Abstract
von Willebrand disease (VWD) disproportionately affects women because of the potential for heavy menstrual bleeding (HMB), delivery complications, and postpartum hemorrhage (PPH). To systematically synthesize the evidence regarding first-line management of HMB, treatment of women requiring or desiring neuraxial analgesia, and management of PPH. We searched Medline and EMBASE through October 2019 for randomized trials, comparative observational studies, and case series comparing the effects of desmopressin, hormonal therapy, and tranexamic acid (TxA) on HMB; comparing different von Willebrand factor (VWF) levels in women with VWD who were undergoing labor and receiving neuraxial anesthesia; and measuring the effects of TxA on PPH. We conducted duplicate study selection, data abstraction, and appraisal of risk of bias. Whenever possible, we conducted meta-analyses. We assessed the quality of the evidence using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach. We included 1 randomized trial, 3 comparative observational studies, and 10 case series. Moderate-certainty evidence showed that desmopressin resulted in a smaller reduction of menstrual blood loss (difference in mean change from baseline, 41.6 [95% confidence interval, 16.6-63.6] points in a pictorial blood assessment chart score) as compared with TxA. There was very-low-certainty evidence about how first-line treatments compare against each other, the effects of different VWF levels in women receiving neuraxial anesthesia, and the effects of postpartum administration of TxA. Most of the evidence relevant to the gynecologic and obstetric management of women with VWD addressed by most guidelines is very low quality. Future studies that address research priorities will be key when updating such guidelines.
Introduction
Von Willebrand disease (VWD) is a common bleeding disorder that typically manifests with mucosal bleeding.1 Although women and men are equally likely to be affected, women with VWD are more likely to have bleeding symptoms related to the high prevalence of heavy menstrual bleeding (HMB). Data from studies of women with H MB show that between 5% and 24% have VWD.2,3 Conversely, studies of women with VWD show that 50% to 92% experience HMB.4,5 Management is challenging, and the diagnosis is delayed in many women, with an average of 16 years from onset of symptoms to diagnosis.6
HMB is not the only challenge for women with VWD.7 Pregnancy is associated with a higher VWF level in some women with VWD; typically those with type 1 VWD. The higher level, however, is not of the same magnitude as seen in pregnant women without VWD, resulting in an increased risk of primary postpartum hemorrhage (PPH) in patients with type 1 VWD.8 Furthermore, the pregnancy-induced rise in the level of VWF occurs within the first postpartum week, then reaches baseline by the third postpartum week, predisposing women with VWD to a risk of secondary PPH.9-12 In addition, management of neuraxial anesthesia may be complicated, given that there is no evidence regarding the optimal target VWF level to safeguard against bleeding complications without increasing the potential risk of thrombosis in pregnant women.13,14 Treatment options for HMB include desmopressin, administration of VWF concentrate, hormonal therapy, and antifibrinolytic therapy (eg, tranexamic [TxA] acid).15-17
In 2017, the American Society of Hematology (ASH), the International Society on Thrombosis and Haemostasis (ISTH), the National Hemophilia Foundation (NHF), and the World Federation of Hemophilia (WFH) convened a working group to define the scope and priority areas of focus for updated guidelines on VWD.18 The topic of guidelines for women with VWD was rated the highest priority among all available priorities.18 The purpose of this article is to describe the methods and the results of the systematic reviews (SRs) conducted to inform the 3 recommendations relevant to obstetrics and gynecology of the ASH ISTH NHF WFH Guidelines on the Management of VWD.19
Methods
We did not register a protocol but followed prespecified evidence synthesis methods standard to ASH20 and established eligibility criteria for inclusion of studies based on the recommended questions prioritized by the panel.
This article addresses 3 SR questions:
- 1.
What are the comparative effects of desmopressin (DDAVP), hormonal therapy, and TxA as first-line treatments for women with HMB? (SR1)
- 2.
What are the comparative effects of increasing VWF to 0.50 to 1.50 IU/mL vs >1.50 IU/mL in women with VWD who are in labor and receiving neuraxial anesthesia? (SR2)
- 3.
