-84) | 63 (31-69) | 59.5 (26-84) | .022 | |||||
| Gender | Female | 7 | 29.2% | 4 | 44.4% | 2 | 40% | 13 | 34.2% | .68 |
| Male | 17 | 70.8% | 5 | 55.6% | 3 | 60% | 25 | 65.8% | ||
| Disease | AML | 10 | 41.7% | 2 | 22.2% | 2 | 40% | 14 | 36.8% | .55 |
| MDS | 4 | 16.7% | 4 | 44.4% | 1 | 20% | 9 | 23.7% | ||
| MN | 3 | 12.5% | 0 | 0% | 1 | 20% | 4 | 10.5% | ||
| ALL | 3 | 12.5% | 0 | 0% | 1 | 20% | 4 | 10.5% | ||
| NHL | 3 | 12.5% | 2 | 22.2% | 0 | 0% | 5 | 13.2% | ||
| HL | 1 | 4.2% | 0 | 0% | 0 | 0% | 1 | 2.6% | ||
| MM | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | ||
| HCT-CI, median (range) | 3.5 (0-8) | 4 (2-5) | 6 (1-9) | 4 (0-9) | .59 | |||||
| KPS | 80%-100% | 22 | 91.7% | 6 | 66.7% | 3 | 60% | 31 | 81.6% | .21 |
| <80% | 2 | 8.3% | 2 | 22.2% | 1 | 20% | 5 | 13.2% | ||
| Missing | 0 | 0% | 1 | 11.1% | 1 | 20% | 2 | 5.3% | ||
| Graft source | BM | 0 | 0% | 0 | 0% | 1 | 20% | 1 | 2.6% | .0003 |
| PBSC | 24 | 100% | 9 | 100% | 2 | 40% | 35 | 92.1% | ||
| UCB | 0 | 0% | 0 | 0% | 2 | 40% | 2 | 5.3% | ||
| Graft type | MMUD | 2 | 8.3% | 1 | 11.1% | 0 | 0% | 3 | 7.9% | .013 |
| MRD | 5 | 20.8% | 2 | 22.2% | 2 | 40% | 9 | 23.7% | ||
| MUD | 17 | 70.8% | 6 | 66.7% | 1 | 20% | 24 | 63.2% | ||
| UCB | 0 | 0% | 0 | 0% | 2 | 40% | 2 | 5.3% | ||
| Variables . | Moffitt (n = 24) . | FHCRC (n = 9) . | UMN (n = 5) . | Total (n = 38) . | P value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, median year (range) | 54.5 (26-73) | 69 (38-84) | 63 (31-69) | 59.5 (26-84) | .022 | |||||
| Gender | Female | 7 | 29.2% | 4 | 44.4% | 2 | 40% | 13 | 34.2% | .68 |
| Male | 17 | 70.8% | 5 | 55.6% | 3 | 60% | 25 | 65.8% | ||
| Disease | AML | 10 | 41.7% | 2 | 22.2% | 2 | 40% | 14 | 36.8% | .55 |
| MDS | 4 | 16.7% | 4 | 44.4% | 1 | 20% | 9 | 23.7% | ||
| MN | 3 | 12.5% | 0 | 0% | 1 | 20% | 4 | 10.5% | ||
| ALL | 3 | 12.5% | 0 | 0% | 1 | 20% | 4 | 10.5% | ||
| NHL | 3 | 12.5% | 2 | 22.2% | 0 | 0% | 5 | 13.2% | ||
| HL | 1 | 4.2% | 0 | 0% | 0 | 0% | 1 | 2.6% | ||
| MM | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | ||
| HCT-CI, median (range) | 3.5 (0-8) | 4 (2-5) | 6 (1-9) | 4 (0-9) | .59 | |||||
| KPS | 80%-100% | 22 | 91.7% | 6 | 66.7% | 3 | 60% | 31 | 81.6% | .21 |
| <80% | 2 | 8.3% | 2 | 22.2% | 1 | 20% | 5 | 13.2% | ||
| Missing | 0 | 0% | 1 | 11.1% | 1 | 20% | 2 | 5.3% | ||
| Graft source | BM | 0 | 0% | 0 | 0% | 1 | 20% | 1 | 2.6% | .0003 |
| PBSC | 24 | 100% | 9 | 100% | 2 | 40% | 35 | 92.1% | ||
| UCB | 0 | 0% | 0 | 0% | 2 | 40% | 2 | 5.3% | ||
| Graft type | MMUD | 2 | 8.3% | 1 | 11.1% | 0 | 0% | 3 | 7.9% | .013 |
| MRD | 5 | 20.8% | 2 | 22.2% | 2 | 40% | 9 | 23.7% | ||
| MUD | 17 | 70.8% | 6 | 66.7% | 1 | 20% | 24 | 63.2% | ||
| UCB | 0 | 0% | 0 | 0% | 2 | 40% | 2 | 5.3% | ||
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BM, bone marrow; FHCRC, Fred Hutchinson Cancer Research Center; HCT-CI, HCT comorbidity index; HL, Hodgkin lymphoma; KPS, Karnofsky performance status; MN, myeloproliferative neoplasms (including chronic myeloid leukemia, and primary myelofibrosis); MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; NHL, non-Hodgkin lymphoma (including follicular lymphoma, mantle cell lymphoma, and small lymphocytic lymphoma); PBSC, peripheral blood mobilized stem cells; UCB, umbilical cord blood; UMN, University of Minnesota.
