Key Points
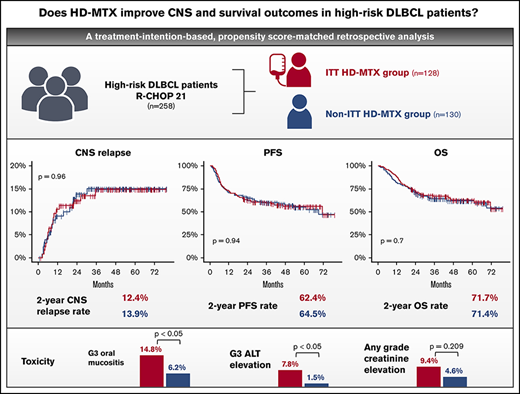
In high-risk DLBCL patients, prophylactic HD-MTX did not improve CNS or survival outcomes but was associated with increased toxicities.
Abstract
Despite central nervous system (CNS) relapse occurring in >10% of high-risk diffuse large B-cell lymphoma (DLBCL) patients, the role of CNS-directed prophylaxis is controversial in the absence of randomized controlled trials. In this retrospective study, we aimed to evaluate the safety and efficacy of prophylactic high-dose methotrexate (HD-MTX) on CNS relapse and survival outcomes in 258 newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated high-risk DLBCL patients, based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX. Patients were classified into an ITT HD-MTX group (n = 128) and a non-ITT HD-MTX group (n = 130). The CNS relapse rate was not significantly different between these groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = 0.96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs 64.5% (P = 0.94) and 71.7% vs 71.4% (P = 0.7), respectively. Also, propensity score–matched analyses showed no significant differences in the time-to-CNS-relapse, progression-free survival, or overall survival. The ITT HD-MTX group showed a higher incidence of grade ≥ 3 oral mucositis and elevated alanine aminotransferase. Prophylactic HD-MTX does not improve CNS relapse rate or survival outcomes in high-risk DLBCL patients, and it is accompanied by increased toxicities.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma, accounting for approximately one third of all newly diagnosed cases.1 The addition of rituximab, an anti-CD20 monoclonal antibody, to cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improved survival outcomes in DLBCL patients, with a complete response rate of 60% to 76% and a 3-year progression-free survival (PFS) rate of 67% to 73%.2-4 Despite improvements in treatment outcomes, a subpopulation of patients experiences central nervous system (CNS) relapse, which is associated with a grave prognosis, with a median survival of only 2 to 5 months from the diagnosis of CNS relapse.5
The incidence of CNS relapse varies according to clinical characteristics. The CNS International Prognostic Index (IPI) is a widely accepted prognostic model that predicts CNS relapse in DLBCL patients based on the variables included in the IPI (age, performance, serum lactate dehydrogenase [LDH] level, stage, and the number of extranodal sites involved) and kidney or adrenal involvement.6 According to the CNS IPI, the 2-year CNS relapse rate varies from <1% in the low-risk group to 10% to 12% in the high-risk group. Several studies have also reported that the involvement of specific extranodal sites, such as the testis or the breast, is associated with a high rate of CNS relapse.7-13 In addition, specific biological features, such as double-hit lymphoma or double-expressor lymphoma, have been identified as high-risk factors for CNS relapse.14-16
To reduce the incidence of CNS relapse in high-risk patients, prophylactic IV high-dose methotrexate (HD-MTX) has been considered an alternative to intrathecal methotrexate, because recent data have shown that intrathecal prophylaxis provides insufficient benefit.17,18 Also, several studies have reported that the addition of CNS-directed treatment to the standard regimen may improve survival outcomes.19,20 However, no randomized controlled trial has been specifically conducted to evaluate whether prophylactic HD-MTX treatment can reduce the risk of CNS relapse and improve survival outcomes. Current data supporting the role of HD-MTX are mostly from retrospective studies20-23 or small-sized prospective studies, which lack a control arm,24,25 limiting the level of evidence.
This study aimed to evaluate the protective effect of IV HD-MTX on CNS relapse and on survival outcomes in newly diagnosed DLBCL patients at a high risk for CNS relapse who were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
Methods
Patients
Patients with newly diagnosed DLBCL at a high risk for CNS relapse who were treated with first-line R-CHOP chemotherapy between August 2013 and July 2018 at Asan Medical Center were retrospectively identified. A high risk for CNS relapse was defined as follows: (1) CNS IPI score ≥4 or involvement of the kidney or the adrenal gland,6 (2) involvement of >1 extranodal site and elevated LDH level,17 (3) involvement of the testis, the breast, the epidural space, or the paranasal sinus,7-11,26,27 (4) HIV+ lymphoma,28 (5) double-expressor lymphoma with coexpression of MYC and BCL2, identified on immunohistochemical analysis and IPI score ≥2,16 or (6) double-hit or triple-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements, identified on fluorescence in situ hybridization.14,15 To minimize selection bias, patients who could not tolerate HD-MTX, those aged ≥80 years at diagnosis, or those with baseline serum creatinine levels ≥1.5 mg/dL were excluded. We also carefully reviewed each patient’s medical record and identified the physician’s initial intent regarding prophylactic HD-MTX treatment. Patients with an unidentifiable initial intent of prophylactic HD-MTX and those who were lost to follow-up during chemotherapy were excluded. This study was approved by the institutional review board of Asan Medical Center and performed following the ethical standards of the institutional research committee and the Declaration of Helsinki. The institutional review board granted a waiver of informed consent for this retrospective study. A subset of patients in the current study was included in a previous study.23 Nevertheless, the current study differs from the previous study in terms of inclusion criteria and methodology.
