Key Points
Caplacizumab reduces refractoriness, recurrences, and death during treatment, in addition to reducing time-to-platelet-count response.
No new safety signals were identified for caplacizumab, with mucocutaneous bleeding being the most frequent adverse event.
Abstract
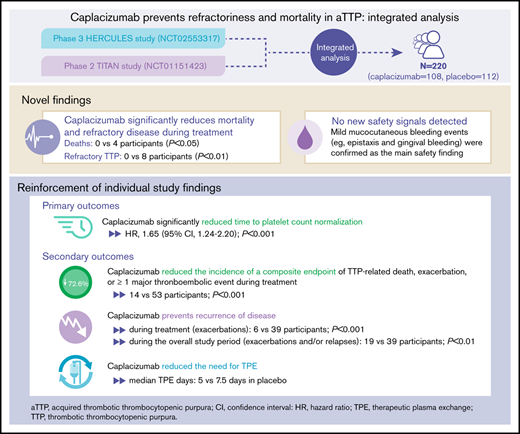
The efficacy and safety of caplacizumab in individuals with acquired thrombotic thrombocytopenic purpura (aTTP) have been established in the phase 2 TITAN and phase 3 HERCULES trials. Integrated analysis of data from both trials was conducted to increase statistical power for assessing treatment differences in efficacy and safety outcomes. Caplacizumab was associated with a significant reduction in the number of deaths (0 vs 4; P < .05) and a significantly lower incidence of refractory TTP (0 vs 8; P < .05) vs placebo during the treatment period. Consistent with the individual trials, treatment with caplacizumab resulted in a faster time to platelet count response (hazard ratio, 1.65; P < .001), a 72.6% reduction in the proportion of patients with the composite end point of TTP-related death, TTP exacerbation, or occurrence of at least 1 treatment-emergent major thromboembolic event during the treatment period (13.0% vs 47.3%; P < .001), and a 33.3% reduction in the median number of therapeutic plasma exchange days (5.0 vs 7.5 days) vs placebo. No new safety signals were identified; mild mucocutaneous bleeding was the main safety finding. This integrated analysis provided new evidence that caplacizumab prevents mortality and refractory disease in acquired TTP and strengthened individual trial findings, with a confirmed favorable safety and tolerability profile. These trials were registered at www.clinicaltrials.gov as #NCT01151423 and #NCT02553317.
Introduction
Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare, life-threatening immune-mediated thrombotic microangiopathy1 that is associated with an acute mortality rate of 8% to 20% despite treatment with therapeutic plasma exchange (TPE) and immunosuppression.2,3 Refractory disease occurs in up to 42% of patients and may lead to poor outcomes.4-6 There remains a need for targeted, rapid-acting treatments to prevent early mortality and morbidity.7,8
Caplacizumab demonstrated efficacy and safety in individuals with aTTP in the placebo-controlled phase 2 TITAN and phase 3 HERCULES studies.9,10 This integrated analysis of TITAN and HERCULES trials assessed efficacy and safety outcomes that may have gone undetected in the individual studies.
Methods
Details of the TITAN and HERCULES studies have been described previously (supplemental Figure 1).9,10 Adults were eligible if they had an acute episode of aTTP diagnosed on the basis of clinical presentation; deficiency of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) was not an eligibility criterion, but a sample was collected. Participants were randomly assigned to receive caplacizumab or placebo in addition to TPE and immunosuppression, during TPE treatment, and after TPE cessation for 30 days in the TITAN study and 30 to 58 days in the HERCULES study, in which treatment extension was allowed for up to 4 weeks in case of unresolved autoimmune disease (eg, ADAMTS13 <10%). Our integrated analysis included all randomly assigned participants (intent-to-treat population) from both studies.
Time-to-platelet-count response (primary end point) was assessed for the blinded study periods. Treatment groups were compared by using a 2-sided log-rank test stratified by trial based on Kaplan-Meier analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by using a Cox proportional hazards regression model with time-to-platelet-count response as a dependent variable, treatment group as an independent variable, and study as a random effect.
Secondary efficacy end points (assessed during blinded study periods unless otherwise specified) included time to normalization of organ damage markers, number of TPE days, and proportion of participants with TTP-related death, TTP recurrence, or ≥1 treatment-emergent major thromboembolic events (MTEs; assessed as a composite end point and as individual events), TTP recurrence (occurring ≤30 days after the end of daily TPE [exacerbation] or >30 days after end of daily TPE [relapse]) during the blinded treatment period and the overall study period, and refractory TTP defined according to the international consensus definition.11 To compare treatment groups, a stratified Cochran-Mantel-Haenszel test was used as a stratification factor in the trials. Assessment of time to first normalization of organ damage marker was similar to that for the primary end point.