Baseline cGVHD characteristics
| Variables . | Moffitt (n = 24) . | FHCRC (n = 9) . | UMN (n = 5) . | Total (n = 38) . | P value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Platelets, median (range) | 195 (61-516) | 155 (78-256) | 207 (187-238) | 196 (61-516) | .31 | |||||
| Bilirubin, median (range) | 0.4 (0.2-6.9) | 0.7 (0.3-1.5) | 0.3 (0.3-0.8) | 0.4 (0.2-6.9) | .040 | |||||
| cGVHD onset | De novo | 1 | 4.2% | 4 | 44.4% | 2 | 40% | 7 | 18.4% | .038 |
| Quiescent | 20 | 83.3% | 5 | 55.6% | 2 | 40% | 27 | 71.1% | ||
| Progressive | 3 | 12.5% | 0 | 0% | 1 | 20% | 4 | 10.5% | ||
| cGVHD type | Classic | 8 | 33.3% | 2 | 22.2% | 0 | 0% | 10 | 26.3% | .29 |
| Overlap | 16 | 66.7% | 7 | 77.8% | 5 | 100% | 28 | 73.7% | ||
| Walk, median (range) | 409 (140-574) | 520 (342-628) | 275 (125-300) | 409 (125-628) | .001 | |||||
| IST | Cyclosporine | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | .014 |
| Prednisone | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | ||
| Sirolimus | 5 | 20.8% | 4 | 44.4% | 0 | 0% | 9 | 23.7% | ||
| Tacrolimus | 4 | 16.7% | 2 | 22.2% | 2 | 40% | 8 | 21.1% | ||
| Tacrolimus/sirolimus | 11 | 45.8% | 0 | 0% | 0 | 0% | 11 | 28.9% | ||
| Tacrolimus/sirolimus/prednisone | 2 | 8.3% | 0 | 0% | 0 | 0% | 2 | 5.3% | ||
| None | 2 | 8.3% | 1 | 11.1% | 3 | 60% | 6 | 15.8% | ||
| NIH scores Skin | 0 | 6 | 25.0% | 0 | 0% | 3 | 60% | 9 | 23.7% | .17 |
| 1 | 2 | 8.3% | 1 | 11.1% | 1 | 20% | 4 | 10.5% | ||
| 2 | 11 | 45.8% | 4 | 44.4% | 1 | 20% | 16 | 42.1% | ||
| 3 | 5 | 20.8% | 4 | 44.4% | 0 | 0% | 9 | 23.7% | ||
| Mouth | 0 | 7 | 29.2% | 3 | 33.3% | 1 | 20% | 11 | 28.9% | .81 |
| 1 | 13 | 54.2% | 5 | 55.6% | 4 | 80% | 22 | 57.9% | ||
| 2 | 4 | 16.7% | 1 | 11.1% | 0 | 0% | 5 | 13.2% | ||
| Eye | 0 | 6 | 25.0% | 4 | 44.4% | 2 | 40% | 12 | 31.6% | .52 |
| 1 | 14 | 58.3% | 5 | 55.6% | 3 | 60% | 22 | 57.9% | ||
| 2 | 4 | 16.7% | 0 | 0% | 0 | 0% | 4 | 10.5% | ||
| Lung | 0 | 20 | 83.3% | 7 | 77.8% | 3 | 60% | 30 | 78.9% | .28 |
| 1 | 4 | 16.7% | 1 | 11.1% | 2 | 40% | 7 | 18.4% | ||
| 2 | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | ||
| GI | 0 | 13 | 54.2% | 6 | 66.7% | 2 | 40% | 21 | 55.3% | .16 |
| 1 | 10 | 41.7% | 2 | 22.2% | 1 | 20% | 13 | 34.2% | ||
| 2 | 1 | 4.2% | 1 | 11.1% | 2 | 40% | 4 | 10.5% | ||
| Liver | 0 | 13 | 54.2% | 3 | 33.3% | 3 | 60% | 19 | 50.0% | .41 |
| 1 | 5 | 20.8% | 5 | 55.6% | 2 | 40% | 12 | 31.6% | ||
| 2 | 2 | 8.3% | 1 | 11.1% | 0 | 0% | 3 | 7.9% | ||
| 3 | 4 | 16.7% | 0 | 0% | 0 | 0% | 4 | 10.5% | ||
| Genital | 0 | 22 | 91.7% | 8 | 88.9% | 5 | 100% | 35 | 92.1% | .82 |
| 1 | 1 | 4.2% | 1 | 11.1% | 0 | 0% | 2 | 5.3% | ||
| 2 | 1 | 4.2% | 0 | 0% | 0 | 0% | 1 | 2.6% | ||
| Joint/fascia | 0 | 9 | 37.5% | 5 | 55.6% | 4 | 80% | 18 | 47.4% | .32 |
| 1 | 7 | 29.2% | 3 | 33.3% | 1 | 20% | 11 | 28.9% | ||
| 2 | 8 | 33.3% | 1 | 11.1% | 0 | 0% | 9 | 23.7% | ||
| Overall severity | 2 (moderate) | 15 | 62.5% | 4 | 44.4% | 5 | 100% | 24 | 63.2% | .12 |