What are the effects of TxA in women in the postpartum period? (SR3)
Eligibility criteria
We included randomized clinical trials and comparative observational studies of any design for all the questions of interest. When none of these study designs was available, we included case series. We included studies in which researchers enrolled women with all types of VWD, hemophilia, or inherited bleeding disorders. We excluded women with acquired VWD. We included studies that addressed any clinical outcome. We excluded studies that provided only information about physiological outcomes, such as VWF levels. We excluded studies published only as conference abstracts. Specific eligibility criteria for each of the questions is described below:
- 1.
SR1: we included women who were seeking first-line therapy for HMB and were enrolled in studies that compared any regimen of desmopressin, hormonal therapy (including oral combined therapy and levonorgestrel-releasing intrauterine system [LNG-IUS]; eg, Mirena), or TxA with each other. We included case series of women who received LNG-IUS.
- 2.
SR2: we included women in labor who required or desired to receive any type of neuraxial anesthesia. We included studies that compared an increase in VWF to 0.50 to 1.50 IU/mL with increasing the level to >1.50 IU/mL, using any intervention, and case series in which women received either of the interventions.
- 3.
SR3: we included women in the postpartum period (ie, up to 6 weeks after giving birth) who were enrolled in studies comparing any TxA regimen or any other antifibrinolytic to no treatment.
For all SRs, we included studies reporting any clinical outcome.
Information sources
We searched in Medline (OVID) and EMBASE from inception through October 2019. We conducted an umbrella search encompassing all recommendation questions addressed in the guidelines (supplemental Appendix 1). We did not limit by date or language of publication. We also searched in the reference list of included studies and contacted the panel of experts to obtain relevant studies. We searched for gray literature using Open Gray.
Study selection and data abstraction
Pairs of independent reviewers screened the titles and abstracts of all citations for all the SRs. We included all studies identified as potentially relevant and performed duplicate screening of the full texts for each of the SRs separately. Reviewers resolved disagreements by discussion with a third reviewer or the clinical experts.
We abstracted data in duplicate. For each study, we abstracted information about the setting, participant characteristics (mean age, distribution according to type of bleeding disorder), interventions received (specific agent and regimen), and outcome data for all clinical outcomes reported, with any method of measurement, at any time point.
Reviewers used standardized, piloted data abstraction forms and underwent training and calibration at all stages.
Data synthesis
For dichotomous outcomes, we used the risk ratio (RR) and its 95% confidence interval (CI) as the effect measure in comparative studies and proportions and their 95% CI in single-arm studies. For continuous outcomes, we used the mean difference and its 95% CI for comparative studies and the mean and standard deviation for noncomparative studies. When studies did not report sufficient data to present the effect estimates using these measures, we used what was available.
Assessment of quality of the evidence
We assessed the quality of the evidence for each of the outcomes by using the Grading of Recommendations Assessments, Development, and Evaluation (GRADE) approach.23 The quality of the evidence assessment considered study design and risk of bias, inconsistency, indirectness, imprecision, publication bias, presence of large effects, dose-response gradient, and residual confounding. We assessed risk of bias by using the Cochrane Risk of Bias tool for randomized clinical trials and the Risk of Bias in Nonrandomized Studies of Interventions tool for comparative observational studies. Because of the lack of a comparison group in the single-arm studies that we used to make inferences about how treatments compare, their risk of bias was judged as high by default. We assessed inconsistency by comparing the point estimates and CIs across studies, as well as by using statistical measures (χ2and I2). We assessed indirectness by focusing on characteristics of the population, particularly the proportion of participants who had VWD instead of other bleeding disorders. We assessed imprecision using a noncontextualized approach and the null effect as the threshold of interest, as well as the optimal information size.24 We planned to assess publication bias by using funnel plots if a meta-analysis had 10 studies or more.