Integrated safety analysis included all randomly assigned participants who received ≥1 dose of the study drug. Mortality, refractoriness, and safety were also assessed in subgroups by confirmation of aTTP diagnosis (ADAMTS13 activity <10% at baseline or at any time during the study, a previous history of aTTP, or a recurrence of aTTP).
The TITAN and HERCULES studies were approved by the relevant ethics committee at each participating site, and they complied with the principles of good clinical practice. All participants provided written informed consent in accordance with the Declaration of Helsinki. Independent data and safety monitoring boards reviewed safety data during the trials.
Results and discussion
This integrated analysis of 220 participants (108 in the caplacizumab group; 112 in the placebo group; supplemental Tables 1 and 2) enrolled in the TITAN and HERCULES trials9,10 provides new evidence regarding the benefits of caplacizumab on mortality and refractory disease in individuals with aTTP without any new safety signals.
Caplacizumab was associated with significant reduction in the number of deaths (0 vs 4; P < .05) and incidence of refractory TTP (0 vs 8; P < .01; Table 1) vs placebo during the treatment period. Time to death ranged from 7 to 11 days after treatment initiation. One patient each in the caplacizumab and placebo groups died outside the treatment period (Table 1). All deaths were considered to be related to TTP by the investigator, but in 1 case, ADAMTS13 deficiency could not be confirmed (supplemental Table 3). The benefit of caplacizumab on mortality and refractoriness was also evident in a subgroup of participants with a confirmed diagnosis of aTTP.
Integrated efficacy end points for the overall ITT population
| Efficacy end points . | Caplacizumab (n = 108) . | Placebo (n = 112) . | P . |
|---|---|---|---|
| Proportion of participants with ≥1 of the events below while receiving blinded study drug treatment | 14 (13.0) | 53 (47.3) | <.001 |
| TTP-related death | 0 (0.0) | 4 (3.6) | |
| Major thromboembolic event* | 8 (7.4) | 14 (12.5) | |
| TTP exacerbation | 6 (5.6) | 39 (34.8) | |
| TTP recurrences† | |||
| TTP exacerbation during blinded treatment period | 6 (5.6) | 39 (34.8) | <.001 |
| TTP relapses during treatment-free follow-up period | 14 (13.0) | 0 (0.0) | n/s |
| TTP exacerbation or relapse during overall study | 19 (17.6) | 39 (34.8) | .0040 |
| Refractory to treatment‡ | 0 (0.0) | 8 (7.1) | <.01 |
| Mortality rate | |||
| During the blinded treatment periods | 0 (0.0) | 4 (3.6) | .0477 |
| During the overall study period | 1 (0.9)§ | 5 (4.5)§ | n/s |
| Efficacy end points . | Caplacizumab (n = 108) . | Placebo (n = 112) . | P . |
|---|---|---|---|
| Proportion of participants with ≥1 of the events below while receiving blinded study drug treatment | 14 (13.0) | 53 (47.3) | <.001 |
| TTP-related death | 0 (0.0) | 4 (3.6) | |
| Major thromboembolic event* | 8 (7.4) | 14 (12.5) | |
| TTP exacerbation | 6 (5.6) | 39 (34.8) | |
| TTP recurrences† | |||
| TTP exacerbation during blinded treatment period | 6 (5.6) | 39 (34.8) | <.001 |
| TTP relapses during treatment-free follow-up period | 14 (13.0) | 0 (0.0) | n/s |
| TTP exacerbation or relapse during overall study | 19 (17.6) | 39 (34.8) | .0040 |
| Refractory to treatment‡ | 0 (0.0) | 8 (7.1) | <.01 |
| Mortality rate | |||
| During the blinded treatment periods | 0 (0.0) | 4 (3.6) | .0477 |
| During the overall study period | 1 (0.9)§ | 5 (4.5)§ | n/s |
Data are presented as n (%).
ITT, intent to treat; n/s, not statistically significant (P ≥ .05).
Based on standardized MedDRA (Medical Dictionary for Regulatory Activities) queries (Standardized MedDRA Query) in the TITAN trial (post hoc); confirmed after adjudication in the HERCULES trial.