| 3 (severe) | 9 | 37.5% | 5 | 55.6% | 0 | 0% | 14 | 36.8% | ||
| Variables . | Moffitt (n = 24) . | FHCRC (n = 9) . | UMN (n = 5) . | Total (n = 38) . | P value . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Platelets, median (range) | 195 (61-516) | 155 (78-256) | 207 (187-238) | 196 (61-516) | .31 | |||||
| Bilirubin, median (range) | 0.4 (0.2-6.9) | 0.7 (0.3-1.5) | 0.3 (0.3-0.8) | 0.4 (0.2-6.9) | .040 | |||||
| cGVHD onset | De novo | 1 | 4.2% | 4 | 44.4% | 2 | 40% | 7 | 18.4% | .038 |
| Quiescent | 20 | 83.3% | 5 | 55.6% | 2 | 40% | 27 | 71.1% | ||
| Progressive | 3 | 12.5% | 0 | 0% | 1 | 20% | 4 | 10.5% | ||
| cGVHD type | Classic | 8 | 33.3% | 2 | 22.2% | 0 | 0% | 10 | 26.3% | .29 |
| Overlap | 16 | 66.7% | 7 | 77.8% | 5 | 100% | 28 | 73.7% | ||
| Walk, median (range) | 409 (140-574) | 520 (342-628) | 275 (125-300) | 409 (125-628) | .001 | |||||
| IST | Cyclosporine | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | .014 |
| Prednisone | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | ||
| Sirolimus | 5 | 20.8% | 4 | 44.4% | 0 | 0% | 9 | 23.7% | ||
| Tacrolimus | 4 | 16.7% | 2 | 22.2% | 2 | 40% | 8 | 21.1% | ||
| Tacrolimus/sirolimus | 11 | 45.8% | 0 | 0% | 0 | 0% | 11 | 28.9% | ||
| Tacrolimus/sirolimus/prednisone | 2 | 8.3% | 0 | 0% | 0 | 0% | 2 | 5.3% | ||
| None | 2 | 8.3% | 1 | 11.1% | 3 | 60% | 6 | 15.8% | ||
| NIH scores Skin | 0 | 6 | 25.0% | 0 | 0% | 3 | 60% | 9 | 23.7% | .17 |
| 1 | 2 | 8.3% | 1 | 11.1% | 1 | 20% | 4 | 10.5% | ||
| 2 | 11 | 45.8% | 4 | 44.4% | 1 | 20% | 16 | 42.1% | ||
| 3 | 5 | 20.8% | 4 | 44.4% | 0 | 0% | 9 | 23.7% | ||
| Mouth | 0 | 7 | 29.2% | 3 | 33.3% | 1 | 20% | 11 | 28.9% | .81 |
| 1 | 13 | 54.2% | 5 | 55.6% | 4 | 80% | 22 | 57.9% | ||
| 2 | 4 | 16.7% | 1 | 11.1% | 0 | 0% | 5 | 13.2% | ||
| Eye | 0 | 6 | 25.0% | 4 | 44.4% | 2 | 40% | 12 | 31.6% | .52 |
| 1 | 14 | 58.3% | 5 | 55.6% | 3 | 60% | 22 | 57.9% | ||
| 2 | 4 | 16.7% | 0 | 0% | 0 | 0% | 4 | 10.5% | ||
| Lung | 0 | 20 | 83.3% | 7 | 77.8% | 3 | 60% | 30 | 78.9% | .28 |
| 1 | 4 | 16.7% | 1 | 11.1% | 2 | 40% | 7 | 18.4% | ||
| 2 | 0 | 0% | 1 | 11.1% | 0 | 0% | 1 | 2.6% | ||
| GI | 0 | 13 | 54.2% | 6 | 66.7% | 2 | 40% | 21 | 55.3% | .16 |
| 1 | 10 | 41.7% | 2 | 22.2% | 1 | 20% | 13 | 34.2% | ||
| 2 | 1 | 4.2% | 1 | 11.1% | 2 | 40% | 4 | 10.5% | ||
| Liver | 0 | 13 | 54.2% | 3 | 33.3% | 3 | 60% | 19 | 50.0% | .41 |
| 1 | 5 | 20.8% | 5 | 55.6% | 2 | 40% | 12 | 31.6% | ||
| 2 | 2 | 8.3% | 1 | 11.1% | 0 | 0% | 3 | 7.9% | ||
| 3 | 4 | 16.7% | 0 | 0% | 0 | 0% | 4 | 10.5% | ||
| Genital | 0 | 22 | 91.7% | 8 | 88.9% | 5 | 100% | 35 | 92.1% | .82 |
| 1 | 1 | 4.2% | 1 | 11.1% | 0 | 0% | 2 | 5.3% | ||
| 2 | 1 | 4.2% | 0 | 0% | 0 | 0% | 1 | 2.6% | ||
| Joint/fascia | 0 | 9 | 37.5% | 5 | 55.6% | 4 | 80% | 18 | 47.4% | .32 |
| 1 | 7 | 29.2% | 3 | 33.3% | 1 | 20% | 11 | 28.9% | ||
| 2 | 8 | 33.3% | 1 | 11.1% | 0 | 0% | 9 | 23.7% | ||
| Overall severity | 2 (moderate) | 15 | 62.5% | 4 | 44.4% | 5 | 100% | 24 | 63.2% | .12 |