Treatment and response assessment
Patients with an initial treatment intent (ITT) with prophylactic HD-MTX were assigned to the ITT HD-MTX group. In contrast, those without an ITT with prophylactic HD-MTX were classified as the non-ITT HD-MTX group. Patients were treated with 6 to 8 cycles of R-CHOP every 3 weeks. HD-MTX as CNS prophylaxis was administered as 3 to 3.5 mg/m2 IV infusion for 2 or 3 cycles on day 15 of every other R-CHOP cycle (intercalating schedule) or after completing the preplanned 6 to 8 cycles of R-CHOP (end-of-treatment schedule). HD-MTX prophylaxis at the end of treatment was only given to patients who achieved a complete metabolic response after completing the preplanned 6 to 8 cycles of R-CHOP. Response to treatment was assessed according to the 2014 Lugano classification.29 Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
Time to CNS relapse (TT-CNS) was defined as the time from the start date of chemotherapy to the date of CNS relapse. PFS was defined as the time from the start date of chemotherapy to the date of disease progression or death from any cause, whichever occurred first. Overall survival (OS) was defined as the time from the start date of chemotherapy to the date of death from any cause. Survival rates and corresponding standard errors were estimated using the Kaplan-Meier method, and the survival curves were compared between groups using the log-rank test. Univariate and multivariate analyses for TT-CNS, PFS, and OS were performed using the Cox proportional hazards regression method. We used a propensity score, estimated using multiple logistic regression on the patients’ baseline characteristics, to minimize bias. For outcome analysis, one-to-one nearest neighbor matching with caliper widths of 0.2 and an inverse probability of treatment weighting (IPTW) technique were used. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
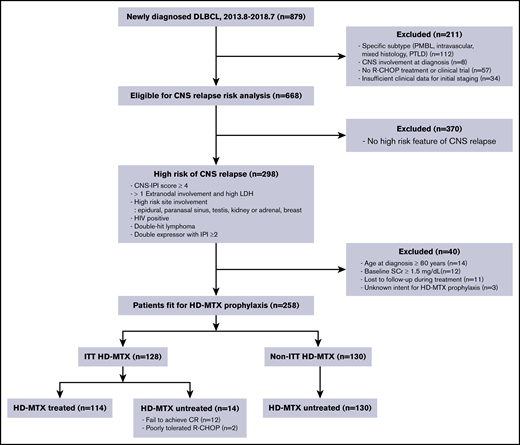
We identified 879 patients with newly diagnosed DLBCL during the study period. Of them, 298 were classified as patients at a “high risk for CNS relapse” according to our predefined criteria (Figure 1). After excluding patients who were ineligible for HD-MTX treatment because of old age (n = 14), increased creatinine at baseline (n = 12), loss to follow-up (n = 11), or unidentifiable initial intent for HD-MTX prophylaxis (n = 3), 258 patients were included in the analysis. One hundred and twenty-eight patients were assigned to the ITT HD-MTX group, and 130 patients were included in the non-ITT HD-MTX group. In the ITT HD-MTX group, 14 of 128 patients did not receive HD-MTX prophylaxis because of failure to achieve complete remission (n = 12) or intolerance of R-CHOP chemotherapy (n = 2). None of the patients in the non-ITT HD-MTX group received HD-MTX prophylaxis. The baseline characteristics of the patients are presented in Table 1. IPI and CNS IPI scores were similar between groups; however, compared with patients in the non-ITT HD-MTX group, a higher proportion of patients in the ITT HD-MTX group were male, had involvement of >1 extranodal site, had stage ≥3 disease, and had testicular involvement. Among the 114 patients who received HD-MTX treatment, the median cumulative dose was 7 g/m2 (range, 1.5-17.5). With regard to the treatment schedule, 69 patients received HD-MTX intercalated with R-CHOP, and 45 patients received HD-MTX after completion of preplanned R-CHOP chemotherapy.
Consort flow diagram. CR, complete remission; PMBL, primary mediastinal B-cell lymphoma; PTLD, posttransplant lymphoproliferative disorder; SCr, serum creatinine; 2013.8-2018.7, August 2013 to July 2018.