We constructed summary-of-findings tables by using GRADEpro. Whenever possible, we present absolute and relative estimates of effects. We calculated absolute estimates using the results from the studies included to obtain a baseline risk. We included in the tables all outcomes for which there was evidence and outcomes that were considered critical or important for decision-making by the guideline panel, but for which there was no information.
Subgroup and sensitivity analyses
We planned to conduct subgroup analyses based on the risk of bias of the studies and the populations included. We did not plan any sensitivity analyses.
Results
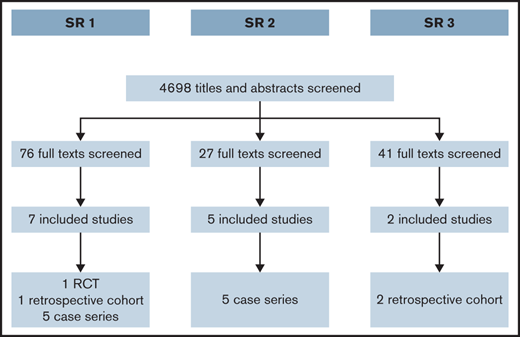
We screened a total of 4698 titles and abstracts. Figure 1 summarizes the results of the search and study selection process. We present the result of each of the SRs below.
SR1: first-line therapy for HMB
After reviewing 76 full texts, we included 2 comparative studies: 1 randomized clinical trial comparing TxA with desmopressin25 and 1 observational study (retrospective cohort) comparing hormonal therapy with desmopressin.26 A third eligible study comparing hormonal therapy with desmopressin27 did not report outcome data clearly, and we were not able to get more information from the researchers to include in this evidence synthesis. In addition, we included evidence from 5 case series of LNG-IUS.28-32 Supplemental Appendix 2 presents the characteristics of the included studies. Tables 1 and 2 summarize the effects of the interventions for the comparative studies. Table 3 summarizes the evidence regarding LNG-IUS.
Summary of findings of comparative effects of DDAVP vs TxA in women with VWD and HMB
| . | ||||||
|---|---|---|---|---|---|---|
| Outcome . | Anticipated absolute effects (95% CI)* . | Relative effect (95% CI) . | Participants, n (studies) . | Certainty of the evidence (GRADE) . | Comments . | |
| Risk with TxA . | Risk with DDAVP (range) . | |||||
| Change in menstrual blood loss assessed as the change from baseline on PBAC follow-up, at 2 mo | The mean change in menstrual blood loss was 105.9. | MD 41.6 higher (19.6-63.6 higher) | — | 116 (1 RCT) | ⨁⨁⨁◯ MODERATE†,‡ | DDAVP probably reduces menstrual blood loss less than TxA. |
| Quality of life assessed with several scales (HRQoL, SF-36, CES-D, RUTA); at follow-up, 2 mo | The researchers did not provide an explicit comparison between the groups. Scores across instruments and domains suggested improvement for both interventions, but this was the only category to show a statistically significant difference. | — | 116 (1 RCT) | ⨁⨁◯◯ LOW‡,§ | There may be no important differences in HRQoL between the interventions. | |
| Side effects (most common, headaches) assessed at 2 mo follow-up | 52 per 1000 | 0 per 1000 (0-0) | Not estimable | 232 (1 RCT) | ⨁⨁◯◯ LOW†,‡,ǁ | There may be no important differences in side effects between the interventions. |
| Severe side effects follow-up at 2 mo | 0 per 1000 | 0 per 1000 (0-0) | Not estimable | 232 (1 RCT) | ⨁⨁◯◯ LOW†,‡,ǁ | There may be no important differences in severe side effects between the interventions. |
| Major bleeding, not reported | — | — | — | — | — | — |
| Need for surgery, not reported | — | — | — | — | — | — |
| Need for additional treatment, not reported | — | — | — | — | — | — |
| Menstruation duration, not reported | — | — | — | — | — | — |
| Absence from school, work, and other necessary activities, not reported | — | — | — | — | — | — |
| . | ||||||
|---|---|---|---|---|---|---|
| Outcome . | Anticipated absolute effects (95% CI)* . | Relative effect (95% CI) . | Participants, n (studies) . | Certainty of the evidence (GRADE) . | Comments . | |
| Risk with TxA . | Risk with DDAVP (range) . | |||||
| Change in menstrual blood loss assessed as the change from baseline on PBAC follow-up, at 2 mo | The mean change in menstrual blood loss was 105.9. | MD 41.6 higher (19.6-63.6 higher) | — | 116 (1 RCT) | ⨁⨁⨁◯ MODERATE†,‡ | DDAVP probably reduces menstrual blood loss less than TxA. |