| 3 (severe) | 9 | 37.5% | 5 | 55.6% | 0 | 0% | 14 | 36.8% | ||
Study therapy and toxicity
Prednisone therapy was started at 1 mg/kg per day, and ofatumumab was given at baseline and 14 days later. Infusion-related toxicity of ofatumumab was expected and occurred in 17 (44.7%) subjects (grade 1 in 4 patients and grade 2 in 13 patients) These included infusion reaction (n = 9), abdominal discomfort (n = 2), and (n = 1 for each) atrial fibrillation, diaphoresis, hypotension, pre-syncope, sore throat, and urticaria. With symptom management, these toxicities resolved and only 1 patient did not receive the second planned dose of ofatumumab. Subsequent AEs, including infectious morbidity, are summarized in Table 3. The most common infections included respiratory viral infections documented by nasopharyngeal swab together with pneumonia, bloodstream infections, and cellulitis, among others (supplemental Table 1). A total of 15 subjects had viral infections (with 9 having multiple); 11 subjects experienced bacterial infections (with 2 of these subjects having 2 distinct bacterial infections); 5 subjects had fungal infections (with 1 subject having multiple). Of note, these infectious events were captured over the full extent of follow-up for all treated subjects (with median follow-up time on trial for all subjects of 23 months, range 1-46 months).
Adverse events by major toxicities
| Toxicity group . | Adverse events . | Grade . | Total . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||||||||
| Hematologic | Anemia | 0 | 0% | 0 | 0% | 1 | 2.6% | 0 | 0% | 1 | 2.6% |
| Neutropenia | 0 | 0% | 0 | 0% | 1 | 2.6% | 1 | 2.6% | 2 | 5.3% | |
| Cardiovascular | Heart failure | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% |
| Gastrointestinal/liver | Abdominal pain | 0 | 0% | 0 | 0% | 1 | 2.6% | 0 | 0% | 1 | 2.6% |
| Diarrhea | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Dysphagia | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Transaminitis | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| General and infusion related | Fatigue | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% |
| Urticaria | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Edema in limbs | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Infection | Bacterial | 0 | 0% | 5 | 13.2% | 7 | 18.4% | 1 | 2.6% | 13 | 34.2% |
| Viral | 1 | 2.6% | 16 | 42.1% | 6 | 15.8% | 3 | 7.9% | 26 | 68.4% | |
| Fungal | 1 | 2.6% | 2 | 5.3% | 2 | 5.3% | 3 | 7.9% | 8 | 21.1% | |
| Unidentified | 2 | 5.3% | 5 | 13.2% | 7 | 18.4% | 0 | 0% | 14 | 36.8% | |
| Electrolyte abnormalities | Hypocalcemia | 0 | 0% | 0 | 0% | 1 | 2.6% | 0 | 0% | 1 | 2.6% |
| Neurologic | Pre-syncope | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% |
| Toxicity group . | Adverse events . | Grade . | Total . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||||||||
| Hematologic | Anemia | 0 | 0% | 0 | 0% | 1 | 2.6% | 0 | 0% | 1 | 2.6% |