Recurrence was defined as a new drop in platelet count after initial platelet count normalization, necessitating re-initiation of TPE. Exacerbation is a recurrence within 30 days after the end of daily TPE. Relapse is a recurrence occurring more than 30 days after the end of daily TPE.
Defined as a lack of sustained platelet count increment or platelet counts <50 × 109/L throughout the assessment period and persistently increased lactate dehydrogenase (>1.5 × upper limit of normal) despite 5 TPEs and steroid treatment.11
In the TITAN trial, 1 participant in the placebo group died 2 days after study drug treatment was permanently discontinued by physician decision and was therefore counted as a death during the follow-up period, despite dying from severe refractory disease in the acute setting. In the HERCULES trial, 1 participant in the caplacizumab group died in the follow-up period after completing treatment and reaching complete remission. Both participants had a confirmed TTP diagnosis (ADAMTS13 <10%).
The mortality rate with placebo was lower than rates reported in the literature with TPE and immunosuppression2,3 ; this is expected, given the controlled setting of a clinical trial. Nevertheless, these findings are consistent with a recent real-world study, which met the primary end point of a significant reduction in the composite of mortality and refractoriness in individuals with aTTP who received first-line caplacizumab, TPE, and immunosuppression vs a historical cohort (2.2% vs 12.2%; P = .01).12 Altogether, these data consistently highlight the significant impact of caplacizumab treatment on mortality and refractoriness, outcomes that have not changed much since the hallmark publication in 1991 comparing TPE and plasma infusion.13
Consistent with the individual study findings,9,10 caplacizumab significantly reduced time to platelet count normalization (HR, 1.65; 95% CI, 1.24-2.20; P < .001; supplemental Table 4; supplemental Figure 2). Furthermore, caplacizumab significantly reduced time to normalization of the organ damage marker lactate dehydrogenase (HR, 1.43; 95% CI, 1.04-1.96; P = .03) and induced a faster normalization of troponin (HR, 1.32; 95% CI, 0.86-2.04; P = .29) and serum creatinine (HR, 1.68; 95% CI, 0.89-3.15; P = .14; supplemental Figure 3). During the overall treatment period, there was a 33.3% reduction in the median number of TPE days with caplacizumab vs placebo (5.0 days [range, 1-35 days] vs 7.5 days [range, 2-46 days], respectively).
The composite end point of TTP-related death, exacerbation, or any treatment-emergent MTE was significantly reduced with caplacizumab compared with placebo (72.6% reduction; P < .001; Table 1). Incidence of MTEs was numerically reduced by 40.8% with caplacizumab vs placebo (7.4% vs 12.5%; supplemental Table 5). Caplacizumab was also associated with 84.0% reduction in the incidence of exacerbations during the blinded treatment period (6 vs 39 participants; P < .001; Table 1). Exacerbations during caplacizumab treatment were not linked to inadequate caplacizumab exposure but were likely caused by concurrent infections in 4 participants and noncompliance with therapy in 1 participant (unexplained in 1 participant). With placebo, exacerbations occurred mostly in participants with ADAMTS13 activity <10% when daily TPE was stopped. Exacerbations lead to rehospitalization and restarting TPE, which again exposed patients to the risks of the disease. Two participants who received placebo experienced an MTE, which coincided with the onset of the exacerbation (acute myocardial infarction and venous thrombosis). Although no exacerbations led to death in either HERCULES or TITAN, a real-world study reported 20 deaths among 140 patients with TTP exacerbations.14 Together, this underscores the importance of preventing exacerbations.
In both trials, more relapses occurred with caplacizumab compared with placebo (14 vs 0 participants); 13 of 14 relapses occurred within 10 days of stopping caplacizumab in participants with persistent ADAMTS13 levels <10%, highlighting the importance of continuing caplacizumab treatment until resolution of the underlying autoimmune disease, which is achieved through immunosuppression. Importantly, by allowing treatment extension in the HERCULES trial, the overall incidence of recurrences (exacerbation or relapse) during the full study period of the integrated analysis remained 49.4% lower in the caplacizumab group (19 vs 39 participants; P < .01; Table 1).