| Quality of life assessed with several scales (HRQoL, SF-36, CES-D, RUTA); at follow-up, 2 mo | The researchers did not provide an explicit comparison between the groups. Scores across instruments and domains suggested improvement for both interventions, but this was the only category to show a statistically significant difference. | — | 116 (1 RCT) | ⨁⨁◯◯ LOW‡,§ | There may be no important differences in HRQoL between the interventions. | |
| Side effects (most common, headaches) assessed at 2 mo follow-up | 52 per 1000 | 0 per 1000 (0-0) | Not estimable | 232 (1 RCT) | ⨁⨁◯◯ LOW†,‡,ǁ | There may be no important differences in side effects between the interventions. |
| Severe side effects follow-up at 2 mo | 0 per 1000 | 0 per 1000 (0-0) | Not estimable | 232 (1 RCT) | ⨁⨁◯◯ LOW†,‡,ǁ | There may be no important differences in severe side effects between the interventions. |
| Major bleeding, not reported | — | — | — | — | — | — |
| Need for surgery, not reported | — | — | — | — | — | — |
| Need for additional treatment, not reported | — | — | — | — | — | — |
| Menstruation duration, not reported | — | — | — | — | — | — |
| Absence from school, work, and other necessary activities, not reported | — | — | — | — | — | — |
GRADE Working Group grades of evidence:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
CES-D, Center for Epidemiologic Studies Depression scale; HRQoL, health-related quality of life; MD, mean difference; RCT, randomized control trial; SF-36, short form 36-question health survey.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Allocation sequence generation and concealment unclear in the publication. However, we clarified with the researchers the procedures they used.
Patients analyzed had not responded to treatment with oral contraceptives. Patients seeking for first line treatment may be importantly different from those seeking second-line treatment.
Lack of blinding could have affected the reporting of this subjective outcome.
Few events; results are likely to be fragile.
Summary of findings of comparative effects of DDAVP vs hormonal therapy in women with VDB and HMB
| . | |||||
|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects (95% CI)* . | Relative effect (95% CI) . | Participants, n (studies) . | Certainty of the evidence (GRADE) . | |
| Risk with hormonal therapy . | Risk with DDAVP . | ||||
| Effectiveness assessed according to alleviation of symptoms; median follow-up, 30 mo | 857 per 1000 | 771 per 1000 (566-1000) | RR 0.90 (0.66-1.23) | 36 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡ |
| Menstrual flow assessed according to mean PBAC score over median follow-up of 30 mo | The mean menstrual flow was 105.1 points | MD 0.9 points higher (9.89 lower to 11.69 higher) | — | 36 (1 observational study) | ⨁◯◯◯ VERY LOW† |
| Adverse events (not serious) assessed by patient self-reports at median follow-up of 30 mo | 0 per 1000 | 0 per 1000 (0-0) | RR 5.87 (0.34-101.31) | 36 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡ |
| . | |||||
|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects (95% CI)* . | Relative effect (95% CI) . | Participants, n (studies) . | Certainty of the evidence (GRADE) . | |
| Risk with hormonal therapy . | Risk with DDAVP . | ||||
| Effectiveness assessed according to alleviation of symptoms; median follow-up, 30 mo | 857 per 1000 | 771 per 1000 (566-1000) | RR 0.90 (0.66-1.23) | 36 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡ |
| Menstrual flow assessed according to mean PBAC score over median follow-up of 30 mo | The mean menstrual flow was 105.1 points | MD 0.9 points higher (9.89 lower to 11.69 higher) | — | 36 (1 observational study) | ⨁◯◯◯ VERY LOW† |
| Adverse events (not serious) assessed by patient self-reports at median follow-up of 30 mo | 0 per 1000 | 0 per 1000 (0-0) | RR 5.87 (0.34-101.31) | 36 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡ |
In all of the studies, we were very uncertain about the evidence for the comparative effects of the 2 treatments. The following outcomes: major bleeding, the need for surgery, the need for additional treatment, menstruation duration, and absence from school, work, and other necessary activities were not reported in any of the studies.