| Neutropenia | 0 | 0% | 0 | 0% | 1 | 2.6% | 1 | 2.6% | 2 | 5.3% | |
| Cardiovascular | Heart failure | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% |
| Gastrointestinal/liver | Abdominal pain | 0 | 0% | 0 | 0% | 1 | 2.6% | 0 | 0% | 1 | 2.6% |
| Diarrhea | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Dysphagia | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Transaminitis | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| General and infusion related | Fatigue | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% |
| Urticaria | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Edema in limbs | 1 | 2.6% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.6% | |
| Infection | Bacterial | 0 | 0% | 5 | 13.2% | 7 | 18.4% | 1 | 2.6% | 13 | 34.2% |
| Viral | 1 | 2.6% | 16 | 42.1% | 6 | 15.8% | 3 | 7.9% | 26 | 68.4% | |
| Fungal | 1 | 2.6% | 2 | 5.3% | 2 | 5.3% | 3 | 7.9% | 8 | 21.1% | |
| Unidentified | 2 | 5.3% | 5 | 13.2% | 7 | 18.4% | 0 | 0% | 14 | 36.8% | |
| Electrolyte abnormalities | Hypocalcemia | 0 | 0% | 0 | 0% | 1 | 2.6% | 0 | 0% | 1 | 2.6% |
| Neurologic | Pre-syncope | 0 | 0% | 1 | 2.6% | 0 | 0% | 0 | 0% | 1 | 2.6% |
Efficacy
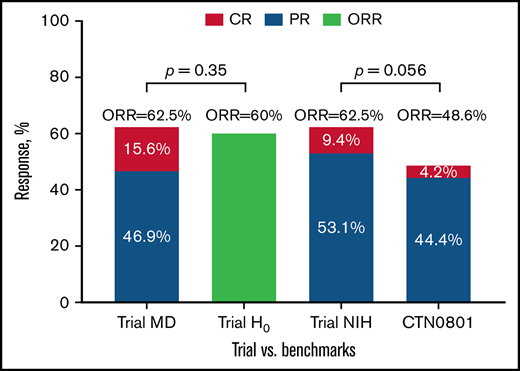
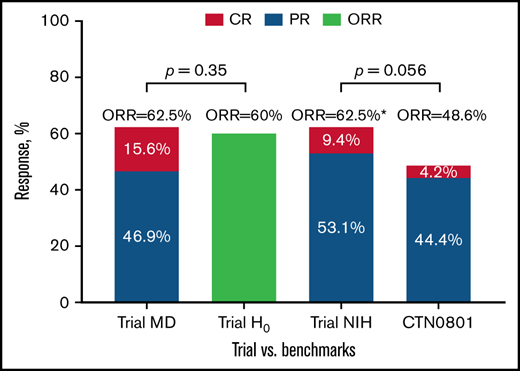
The 6-month clinician-reported ORR was 62.5% (95% CI 44%-79%), yielding no superiority of our combined therapy approach compared with prespecified historic null hypothesis of 60% (H0 failed to be rejected with 1-sided P = .35; Figure 1). The 6-month ORR by 2014 NIH response criteria was also 62.5% (9.4% CR; 53.1% PR) with high correlation between clinician- and NIH-defined CR vs PR responses (Kendall τ = 0.82; Figure 2).
Clinician- and NIH-based responses at 6 months among evaluable subjects compared with the benchmarks. Lower limit of 1-sided 90% CI for both MD- and NIH-defined ORR of 62.5% was 51.5% (Atkinson-Brown estimate), which exceeded benchmark estimate from BMT CTN 0801 (48.6%). Trial MD was the trial clinician–assessed response rate at 6 months; Trial H0 was the trial null hypothesis based on prior published clinician-assessed 6-month response rates in phase III cGVHD therapy trials; Trial NIH was the trial NIH 6-month response rate; CTN 0801 was the published NIH 6-month response rate in BMT CTN 0801 trial. *Atkinson-Brown 1-sided CI limit was 51.5%.
Clinician- and NIH-based responses at 6 months among evaluable subjects compared with the benchmarks. Lower limit of 1-sided 90% CI for both MD- and NIH-defined ORR of 62.5% was 51.5% (Atkinson-Brown estimate), which exceeded benchmark estimate from BMT CTN 0801 (48.6%). Trial MD was the trial clinician–assessed response rate at 6 months; Trial H0 was the trial null hypothesis based on prior published clinician-assessed 6-month response rates in phase III cGVHD therapy trials; Trial NIH was the trial NIH 6-month response rate; CTN 0801 was the published NIH 6-month response rate in BMT CTN 0801 trial. *Atkinson-Brown 1-sided CI limit was 51.5%.