A total of 216 participants (106 in the caplacizumab group; 110 in the placebo group) were included in the integrated safety analysis. TPE-related treatment-emergent adverse events (TEAEs) occurred in 40.6% (43 of 106) and 45.5% (50 of 11) in the caplacizumab and placebo groups, respectively; serious TPE-related TEAEs occurred in 3.8% (4 of 106) and 6.4% (7 of 110), respectively (supplemental Table 6). As expected, bleeding TEAEs were the most common adverse events associated with caplacizumab treatment (Table 2; supplemental Tables 6 and 7). Bleeding events were mainly mucocutaneous (epistaxis and gingival bleeding; supplemental Table 8), and most occurrences were mild to moderate and self-limited. Importantly, severe TEAEs were uncommon (supplementary Table 6); no new safety signals were identified. Among 8 participants in the caplacizumab group with an unconfirmed aTTP diagnosis, any bleeding TEAEs occurred in 6 participants and serious bleeding TEAEs occurred in 4 participants (50%; supplemental Table 9). The type and nature of bleeding events were similar to those observed in the confirmed TTP population (with epistaxis [n = 2] and gingival bleeding [n = 3] occurring most frequently; ecchymosis, gastric ulcer hemorrhage, and rectal hemorrhage were reported in 1 patient each). Comparison between subgroups is difficult because of the small numbers of patients. Yet as expected, the data suggest that patients with non-deficient ADAMTS13 also have an increased bleeding risk with caplacizumab, which also seems to be mainly mucocutaneous in nature.
Summary of TEAEs occurring in the blinded study periods (safety analysis set)
| Preferred term . | Overall . | |||
|---|---|---|---|---|
| Caplacizumab (n = 106) . | Placebo (n = 110) . | |||
| n (%) . | No. of events . | n (%) . | No. of events . | |
| At least 1 TEAE | 102 (96.2) | 790 | 105 (95.5) | 897 |
| At least 1 serious TEAE | 31 (29.2) | 44 | 54 (49.1) | 72 |
| Any bleeding TEAE excluding TTP* | 62 (58.5) | 157 | 47 (42.7) | 94 |
| Any serious bleeding TEAE excluding TTP | 12 (11.3) | 15 | 2 (1.8) | 2 |
| Epistaxis | 4 (3.8) | 4 | 0 | 0 |
| Subarachnoid hemorrhage | 2 (1.9) | 2 | 0 | 0 |
| Gingival bleeding | 1 (0.9) | 1 | 0 | 0 |
| Gastric ulcer hemorrhage | 1 (0.9) | 1 | 0 | 0 |
| Hematemesis | 1 (0.9) | 1 | 0 | 0 |
| Upper gastrointestinal hemorrhage | 1 (0.9) | 1 | 0 | 0 |
| Retinal hemorrhage | 1 (0.9) | 1 | 0 | 0 |
| Metrorrhagia | 1 (0.9) | 2 | 0 | 0 |
| Mennorrhagia | 1 (0.9) | 1 | 0 | 0 |
| Hemorrhagic ovarian cyst | 1 (0.9) | 1 | 0 | 0 |
| Cerebral hemorrhage | 0 | 0 | 1 (0.9) | 1 |
| Hemorrhagic transformation Stroke | 0 | 0 | 1 (0.9) | 1 |
| Preferred term . | Overall . | |||
|---|---|---|---|---|
| Caplacizumab (n = 106) . | Placebo (n = 110) . | |||
| n (%) . | No. of events . | n (%) . | No. of events . | |
| At least 1 TEAE | 102 (96.2) | 790 | 105 (95.5) | 897 |
| At least 1 serious TEAE | 31 (29.2) | 44 | 54 (49.1) | 72 |
| Any bleeding TEAE excluding TTP* | 62 (58.5) | 157 | 47 (42.7) | 94 |
| Any serious bleeding TEAE excluding TTP | 12 (11.3) | 15 | 2 (1.8) | 2 |
| Epistaxis | 4 (3.8) | 4 | 0 | 0 |
| Subarachnoid hemorrhage | 2 (1.9) | 2 | 0 | 0 |
| Gingival bleeding | 1 (0.9) | 1 | 0 | 0 |
| Gastric ulcer hemorrhage | 1 (0.9) | 1 | 0 | 0 |
| Hematemesis | 1 (0.9) | 1 | 0 | 0 |
| Upper gastrointestinal hemorrhage | 1 (0.9) | 1 | 0 | 0 |
| Retinal hemorrhage | 1 (0.9) | 1 | 0 | 0 |
| Metrorrhagia | 1 (0.9) | 2 | 0 | 0 |
| Mennorrhagia | 1 (0.9) | 1 | 0 | 0 |
| Hemorrhagic ovarian cyst | 1 (0.9) | 1 | 0 | 0 |
| Cerebral hemorrhage | 0 | 0 | 1 (0.9) | 1 |
| Hemorrhagic transformation Stroke | 0 | 0 | 1 (0.9) | 1 |
Participants may have had more than 1 adverse event (AE) per preferred term. An individual was counted only once if he or she experienced 1 or more AE.