GRADE Working Group grades of evidence:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
MD, mean difference.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Assignment to treatment was according to clinician and patient preference. No matching or control of confounding.
The CI suggests the possibility of appreciable benefit and appreciable harm. Small sample size.
Summary of findings of effects of VWF levels in women receiving neuraxial anesthesia during delivery
| . | |||
|---|---|---|---|
| Outcomes . | Impact . | Participants, n (studies) . | Certainty of the evidence (GRADE) . |
| Complications of epidural assessed as number of events/administration | The pooled proportion of complications of an epidural was 6% (5/83 deliveries). In 4 studies, the types of complications were not reported. In 1 of the studies, the complications reported were hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia. | 83 (5 observational studies) | ⨁◯◯◯ VERY LOW*,† |
| Failed procedure assessed as number of events/administration | In the study that reported this outcome, the proportion of deliveries in which it occurred was 2.4% (1/41 deliveries). | 41 (1 observational study) | ⨁◯◯◯ VERY LOW*,† |
| . | |||
|---|---|---|---|
| Outcomes . | Impact . | Participants, n (studies) . | Certainty of the evidence (GRADE) . |
| Complications of epidural assessed as number of events/administration | The pooled proportion of complications of an epidural was 6% (5/83 deliveries). In 4 studies, the types of complications were not reported. In 1 of the studies, the complications reported were hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia. | 83 (5 observational studies) | ⨁◯◯◯ VERY LOW*,† |
| Failed procedure assessed as number of events/administration | In the study that reported this outcome, the proportion of deliveries in which it occurred was 2.4% (1/41 deliveries). | 41 (1 observational study) | ⨁◯◯◯ VERY LOW*,† |
VWF levels 50-150 IU/dL compared with VWF levels >150 IU/dL in women with VWD in labor who require or desire epidural anesthesia. The following outcomes: major bleeding, adverse events in mother, spinal hematoma, mortality, thrombotic events, and transfusion were not reported in any of the studies.
GRADE Working Group grades of evidence:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
No control group.
Very few events and patients.
Comparison 1: desmopressin vs TxA.
Moderate-certainty evidence from a randomized controlled trial with 232 patients showed that desmopressin probably results in less reduction of menstrual blood loss (difference in mean change from baseline, 41.6 [95% CI, 16.6-63.6] points in pictorial blood assessment chart [PBAC] score) as compared with TxA (a score of >100 is considered HMB).33 Low-certainty evidence suggested no important differences in side effects and severe side effects (Table 1).
Comparison 2: desmopressin vs hormonal therapy.
Very-low-certainty evidence from a single observational study with 36 patients suggested that desmopressin may be less effective at alleviating symptoms (RR 0.90; 95% CI, 0.66-1.23) when compared with hormonal therapy. There was very low certainty regarding the effects on menstrual flow and adverse events (Table 2).
Intervention: LNG-IUS.
The case series reported information about the outcomes in different ways and could not be included in the meta-analysis. There was very low certainty for the comparative effectiveness of LNG-IUS and the other therapies for control of HMB, duration of menstruation, health-related quality of life, anemia, absence from necessary activities, complications, and adverse effects (supplemental Appendix 3).
SR2: VWF levels in women receiving neuraxial anesthesia during labor
After screening the full text of 27 studies, we included 5 case series.9,34-37 Most of the studies included information about carriers of hemophilia. Supplemental Appendix 4 presents the characteristics of the included studies.