Consort flow diagram. CR, complete remission; PMBL, primary mediastinal B-cell lymphoma; PTLD, posttransplant lymphoproliferative disorder; SCr, serum creatinine; 2013.8-2018.7, August 2013 to July 2018.
Baseline patient characteristics
| Characteristics . | Overall population (N = 258) . | ITT HD-MTX (n = 128) . | Non-ITT HD-MTX (n = 130) . | P . |
|---|---|---|---|---|
| Age, median (range), y | 62 (25-79) | 61 (25-79) | 65 (29-79) | .059 |
| Sex | ||||
| Male | 146 (56.6) | 83 (64.8) | 63 (48.5) | .011 |
| Female | 112 (43.4) | 45 (35.2) | 67 (51.5) | |
| IPI risk | ||||
| Low (0-1) | 27 (10.4) | 12 (9.4) | 15 (11.5) | .395 |
| Low-intermediate (2) | 41 (15.9) | 16 (12.5) | 25 (19.2) | |
| High-intermediate (3) | 90 (34.9) | 46 (35.9) | 44 (33.9) | |
| High (4-5) | 100 (38.8) | 54 (42.2) | 46 (35.4) | |
| IPI risk factors | ||||
| Age >60 y | 150 (58.1) | 69 (53.9) | 81 (62.3) | .214 |
| ECOG PS >1 | 34 (13.2) | 17 (13.3) | 17 (13.1) | 1 |
| LDH > ULN | 196 (76.0) | 98 (76.6) | 98 (75.4) | .94 |
| EN >1 | 189 (73.3) | 107 (83.6) | 82 (63.1) | <.001 |
| Stage ≥3 | 214 (82.9) | 113 (88.3) | 101 (77.7) | .036 |
| CNS IPI | ||||
| Low (0-1) | 25 (9.7) | 12 (9.4) | 13 (10.0) | .525 |
| Intermediate (2-3) | 107 (41.5) | 49 (38.3) | 58 (44.6) | |
| High (4-6) or kidney/adrenal involvement | 126 (48.8) | 67 (52.3) | 59 (45.4) | |
| EN >1 and high LDH | 155 (60.1) | 88 (68.8) | 67 (51.5) | .007 |
| High-risk site involvement | ||||
| Yes | 99 (38.4) | 57 (44.5) | 42 (32.3) | .059 |
| Kidney/adrenal | 38 (14.7) | 21 (16.4) | 17 (13.1) | .563 |
| Testicular | 18 (7.0) | 18 (14.1) | 0 (0.0) | <.001 |
| Breast | 23 (8.9) | 10 (7.8) | 13 (10.0) | .691 |
| Paranasal | 18 (7.0) | 6 (4.7) | 12 (9.2) | .235 |
| Epidural | 10 (3.9) | 8 (6.2) | 2 (1.5) | .101 |
| HIV | ||||
| Negative | 254 (98.4) | 126 (98.4) | 128 (98.4) | .513 |
| Positive | 3 (1.2) | 2 (1.6) | 1 (0.8) | |
| Unknown | 1 (0.4) | 0 (0.0) | 1 (0.8) | |
| Cell of origin | ||||
| GCB | 80 (31.0) | 36 (28.1) | 44 (33.8) | .545 |
| Non-GCB | 166 (64.3) | 85 (66.4) | 81 (62.4) | |
| Unknown | 12 (4.7) | 7 (5.5) | 5 (3.8) | |
| Double expressor | ||||
| No | 123 (47.6) | 63 (49.2) | 60 (46.1) | .845 |
| Yes | 115 (44.6) | 56 (43.8) | 59 (45.4) | |
| With IPI ≥2 | 107 (93.0) | 54 (96.4) | 53 (89.8) | |
| Unknown | 20 (7.8) | 9 (7.0) | 11 (8.5) | |
| Double hit | ||||
| No | 5 (1.9) | 2 (1.6) | 3 (2.3) | .813 |
| Yes | 43 (16.7) | 20 (15.6) | 23 (17.7) | |
| Unknown | 210 (81.4) | 106 (82.8) | 104 (80.0) |
| Characteristics . | Overall population (N = 258) . | ITT HD-MTX (n = 128) . | Non-ITT HD-MTX (n = 130) . | P . |
|---|---|---|---|---|
| Age, median (range), y | 62 (25-79) | 61 (25-79) | 65 (29-79) | .059 |