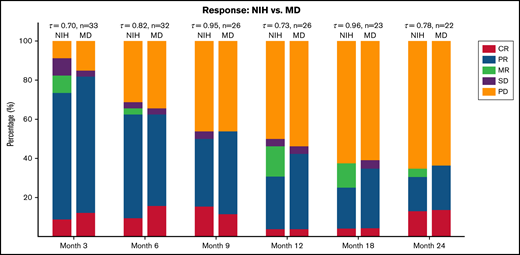
Clinician-reported (MD) or NIH-calculated (NIH) responses at serial time points on trial. MR, mixed response; PD, progressive disease; SD, stable disease.
Clinician-reported (MD) or NIH-calculated (NIH) responses at serial time points on trial. MR, mixed response; PD, progressive disease; SD, stable disease.
Comparison between the 6-month NIH-defined ORR of 62.5% with more contemporaneous benchmark of 48.6% ORR from BMT CTN 0801 suggested difference (lower CI limit of 51.5%, P = .056) favoring the ofatumumab combination (Figure 1; supplemental Table 2). We found no significant association between baseline cGVHD subtype, organ involvement, overall severity, or baseline immune suppression with the primary outcome of 6-month ORR (supplemental Table 3). Among those with clinician-reported ORR at 6 months, subsequent clinician-reported responses (9, 12, 18, 24 months) are presented in supplemental Table 4. There was moderate correlation between clinician-reported responses and NIH-calculated responses at serial time points on the trial (Figure 2). Individual changes in NIH organ scores over time are presented in supplemental Table 5. We also examined the change in grip strength and walk test over time and found no significant association with clinician-reported ORR (supplemental Table 6).
Prednisone taper was rapid for most patients, allowing for median steroid dose reductions from a starting dose of 1 mg/kg per day to 0.49 mg/kg per day by day 30 and to 0.14 mg/kg per day by the response assessment at 6 months (supplemental Figure 3). Treatment failure was modeled as the composite of malignancy relapse, NRM, and requirement of additional systemic IST beyond the study intervention (prednisone + ofatumumab). Among the 38 subjects, the first FFS event included 3 relapses, 5 nonrelapse deaths, and 13 new systemic IST events, while 17 subjects were censored. The 3 relapse events occurred in subjects with acute myelogenous leukemia,1 myelodysplastic syndrome,1 and acute lymphoblastic leukemia.1 The actual systemic immune suppression agents used after the study intervention are provided in supplemental Table 7, and the median time to initiation of second-line therapy was 5.4 months (range 0.9-15.1 months). The cumulative incidence of treatment failure and a Kaplan-Meier plot of OS and FFS are presented in Figure 3. FFS at 6 months was 64.2% (95% CI, 46.5%-77.4%) and at 12 months was 53.1% (95% CI, 35.8%-67.7%), which was overall similar to previously published FFS following prednisone ± other agents for initial cGVHD therapy.16 However, when compared with the benchmark of 15% FFS with CR/PR at 12 months,31 the ofatumumab combination was statistically superior (supplemental Table 2) at 33.5% (P = .019). The group of patients with 12-month FFS with CR/PR (compared with 12-month FFS with non-CR/PR response) had higher likelihood of complete steroid discontinuation by 24 months with odds ratio (OR) of 8 (95% CI, 1.21-52.7; P = .025). Separately, considering any death occurring on study for the OS outcome, causes of death were pneumonia/respiratory failure (N = 2), multiple organ failure (N = 1), GI bleeding (N = 1), unknown (N = 4), and relapsed disease (N = 2).
Long-term study outcomes as measured by treatment failure and OS and FFS. (A) Cumulative incidence of treatment failure (death, relapse, and new systemic IST). (B) Overall and FFS.
Long-term study outcomes as measured by treatment failure and OS and FFS. (A) Cumulative incidence of treatment failure (death, relapse, and new systemic IST). (B) Overall and FFS.
Immune reconstitution
Among the patients enrolled at the Moffitt Cancer Center (n = 24), immune cell subsets and quantitative immunoglobulins were tracked at baseline and 3, 6, and 12 months after start of study therapy (supplemental Figure 2; supplemental Table 8). These data demonstrate marked depletion of total CD19+ B lymphocytes with corresponding decline in immunoglobulins with some minimal recovery by 12 months of follow-up. We calculated infection density (infections per survival time) for 3 distinct time periods (within 30 days, 31-180 days, and 181-365 days) after initiation of study therapy and explored the relationship between infection density and degree of immune compromise (approximated by prednisone exposure and quantitative immunoglobulins). These data demonstrated greater infection density in the 2 earlier time periods and supported increased infection density in the early time frame (within 30 days) for patients with IgG, IgA, and IgE below median values (supplemental Table 9). Otherwise, no significant associations were detected.