The studies did not describe outcomes based on VWF levels, but rather described outcomes of women with VWF levels >0.50 IU/mL (without specifying which proportion of women had levels >1.50 IU/mL). Therefore, there was very-low-certainty evidence about the comparative effects of increasing the VWF levels to 0.50 to 1.50 IU/mL vs >1.50 IU/mL.
Meta-analysis showed that the proportion of anesthesia complications was 6% (95% CI, 0-53; very-low-certainty evidence). These complications included hypotension, accidental dural puncture, inadequate analgesia, bloody tap with no further complications, and failed block requiring general anesthesia. Based on the only study that reported failed anesthetic procedures as an outcome, the proportion of deliveries with failed anesthetic procedures was 2.4% (Table 3).
SR3: TxA during postpartum period
After screening the full text of 41 studies, we included 2 of them.38,39 Both studies included a majority of women who had a diagnosis of VWD and had a retrospective cohort design. Supplemental Appendix 5 presents the main characteristics of the included studies.
Very-low-certainty evidence suggested that TxA reduces the risk of severe primary postpartum hemorrhage (RR, 0.36; 95% CI, 0.05-2.59), primary postpartum hemorrhage (RR, 0.25; 95% CI, 0.04-1.75), and secondary postpartum hemorrhage (RR, 0.42; 95% CI, 0.20-0.91). There was also very-low-certainty evidence of the effects of TxA on blood transfusions, vaginal hematoma, blood loss, and thrombotic complications (Table 4).
Summary of finding of effects of TxA in women with VWD in the postpartum period
| . | |||||
|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects (95% CI)* . | Relative effect (95% CI) . | Participants, n (studies) . | Certainty of the evidence (GRADE) . | |
| Risk with no TxA . | Risk with TxA . | ||||
| Severe primary postpartum hemorrhage assessed according to number of events or deliveries | 313 per 1000 | 112 per 1000 (16-809) | RR 0.36 (0.05 to 2.59) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡,§ |
| Primary postpartum hemorrhage assessed according to number of events/deliveries | 438 per 1000 | 109 per 1000 (18-766) | RR 0.25 (0.04-1.75) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡,§ |
| Secondary postpartum hemorrhage assessed according to number of events/deliveries | 381 per 1000 | 160 per 1000 (76-347) | RR 0.42 (0.20-0.91) | 87 (2 observational studies) | ⨁◯◯◯ VERY LOW†,‡ |
| Blood transfusion assessed according to number of events/deliveries | 188 per 1000 | 45 per 1000 (2-793) | RR 0.24 (0.01-4.23) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,§ |
| Vaginal hematoma assessed according to number of events/deliveries | 125 per 1000 | 43 per 1000 (3-799) | RR 0.34 (0.02-6.39) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,§ |
| Adverse events in mother- Thrombotic complications assessed according to number of events/deliveries | — | — | — | 36 (1 observational study) | ⨁◯◯◯ VERY LOW†,ǁ |
| Blood loss assessed as median per group | The median (range) blood loss after deliveries in people who received TxA was 400 (270-1470) mL, and it was 425 (200-6000) in people who did not receive TxA. | — | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡,¶ | |
| . | |||||
|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects (95% CI)* . | Relative effect (95% CI) . | Participants, n (studies) . | Certainty of the evidence (GRADE) . | |
| Risk with no TxA . | Risk with TxA . | ||||
| Severe primary postpartum hemorrhage assessed according to number of events or deliveries | 313 per 1000 | 112 per 1000 (16-809) | RR 0.36 (0.05 to 2.59) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡,§ |
| Primary postpartum hemorrhage assessed according to number of events/deliveries | 438 per 1000 | 109 per 1000 (18-766) | RR 0.25 (0.04-1.75) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡,§ |
| Secondary postpartum hemorrhage assessed according to number of events/deliveries | 381 per 1000 | 160 per 1000 (76-347) | RR 0.42 (0.20-0.91) | 87 (2 observational studies) | ⨁◯◯◯ VERY LOW†,‡ |
| Blood transfusion assessed according to number of events/deliveries | 188 per 1000 | 45 per 1000 (2-793) | RR 0.24 (0.01-4.23) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,§ |
| Vaginal hematoma assessed according to number of events/deliveries | 125 per 1000 | 43 per 1000 (3-799) | RR 0.34 (0.02-6.39) | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,§ |
| Adverse events in mother- Thrombotic complications assessed according to number of events/deliveries | — | — | — | 36 (1 observational study) | ⨁◯◯◯ VERY LOW†,ǁ |
| Blood loss assessed as median per group | The median (range) blood loss after deliveries in people who received TxA was 400 (270-1470) mL, and it was 425 (200-6000) in people who did not receive TxA. | — | 25 (1 observational study) | ⨁◯◯◯ VERY LOW†,‡,¶ | |
In all of the studies, we were very uncertain about the evidence of the effects of postpartum administration of TxA in patients with VWD. The following outcomes: major bleeding, need for other medical procedures, and mortality were not reported in any of the studies.