| Sex | ||||
| Male | 146 (56.6) | 83 (64.8) | 63 (48.5) | .011 |
| Female | 112 (43.4) | 45 (35.2) | 67 (51.5) | |
| IPI risk | ||||
| Low (0-1) | 27 (10.4) | 12 (9.4) | 15 (11.5) | .395 |
| Low-intermediate (2) | 41 (15.9) | 16 (12.5) | 25 (19.2) | |
| High-intermediate (3) | 90 (34.9) | 46 (35.9) | 44 (33.9) | |
| High (4-5) | 100 (38.8) | 54 (42.2) | 46 (35.4) | |
| IPI risk factors | ||||
| Age >60 y | 150 (58.1) | 69 (53.9) | 81 (62.3) | .214 |
| ECOG PS >1 | 34 (13.2) | 17 (13.3) | 17 (13.1) | 1 |
| LDH > ULN | 196 (76.0) | 98 (76.6) | 98 (75.4) | .94 |
| EN >1 | 189 (73.3) | 107 (83.6) | 82 (63.1) | <.001 |
| Stage ≥3 | 214 (82.9) | 113 (88.3) | 101 (77.7) | .036 |
| CNS IPI | ||||
| Low (0-1) | 25 (9.7) | 12 (9.4) | 13 (10.0) | .525 |
| Intermediate (2-3) | 107 (41.5) | 49 (38.3) | 58 (44.6) | |
| High (4-6) or kidney/adrenal involvement | 126 (48.8) | 67 (52.3) | 59 (45.4) | |
| EN >1 and high LDH | 155 (60.1) | 88 (68.8) | 67 (51.5) | .007 |
| High-risk site involvement | ||||
| Yes | 99 (38.4) | 57 (44.5) | 42 (32.3) | .059 |
| Kidney/adrenal | 38 (14.7) | 21 (16.4) | 17 (13.1) | .563 |
| Testicular | 18 (7.0) | 18 (14.1) | 0 (0.0) | <.001 |
| Breast | 23 (8.9) | 10 (7.8) | 13 (10.0) | .691 |
| Paranasal | 18 (7.0) | 6 (4.7) | 12 (9.2) | .235 |
| Epidural | 10 (3.9) | 8 (6.2) | 2 (1.5) | .101 |
| HIV | ||||
| Negative | 254 (98.4) | 126 (98.4) | 128 (98.4) | .513 |
| Positive | 3 (1.2) | 2 (1.6) | 1 (0.8) | |
| Unknown | 1 (0.4) | 0 (0.0) | 1 (0.8) | |
| Cell of origin | ||||
| GCB | 80 (31.0) | 36 (28.1) | 44 (33.8) | .545 |
| Non-GCB | 166 (64.3) | 85 (66.4) | 81 (62.4) | |
| Unknown | 12 (4.7) | 7 (5.5) | 5 (3.8) | |
| Double expressor | ||||
| No | 123 (47.6) | 63 (49.2) | 60 (46.1) | .845 |
| Yes | 115 (44.6) | 56 (43.8) | 59 (45.4) | |
| With IPI ≥2 | 107 (93.0) | 54 (96.4) | 53 (89.8) | |
| Unknown | 20 (7.8) | 9 (7.0) | 11 (8.5) | |
| Double hit | ||||
| No | 5 (1.9) | 2 (1.6) | 3 (2.3) | .813 |
| Yes | 43 (16.7) | 20 (15.6) | 23 (17.7) | |
| Unknown | 210 (81.4) | 106 (82.8) | 104 (80.0) |
Data are n (%), unless otherwise noted. ECOG PS, Eastern Cooperative Oncology Group performance status; EN, extranodal sites involved; GCB, germinal center B-cell like; ULN, upper limit of normal.
CNS relapse
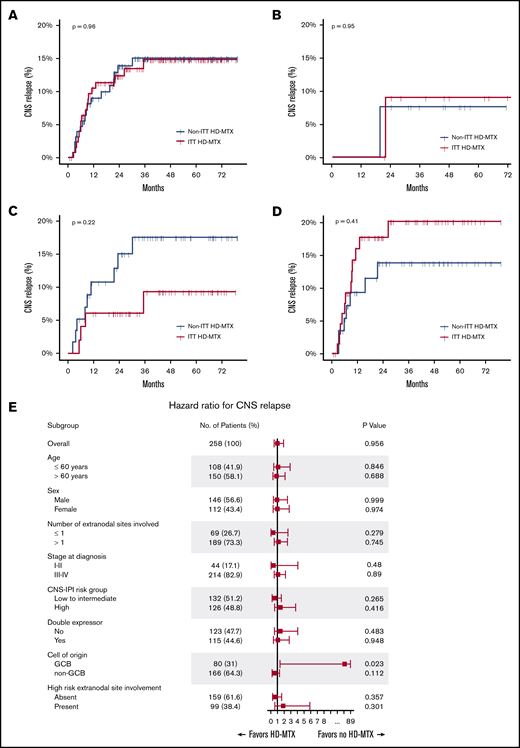