Discussion
Under standard-of-care practices for initial cGVHD therapy, results are poor, with frequent failure and need for additional lines of systemic IST. Supported by a large body of preclinical and clinical trial-based evidence recognizing a key role for B cells in cGVHD pathobiology, this trial aimed to combine a potent B lymphocyte depleting agent, ofatumumab, with standard-of-care prednisone to evaluate whether this approach would provide a benefit worthy of additional study beyond this single-arm phase II trial. Combination therapy was feasible to administer in a multicenter setting given relatively low and tolerable toxicity profile with addition of ofatumumab to prednisone. The observed toxicities largely mirrored those expected with prednisone alone in this setting. Infusion-related reaction from the ofatumumab was distinct and mostly mild and responded to supportive care without preventing delivery of the planned second dose of the drug, except in 1 patient. Long-term toxicity was dominated by infectious complications, particularly respiratory viral infections. We compared rates of severe (grade 3-4) infections on our trial vs that reported in the BMT CTN 0801 trial as a frame of reference. Based on the expected rate of severe infections per number of subjects on that trial, we would expect in our study 36 grade 3 to 4 infectious events per 38 subjects treated. As presented in Table 3, we observed 29 grade 3 to 4 infectious events. Thus, the rate of severe infections on our trial appeared to be within expected limits.15 We also explored whether certain measures or proxies of immune compromise (cumulative prednisone exposure, degree of immune globulin decline) influenced observed infection density. Some association was seen with reduced immunoglobulin levels; however, the trial did not provide specific guidance on use of IV immunoglobulin to address this issue (thus defaulted to standard-of-care practices). We did not observe any serious complications of ofatumumab therapy, such as viral hepatitis reactivation or PML. Within this phase II trial population, we observed a relatively low rate of malignancy relapse after HCT but acknowledge that a larger study population would be required to evaluate it further.
Our trial was initially developed and launched before the implementation of the 2014 NIH Consensus Response Criteria for clinical trials. This trial was powered for a relatively large improvement in physician-reported treatment response (6-month CR/PR rate of 80% vs 60%) compared with a historical benchmark set by prior cGVHD initial therapy trials that similarly used physician-reported response as primary outcome. This large effect may not have been achievable with either ofatumumab or any initial cGVHD therapy approach. Potential contributors to the observed physician-reported response rate include variation in the cGVHD features of enrolled subjects, aggressive prednisone-tapering practices on trial, and interrater variability in physician response assessment. Although cGVHD features may have contributed to the observed response rate, the relatively modest sample size of this single-arm phase II trial was insufficient to clearly identify organ-specific responsiveness to the tested intervention. Furthermore, response also was likely influenced by rapid and heterogeneous prednisone taper practices on trial. Prolonged initial steroid therapy may have perhaps positively influenced the 6-month response assessment. However, clinician judgment and patient tolerance of steroid-associated adverse effects are important and are inherent trade-offs to steroid-based therapy. During prednisone taper, an increasing proportion of stable disease and progressive disease were associated with use of second-line therapy (with median time to second-line therapy of 5.3 months). This trial was conducted in multiple centers with numerous participating investigators and treating physicians. Heterogeneous response assessment by evaluating physician is well established and highlights the limitation of this approach. Nevertheless, we systematically captured organ scores serially using provider forms designated by the NIH Consensus recommendations and computed NIH response as a secondary outcome measure.
Although the trial did not meet its primary endpoint of hypothesized 20% improvement in the ORR at 6 months, it demonstrated potential clinical benefit in secondary and exploratory analyses using contemporary definitions of response and overall clinical benefit according to 2014 NIH Response criteria and FFS with ongoing CR/PR at 12 months. Our observed 6-month NIH CR/PR rate suggests that this combination has potential activity as an initial therapy for cGVHD and compares favorably with current best estimates of response, such as those reported by the BMT CTN 0801 trial. In fact, the 6-month NIH ORR benchmark for the BMT CTN0801 trial was set at 40%, whereas the 6-month ORR was 48.6% in the 2-drug arm and 50% in the 3-drug arm.15 Similarly, in the more recent INTEGRATE phase 3 trial comparing combination of ibrutinib and prednisone vs prednisone, the 6-month ORR was 47%.34,35 Both of these more contemporaneous trials employed NIH-based response assessment. We acknowledge, however, that these comparisons are post hoc and that there is inherent heterogeneity in these patient populations. Also, FFS was comparable to current best estimates in the initial therapy setting, and FFS with CR/PR at 12 months appeared to surpass current best estimates. Consistent with prior data, this subset of patients had greater likelihood of favorable outcome with discontinuation of systemic corticosteroids within 2 years of follow-up. These data supports that a subgroup of patients treated with prednisone plus ofatumumab will achieve durable response and freedom from treatment failure, yet the limited study population did not afford clear insights into patient- and cGVHD-level variables associated with durable treatment success. We note, however, that these comparisons were post hoc and therefore do not fully support the superiority of this approach.