GRADE Working Group grades of evidence:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
No adjustment for any potential confounder.
The panel raised applicability concerns regarding the method of outcome measurement.
Very small number of patients and events. The CI suggests appreciable benefit on one extreme and appreciable harm on the other.
No events for this outcome.
Very small number of patients.
Discussion
We conducted 3 SRs to inform the recommendations on the obstetric and gynecologic management of women with VWD for the ASH, ISTH, NHF, and WFH. The questions were prioritized by the guideline panel based on areas of significant need in the field of VWD, along with input from an international survey of clinicians, patients, and caregivers.18 As part of this project, other SRs were developed to cover the perioperative management40 and VWF prophylaxis in patients with VWD (manuscript submitted).41 In this SR, we found mostly very-low-certainty evidence from comparative observational studies and case series. Further data are needed to determine the optimal hierarchy of treatment of women with VWD and HMB. The evidence suggests that (1) there is very low certainty about the efficacy of different treatment options in women with VWD. Because hormonal therapy is effective in controlling HMB (based on data from women without bleeding disorders),42,43 we believe the most effective strategy to be hormonal therapy with a LNG-IUS or combined oral contraceptives, followed by TxA, and desmopressin; (2) there is high uncertainty about how VWF levels of 0.50 to 1.50 IU/mL compare with VWF levels >1.50 IU/mL in women with VWD who receive epidural anesthesia during labor; and (3) TxA may have benefits in women with VWD in the postpartum period, but there is much uncertainty in this evidence.
Overall, the certainty of the evidence for all the SRs was very low. There was a single randomized clinical trial that enrolled 116 participants addressing one of the comparisons of interest in SR1. The certainty of the evidence that this randomized control trial provided was moderate to low across outcomes because of indirectness (the women enrolled in the trial were seeking second-line therapy) and imprecision. For the other comparisons and SRs, the certainty of the evidence was very low because of the risk of bias (the comparative observational studies did not account for potential confounders, and there was no comparison group in the case series) and imprecision.
There were also important gaps in the literature. Several outcomes deemed critical for decision making by the guideline panel lacked evidence, including major bleeding and need for surgery or additional treatments in SR1; mortality, major bleeding, spinal hematoma, transfusion, and thrombotic events in SR2; and mortality, major bleeding, and need for other medical procedures in SR3.
These SRs have several strengths. First, we conducted them according to rigorous methodological standards.44 Second, we used broad eligibility criteria to include any type of evidence that could be informative. For example, we included evidence from women with bleeding disorders other than VWD, as well as study designs that are not considered optimal for making inferences about treatment effects (such as case series) when these were the only evidence available. Third, we conducted an explicit and transparent assessment of the certainty of the evidence using the GRADE approach and used this assessment to make conclusions that considered not only the effect estimates, but also the certainty of the evidence.