During the median follow-up of 50.2 months (95% confidence interval [CI], 45.6-53.1), CNS relapse occurred in 34 (13.2%) patients (17 in each group). Among the 17 patients with CNS relapse in the ITT HD-MTX group, 3 experienced CNS relapse before administration of prophylactic HD-MTX. In the entire study cohort, the 6-month, 1-year, and 2-year cumulative incidence of CNS relapse was 4.8% (95% CI, 2.7-8.2), 9.8% (95% CI, 6.7-14.3), and 13.1% (95% CI, 9.4-18.2), respectively. The median TT-CNS in patients with CNS relapse was 8.4 months (95% CI, 5.7-10.7). There was no significant difference in the CNS relapse rates between the ITT HD-MTX and non-ITT HD-MTX groups, with a 2-year cumulative incidence of CNS relapse of 12.4% (95% CI, 7.6-19.7) and 13.9% (95% CI, 8.7-21.7), respectively (P = .96; Figure 2A). No significant difference in the CNS relapse rates was observed among the CNS IPI low-risk (0-1 risk factors), intermediate-risk (2-3 risk factors), and high-risk (4-6 risk factors) groups (Figure 2B-D). Additionally, in 244 patients whose ITT and actual treatment with HD-MTX prophylaxis were concordant (patients who were treated with prophylactic HD-MTX in the ITT HD-MTX group, n = 114; patients who were not treated in the non-ITT HD-MTX group, n = 130), there was no statistically significant difference in the CNS relapse rate between the 2 groups (P = .67) (supplemental Figure 1). In a subgroup analysis in which the patients were stratified according to age, sex, the CNS-IPI risk groups, double-expressor status, cell of origin, and presence of high-risk extranodal sites involvement, a benefit of HD-MTX treatment was not observed (Figure 2E).
Cumulative incidence of CNS relapse. In the overall population (A), CNS IPI low-risk (B), CNS IPI intermediate-risk (C), and CNS IPI high-risk (D) groups. (E) Forest plots of subgroup analyses.
Cumulative incidence of CNS relapse. In the overall population (A), CNS IPI low-risk (B), CNS IPI intermediate-risk (C), and CNS IPI high-risk (D) groups. (E) Forest plots of subgroup analyses.
Survival outcomes
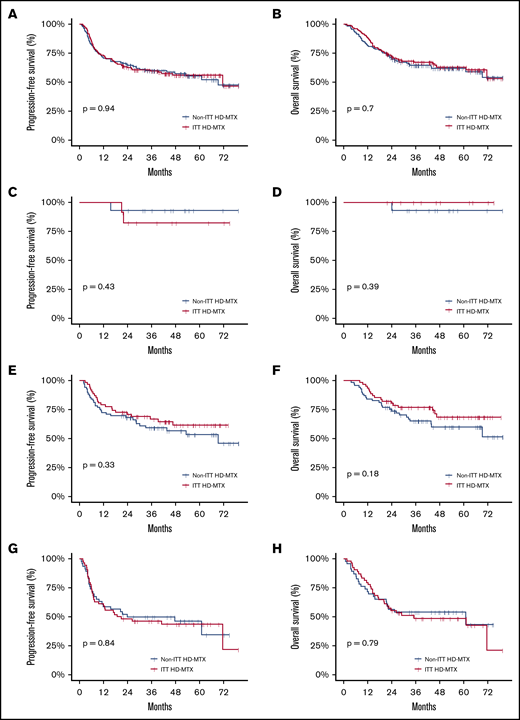
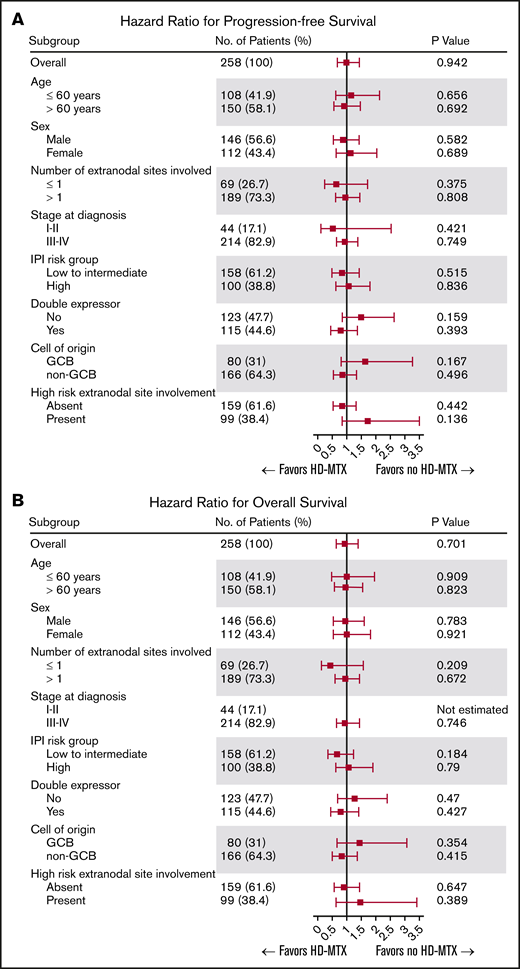
In the overall patient population, the 2-year and 5-year PFS rates were 63.5% (95% CI, 57.2-69.0) and 55.6% (95% CI, 48.8-61.9) and the 2-year and 5-year OS rates were 71.5% (95% CI, 65.6-76.7) and 62.4% (95% CI, 55.8-68.3), respectively. No statistically significant difference in the OS or PFS was noted between the ITT HD-MTX and non-ITT HD-MTX groups (Figure 3A-B). The 2-year PFS and OS rates in the ITT HD-MTX group were 62.4% (95% CI, 53.4-70.2) and 71.7% (95% CI, 62.9-78.7), and those in the non-ITT HD-MTX group were 64.5% (95% CI, 55.6-72.1) and 71.4% (95% CI, 62.8-78.4), respectively (P = .94 for PFS; P = .70 for OS). No statistically significant difference was observed between the groups in terms of PFS and OS in each of the IPI risk groups (Figure 3C-H). In the subgroup analyses of PFS and OS, no benefit of HD-MTX was observed (Figure 4).