The field of cGVHD therapy is rapidly evolving, and both interpretation of this trial’s results and plans for future cGVHD treatment trials require careful deliberation. First, it is possible that prednisone-based combination approaches simply cannot exceed the limited success of current standard-of-care initial therapy (eg, prednisone/calcineurin inhibitor or prednisone/sirolimus) and that different strategies are needed. For example, multiple large, randomized trials testing combined initial therapy approaches (eg, addition of agents such as thalidomide, azathioprine, or mycophenolate mofetil)11-13 have failed to show benefit over standard therapy, and a triple-drug regimen (calcineurin inhibitor/sirolimus/prednisone) was equally effective but more toxic than sirolimus/prednisone alone in BMT CTN 0801.15 Thus, intensification of initial therapy alone may not provide greater long-term treatment success, at least delivered at this point in cGVHD natural history. Second, earlier intervention in cGVHD pathogenesis may ultimately prove more effective in achieving disease control, sparing long-term morbidity and disability from cGVHD, and preventing prolonged IST seen under current practices. Concepts surrounding primary prevention, early diagnosis, and preemptive therapy of cGVHD have been explored in the 2020 NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft versus Host Disease and should inform trial development in the future.36-38 Lastly, another major proposal from this 2020 NIH Consensus Project involved supporting innovative trials testing lower-dose or steroid-free approaches with targeted agents.39 Prior phase II data with the use of rituximab with or without corticosteroids in the frontline setting pointed toward overall high response rates.25,40 In contrast to the study by Malard et al, which allowed rituximab retreatment, we observed no PML in our trial. Taken together, CD20 targeting frontline therapy of cGVHD warrants future randomized trials of potentially steroid-free combination regimens according to the recently proposed NIH guidelines on cGVHD therapy.39 These future therapeutic strategies may offer similar clinical benefit in initial cGVHD therapy while sparing steroid-related toxicity. Rationally applying the right intervention to patients with the greatest likelihood of benefiting (personalized therapy) represents an important future goal. Given the signal of benefit in our trial (and the wealth of published data supporting a role of B cells in cGVHD), ofatumumab or other B-cell targeting agents should be considered in these novel treatment paradigms.
In summary, our single-arm phase II trial demonstrated acceptable safety and efficacy for key NIH-defined treatment outcomes after upfront use of ofatumumab in cGVHD. Future strategies may employ B-cell targeting agents in novel trial designs alone, with low-dose prednisone, or in combination with other targeted therapies.
Acknowledgment
This work was supported in part by Biostatistics Core shared resources at the H. Lee Moffitt Cancer Center & Research Institute, designated a comprehensive cancer center by the National Cancer Institute, National Institutes of Health (P30CA076292).
Authorship
Contribution: J.A.P., A.L., S.L., M. Arora, J.K., and C.A. designed and performed the research and wrote the paper; and B.C.B., F.K., T.N., N.B., H.L., M.A.K.-D., F.L.L., R.G., M.D.J., M.L.D., L.E.P., A.M., A.P.P., K.B., E.A., L.O., O.C.P., R.F., M. Alina, H.E., M.L.N., and H.F. contributed to the conduct of the trial, performed the research, contributed to interpretation of data, and contributed to writing the manuscript.
Conflict-of-interest disclosure: M.A.K.-D. is a consultant for Daiichi Sankyo. S.L. received research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer Syndax, and Takeda, received study medication from Johnson and Johnson, and is on the steering committee for Incyte. F.L.L. has a scientific advisory role at Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, GammaDelta Therapeutics, Iovance, Janssen, Legend Biotech, Novartis, and Wugen, received research funding from Kite Pharma (Institutional), Allogene (Institutional), and Novartis (Institutional), and is a consultant for Cellular Biomedicine Group, Cowen, EcoR1, Emerging Therapy Solutions, and Gerson Lehrman Group. A.M. received grant funding from Novartis. M.D.J. is a consultant for Kite/Gilead, Novartis, BMS, and Takeda. J.A.P. has a consulting and advisory board membership with Syndax, CTI Biopharma, Amgen, Regeneron, and Incyte and received clinical trial funding from Novartis, Amgen, Takeda, Janssen, Johnson and Johnson, Pharmacyclics, Abbvie, CTI Biopharma, and BMS. The remaining authors declare no competing financial interests.
Correspondence: Joseph A. Pidala, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: joseph.pidala@moffitt.org.
References
Author notes
For data sharing, contact the corresponding author: joseph.pidala@moffitt.org.
The full-text version of this article contains a data supplement.