Our SRs have limitations common to all SRs. Finding the relevant studies depends on the quality of the indexing process of the searched databases, and some of the studies included in the SRs were not indexed under the terms describing women with bleeding disorders used in our initial search strategy. Similarly, including studies depends on the clear reporting in title and abstracts, which was not the case for many of these studies. We overcame these limitations by maintaining close collaboration with the guideline panel members who were familiar with the literature and were able to point out studies that may be relevant but had not been originally found because of the limitations described herein. This way, we were certain to include all relevant evidence.
Interpretation and decision making in the context of scarce evidence requires expertise. This SR presents conclusions based only on the evidence and its certainty. When formulating recommendations, however, the guideline panel interpreted the evidence adding their experience and knowledge of indirect evidence. For example, even though there was only very-low-certainty evidence about the effects of TxA in women with VWD in the postpartum period, knowledge of the large body of evidence that shows that TxA reduces the risk of postpartum hemorrhage in the general population led the guideline panel to believe that these benefits in patients with type 1 VWD may be true. The guidelines on the management of VWD19 provide a detailed discussion of the effects of the interventions that includes considerations of contextual factors and indirect evidence that is not presented in this article.
Future research should focus on areas of uncertainty revealed by these SRs. Even in those topics for which there is evidence, the evidence is scarce and insufficient to inform decision-making confidently. Because VWD is a rare disease, it is difficult to conduct large multicenter randomized clinical trials that include a sufficient number of participants to address the questions of interest. Well-designed comparative observational studies in which researchers account for confounding factors may be more feasible and would be more informative than what is available to date. The guideline panel outlined research priorities including studies evaluating combination vs monotherapy for treating women with HMB, the role of platelet-derived VWF in hemostasis during labor and delivery, and the effects of TxA on postpartum hemorrhage in women with VWD.
In summary, there is very-low-certainty evidence to inform decisions about the obstetric and gynecologic management of women with VWD. This evidence, however, is the best available to inform decisions about management. Clinicians seeking advice on how to manage their patients with VWD should refer to the practice guidelines19 and assess to what extent they are applicable to their patients.
Acknowledgments
The authors thank Alice Arapshian, Jean M. Grow, Michael Laffan, Frank W. G. Leebeek, Sarah H. O’Brien, and Alberto Tosetto for invaluable assistance and the ASH, ISTH, NHF, and WFH for their support of the guideline process, with specific thanks to Jenny Castano, Cary Clark, Rob Kunkle, Ellen Riker, Fiona Robinson, and Mark Skinner.
This systematic review was conducted to support the development of the ASH, ISTH, NHF, and the WFH 2020 Guidelines for Management of VWD. The entire guideline development process was funded by the 4 collaborating organizations: ASH, ISTH, NHF, and WFH. Through the Outcomes and Implementation Research Unit at the University of Kansas Medical Center and the McMaster GRADE center, A.E.A., N.H., R.B.-P., and R.A.M. received salary or grant support; others participated to fulfill requirements of an academic degree or program or volunteered their time.
Authorship
Contribution: R.A.M., R.B.-P., N.H., and M.A.K. contributed to the review design, study selection, data extraction, statistical analysis, and interpretation of results; A.E.A., S.S., Y.A., A.B., H.A., H.E.-K., and S.M. contributed to study selection and data extraction; N.H., A.E.A., R.B.-P. and R.A.M. contributed to drafting the report; and J.R., R.A.-K., S.C., P.K., M.L., M.C.O., A.W., V.H.F., N.T.C., and P.D.J., contributed to the interpretation of results, and critical revision of the report.
Conflict-of-interest disclosure: All authors were members of the guideline panel or members of the systematic review team or both. As such, they completed a disclosure-of-interest form, which was reviewed by ASH and is available in supplemental Appendix Files 6 and 7.
Correspondence: Reem A. Mustafa, University of Kansas Medical Center, 4330 Shawnee Mission Parkway, Kansas City, KS 66205; e-mail: rmustafa@kumc.edu.
References
Author notes
For original data, please contact the corresponding author (rmustafa@kumc.edu).
The full-text version of this article contains a data supplement.