Survival outcomes of the study population. PFS (A) and OS (B) in the overall patient population by physicians’ ITT of prophylactic HD-MTX. PFS (C) and OS (D) in the IPI low-risk group. PFS (E) and OS (F) in the IPI intermediate-risk group. PFS (G) and OS (H) in the IPI high-risk group.
Survival outcomes of the study population. PFS (A) and OS (B) in the overall patient population by physicians’ ITT of prophylactic HD-MTX. PFS (C) and OS (D) in the IPI low-risk group. PFS (E) and OS (F) in the IPI intermediate-risk group. PFS (G) and OS (H) in the IPI high-risk group.
ITT HD-MTX did not remain an independent prognostic factor for TT-CNS, PFS, or OS in the multivariate analyses adjusted for each IPI risk factor (age >60 years, Eastern Cooperative Oncology Group Performance Status ≥2, elevated serum LDH level, involvement of >1 extranodal site, stage III-IV disease), the involvement of high-risk sites for CNS relapse (kidney/adrenal gland, testis, breast, epidural space, paranasal sinus), or double-expressor lymphoma (Table 2).
Multivariate analysis and propensity score matching based on ITT HD-MTX for TT-CNS, PFS, and OS
| . | Multivariate analysis . | PS matching . | IPTW . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| TT-CNS | 0.72 (0.34-1.54) | .400 | 1 (0.42-2.40) | 1.000 | 0.82 (0.50-1.33) | .418 |
| PFS | 0.93 (0.62-1.40) | .733 | 0.91 (0.57-1.48) | .714 | 0.97 (0.74-1.26) | .801 |
| OS | 0.85 (0.55-1.32) | .473 | 0.94 (0.56-1.55) | .796 | 0.91 (0.68-1.21) | .506 |
| . | Multivariate analysis . | PS matching . | IPTW . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| TT-CNS | 0.72 (0.34-1.54) | .400 | 1 (0.42-2.40) | 1.000 | 0.82 (0.50-1.33) | .418 |
| PFS | 0.93 (0.62-1.40) | .733 | 0.91 (0.57-1.48) | .714 | 0.97 (0.74-1.26) | .801 |
| OS | 0.85 (0.55-1.32) | .473 | 0.94 (0.56-1.55) | .796 | 0.91 (0.68-1.21) | .506 |
Variables used in the multivariate analysis include age, sex, Eastern Cooperative Oncology Group Performance Status, serum LDH level, number of involved extranodal sites, stage at diagnosis, high-risk extranodal site involvement, and double-expressor status.
HR, hazard ratio; PS, propensity score.
Propensity score–matched analysis
A total of 101 patients in each group were matched in the propensity score–matched analysis. Baseline characteristics were well balanced between the ITT HD-MTX and non-ITT HD-MTX groups in the matched population (supplemental Table 1), but no significant difference in TT-CNS, PFS, or OS was found between groupsTable 2. Similar results were observed in the IPTW analysis, with no significant difference between groups with regard to TT-CNS, PFS, or OS (Table 2).
Toxicity
Adverse events are shown in Table 3. The incidence of grade ≥3 hematologic toxicity was similar between groups; however, febrile neutropenia tended to occur more frequently in the ITT HD-MTX group than in the non-ITT HD-MTX group (17.7% vs 23.4%; P = .323). The frequencies of grade ≥ 3 oral mucositis and elevated alanine aminotransferase (ALT) levels were significantly higher in the ITT HD-MTX group than in the non-ITT HD-MTX group (14.8% vs 6.2%, P = .038 and 7.8% vs 1.5%, P = .036; respectively). The incidence of increased serum creatinine levels of any grade was higher in the ITT HD-MTX group than in the non-ITT HD-MTX group (9.4% vs 4.6%, P = .209 for adverse events of any grade). A delay or a dose reduction in R-CHOP occurred more frequently in the ITT HD-MTX group than in the non-ITT HD-MTX group (31.2% vs 16.9%), and discontinuation of R-CHOP occurred more frequently in the non-ITT HD-MTX group than in the ITT HD-MTX group (10.0% vs 3.9%).
