Page 5580, abstract: “Median relapse-free survival (RFS) was 2.07 years in the azacitidine group vs 1.28 years in the control group (P = .19). There was also no significant difference for overall survival, with a median of 2.52 years vs 3.56 years in the azacitidine and control groups (P = .43), respectively. Cox regression analysis revealed no improvement in RFS or overall survival with the use of azacitidine as maintenance compared with the control group (hazard ratios of 0.86 [95% confidence interval, 0.59-1.3; P = .43] and 0.84 [95% confidence interval, 0.55-1.29; P = .43])” should read “Median relapse-free survival (RFS) was 2.07 years in the azacitidine group vs 1.28 years in the control group (P = .43). There was also no significant difference for overall survival, with a median of 2.52 years vs 2.56 years in the azacitidine and control groups (P = .85), respectively. Multivariate Cox regression analysis revealed no improvement in RFS or overall survival with the use of azacitidine as maintenance compared with the control group (hazard ratios of 0.73 [95% confidence interval, 0.49-1.1; P = .14] and 0.84 [95% confidence interval, 0.55-1.29; P = .43]).”
Page 5582, Table 2: In the “Observation” column heading, the number of patients should read “94.” In the data for “Disease status at HSCT, AML, n (%),” “27 (39.1)” should read “23 (33.3),” “34 (52.3)” should read “33 (50.8),” “12 (17.4)” should read “7 (10.2),” “30 (43.5)” should read “39 (56.5),” and “24 (27.5)” should read “15 (23).”
Page 5583, first paragraph of “Results”: In the next-to-last sentence, “25%” should read “15%.”
Page 5583: The first paragraph under “Median RFS was not improved with azacitidine maintenance compared with observation” should read “Among the 80 patients who survived, the median follow-up was 4.6 years in the azacitidine arm and 4.06 years in the control arm. Median RFS times were not significantly different between the groups: 2.07 years in the azacitidine arm and 1.28 years in the control arm (P = .43) (Figure 2A). Comparative analyses in different disease risk subgroups showed no benefit of azacitidine maintenance after transplant (data not shown). There was also no significant difference in OS, with a median of 2.52 vs 2.56 years in the azacitidine group (Figure 2B) and control group (HR, 0.96; 95% CI, 0.65-1.4; P = .85), respectively.”
Page 5584, Table 3: In row 2, under “RFS,” the HR should be 0.73, the 95% CI should be 0.49-1.10, and P should be .14. In row 10, there should be an asterisk after “Complete remission,” and its corresponding footnote should read, “*Only patients in CR with count recovery were included in this group.”
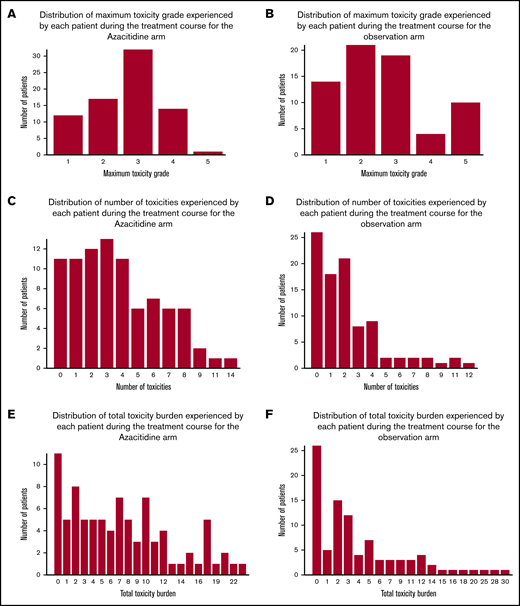
Page 5586, Figure 4: The graphs for the azacitidine and observation arms were in the wrong order. Panel C should feature the graph for the azacitidine arm, and panel D should feature the graph for the observation arm. The corrected Figure 4 is shown below.
Page 5588: Reference 27 was incorrect. The correct reference is the following:
27. Wei AH, Döhner H, Pocock C, et al. The QUAZAR AML-001 maintenance trial: results of a phase III international, randomized, double-blind, placebo-controlled study of CC-486 (oral formulation of azacitidine) in patients with acute myeloid leukemia (AML) in first remission [abstract]. Blood. 2019;134(suppl 2). Abstract LBA-3.
The errors have been corrected in the published article.