A bleeding TEAE was defined as a Standardized MedDRA Query bleeding TEAE (excluding TTP events), with an onset date on or after the first dose of study medication.
This integrated analysis provides important new insights that supplement findings from the individual HERCULES and TITAN studies. Caplacizumab treatment prevents mortality and refractory disease and is generally well tolerated, with mild mucocutaneous bleeding as the main safety finding. Analysis of the larger population in this integrated analysis, together with recent real-world evidence, further demonstrates that caplacizumab may address a serious unmet need in acute aTTP.12,15,16
Presented in oral form (integrated efficacy analysis) and as a poster (integrated safety analysis) at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
Individual participant and supporting data will not be shared.
Acknowledgments
The authors and Sanofi thank the participants in the trials as well as the TITAN and HERCULES steering committees and investigators. Critical review of the manuscript was provided by Rui de Passos Sousa and Dunja Stankovic of Sanofi.
Medical writing and editorial support were provided by Namiko Abe (Fishawack Communications, part of Fishawack Health, Conshohocken, PA) and were funded by Sanofi. This work was supported by Ablynx, a Sanofi company, as were the HERCULES and TITAN studies.
The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Authorship
Contribution: F.P., S.C., M.S., P.C., P.K., J.A.K.H., A.M., J.d.l.R., and K.P. designed or performed the research, contributed to analysis and interpretation of results, and helped prepare the manuscript; J.M.M.E. provided statistical support, helped analyze and interpret the data, and reviewed the manuscript; H.D.W. helped design the study, medically monitor the study, and analyze, interpret, and report the data; F.C. designed the research, provided overview for the research, analyzed and interpreted data, and wrote and reviewed the manuscript; and all authors helped write and review the draft manuscript and approved the final version for publication.
Conflict-of-interest disclosure: F.P. served as a consultant for Kedrion, received honoraria for being a speaker at educational meetings from Ablynx/Sanofi, Grifols, Novo Nordisk, Roche, Shire, and Sobi, and was a member of an advisory board for Ablynx/Sanofi. S.C. received research funding and consulting fees from Ablynx/Sanofi and Alexion. M.S. served on advisory boards and received speaker fees from Ablynx/Sanofi, Alexion, Shire, and Novartis, and received research funding from Shire. P.C. was a member of advisory boards for and received speaker fees from Ablynx/Sanofi, Alexion, Octapharma, and Shire. P.K. served as a consultant, was a member of an advisory board, and received speaker fees from Ablynx/Sanofi, Shire, CSL Behring, Roche, and Novo Nordisk, and received research funding as unrestricted educational grants from Novo Nordisk. J.A.K.H. received research funding from Shire, received honoraria (to employer, Insel Gruppe AG, Department of Hematology) for participation in advisory boards and presentations from Ablynx/Sanofi, CSL Behring, Roche, Shire, and Siemens. A.M. served as a consultant for Ablynx/Sanofi. J.d.l.R. served as a consultant for Ablynx/Sanofi, Amgen, Celgene, and Janssen, and provided expert testimony for Amgen, Celgene, and Janssen. K.P. received research funding for participation in clinical trials from Ablynx (a Sanofi company), Bioverativ (a Sanofi company), Alexion, and Octapharma, and received honoraria for participation in advisory boards and presentations from Ablynx (a Sanofi company), Shire, and Alexion. J.M.M.E. is an employee with Ablynx/Sanofi. H.D.W. was an employee with Ablynx/Sanofi when this research was conducted. F.C. is employed by Sanofi (and was formerly employed by Ablynx/Sanofi).
Correspondence: Filip Callewaert, Medical Rare Blood Disorders, Sanofi, Airport Plaza–Montreal Building, Leonardo Da Vincilaan 19, 1831 Diegem, Ghent, Belgium, e-mail: filip.callewaert@sanofi.com.
References
Author notes
The full-text version of this article contains a data supplement.