Adverse events
| Event . | Non-ITT HD-MTX (N = 130) . | ITT HD-MTX (N = 128) . | P* . | ||
|---|---|---|---|---|---|
| Any grade . | Grade 3 or 4 . | Any grade . | Grade 3 or 4 . | ||
| Hematologic toxicity | |||||
| Leukopenia | 85 (65.4) | 63 (48.5) | 89 (69.5) | 71 (55.5) | .316 |
| Neutropenia | 89 (68.5) | 72 (55.4) | 99 (77.3) | 79 (61.7) | .365 |
| Anemia | 129 (99.2) | 29 (22.3) | 127 (99.2) | 30 (23.4) | .946 |
| Thrombocytopenia | 66 (50.8) | 23 (17.7) | 79 (61.7) | 28 (21.9) | .492 |
| Febrile neutropenia | 23 (17.7) | 30 (23.4) | .323 | ||
| Nonhematologic toxicity | |||||
| Creatinine elevation | 6 (4.6) | 0 (0.0) | 12 (9.4) | 0 (0.0) | .209† |
| AST elevation | 44 (33.8) | 2 (1.5) | 79 (61.7) | 8 (6.2) | .101 |
| ALT elevation | 48 (36.9) | 2 (1.5) | 87 (68.0) | 10 (7.8) | .036 |
| Bilirubin elevation | 5 (3.8) | 1 (0.8) | 11 (8.6) | 3 (2.3) | .603 |
| Oral mucositis | 43 (33.1) | 8 (6.2) | 69 (53.9) | 19 (14.8) | .038 |
| Interruption of R-CHOP‡ | |||||
| Delay or dose reduction | 22 (16.9) | 40 (31.2) | |||
| Discontinuation | 13 (10.0) | 5 (3.9) | |||
| Event . | Non-ITT HD-MTX (N = 130) . | ITT HD-MTX (N = 128) . | P* . | ||
|---|---|---|---|---|---|
| Any grade . | Grade 3 or 4 . | Any grade . | Grade 3 or 4 . | ||
| Hematologic toxicity | |||||
| Leukopenia | 85 (65.4) | 63 (48.5) | 89 (69.5) | 71 (55.5) | .316 |
| Neutropenia | 89 (68.5) | 72 (55.4) | 99 (77.3) | 79 (61.7) | .365 |
| Anemia | 129 (99.2) | 29 (22.3) | 127 (99.2) | 30 (23.4) | .946 |
| Thrombocytopenia | 66 (50.8) | 23 (17.7) | 79 (61.7) | 28 (21.9) | .492 |
| Febrile neutropenia | 23 (17.7) | 30 (23.4) | .323 | ||
| Nonhematologic toxicity | |||||
| Creatinine elevation | 6 (4.6) | 0 (0.0) | 12 (9.4) | 0 (0.0) | .209† |
| AST elevation | 44 (33.8) | 2 (1.5) | 79 (61.7) | 8 (6.2) | .101 |
| ALT elevation | 48 (36.9) | 2 (1.5) | 87 (68.0) | 10 (7.8) | .036 |
| Bilirubin elevation | 5 (3.8) | 1 (0.8) | 11 (8.6) | 3 (2.3) | .603 |
| Oral mucositis | 43 (33.1) | 8 (6.2) | 69 (53.9) | 19 (14.8) | .038 |
| Interruption of R-CHOP‡ | |||||
| Delay or dose reduction | 22 (16.9) | 40 (31.2) | |||
| Discontinuation | 13 (10.0) | 5 (3.9) | |||
Data are n (%).
AST, aspartate aminotransferase.
Grade 3 vs grade 4 adverse events.
P value for comparison of adverse events of any grade.
Excluding treatment discontinuation because of disease progression. Interruption of R-CHOP chemotherapy because of adverse events of HD-MTX occurred in 15 cases.
Discussion
In the absence of randomized controlled trials, the role of HD-MTX as CNS prophylaxis and its effect on survival outcomes in patients with DLBCL at high risk for CNS relapse are controversial. This is the first study to evaluate and compare the efficacy of prophylactic HD-MTX by the ITT. We demonstrated that the addition of HD-MTX to R-CHOP was not associated with a reduced incidence of CNS relapse or improved survival outcomes. After performing propensity score–matched or IPTW analyses to balance the baseline characteristics of the ITT HD-MTX and non-ITT HD-MTX groups, there was no significant difference in the CNS relapse rate or survival outcomes between groups.
In the unweighted cohort, the 2-year cumulative incidence of CNS relapse (12.4% in the ITT HD-MTX group and 13.9% in the non-ITT HD-MTX group) was similar to that reported in previous studies involving patients at high risk for CNS relapse,6 but the difference was not statistically significant between the ITT HD-MTX and non-ITT HD-MTX groups. This is in line with previous studies demonstrating that the addition of HD-MTX to standard treatment did not reduce CNS relapse rate in patients with DLBCL.19,30 In contrast, several other studies have reported the efficacy of prophylactic HD-MTX in reducing the incidence of CNS relapse20,21,25 ; however, these studies lacked control arms, or the baseline characteristics between patients in the HD-MTX and control arms were not balanced, which limited the interpretation of data. In the current study, even after performing propensity score–matched or IPTW analyses, no significant benefit of HD-MTX for CNS relapse was observed, further supporting the lack of CNS prophylactic efficacy of HD-MTX. Moreover, the benefit of HD-MTX for CNS relapse was not observed in any subgroup of patients, including those with high CNS IPI.
Similar to the results for CNS relapse, there was no significant difference in PFS or OS between patients in the ITT HD-MTX and non-ITT HD-MTX groups. This is contradictory to the results of previous studies that demonstrated a survival benefit with HD-MTX in DLBCL patients.19,20 However, it is important to note that patients who were refractory to first-line chemotherapy, who generally have a very poor prognosis, were likely to be included in the group not receiving HD-MTX treatment in these previous studies. In the current study, we classified patients based on the ITT to reduce such selection bias. Moreover, after performing propensity score–matched or IPTW analyses, no survival benefit was observed in the ITT HD-MTX group compared with the non-ITT HD-MTX group. Furthermore, the benefit for PFS or OS was not observed in patients in any of the subgroups, including those with high IPI. These results further support the lack of a survival benefit when adding HD-MTX to R-CHOP in patients at a high risk for CNS relapse.
HD-MTX treatment is associated with various toxicities, such as hepatotoxicity, renal toxicity, and stomatitis.31-33 In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated ALT levels. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group. Furthermore, treatment delay or a dose reduction in R-CHOP was more common in the ITT HD-MTX group, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles. This might result in a reduced dose intensity of R-CHOP and could play a role in the lack of an observed survival benefit with additional HD-MTX.34 Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs. Taken together, in the absence of clear evidence for a benefit of HD-MTX treatment on CNS relapse and survival outcomes, those potential risks should be considered before incorporating HD-MTX into standard R-CHOP chemotherapy.
This study has several limitations. As anticipated for any retrospective study, selection bias may exist. However, we classified patients based on the physician’s initial intent to treat them with HD-MTX to minimize such bias. Also, we performed propensity score–matched and IPTW analyses to overcome potential bias between the groups, because the decision to give HD-MTX prophylaxis was at the discretion of the physician. Despite these careful attempts to minimize selection bias, we acknowledge that effects from unmeasured clinical factors might not be completely adjusted for. The small number of CNS relapse events is another limitation of this study, potentially restricting the power of analysis. However, the estimated CNS relapse rates and their 95% CIs in this study are consistent with previous large-scale data studies, including the original CNS IPI study.6 Therefore, it is likely that the estimated CNS relapse rate in our study reflects the real-world incidence in this population. Also, our results are based on a uniformly treated cohort, one of the largest at high risk for CNS relapse. Despite these limitations, this real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population.
In conclusion, HD-MTX prophylaxis was not associated with reduced CNS relapse rates or improved survival outcomes, and it was accompanied by increased toxicities in DLBCL patients at high risk for CNS relapse.
Data sharing requests should be sent to Dok Hyun Yoon (dhyoon@amc.seoul.kr) or Cheolwon Suh (csuh@amc.seoul.kr).
Authorship
Contribution: H.J. contributed to data curation, formal analysis, and visualization and writing of the original draft; H. Cho. contributed to formal analysis and writing of the original draft; H.K. contributed to data curation and writing of the original draft; H. Chae and K.L. contributed to data curation, data validation, and writing, reviewing, and editing the manuscript; J.-B.L. contributed to formal analysis; S.K. contributed to data curation; S.-w.L., J.-S.R., K.W.K., E.J.C., J.H., and C.-S.P. contributed to data validation, and writing, reviewing, and editing the manuscript; and D.H.Y. and C.S contributed to conceptualization, formal analysis, methodology, supervision, and writing, reviewing, and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dok Hyun Yoon, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Republic of Korea; e-mail: dhyoon@amc.seoul.kr; and Cheolwon Suh, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Republic of Korea; e-mail: csuh@amc.seoul.kr.
References
Author notes
H.J., H. Cho, and H.K. contributed equally to this work and are joint first authors.
D.H.Y. and C.S. contributed equally to this work.
The full-text version of this article contains a data supplement.