Key Points
In Sweden, MCL patients who were never married, were divorced, or had a lower educational level underwent transplantation less often.
Receiving a transplantation reduces the long-term probability of death in MCL patients ≤65 years of age.
Abstract
It is unknown how many mantle cell lymphoma (MCL) patients undergo consolidation with autologous hematopoietic cell transplantation (AHCT), and the reasons governing the decision, are also unknown. The prognostic impact of omitting AHCT is also understudied. We identified all MCL patients diagnosed from 2000 to 2014, aged 18 to 65 years, in the Swedish Lymphoma Register. Odds ratios (ORs) and 95% confidence intervals (CIs) from logistic regression models were used to compare the likelihood of AHCT within 18 months of diagnosis. All-cause mortality was compared between patients treated with/without AHCT using hazard ratios (HRs) and 95% CIs estimated from Cox regression models. Probabilities of being in each of the following states: alive without AHCT, alive with AHCT, dead before AHCT, and dead after AHCT, were estimated over time from an illness-death model. Among 369 patients, 148 (40%) were not treated with AHCT within 18 months. Compared with married patients, never married and divorced patients had lower likelihood of undergoing AHCT, as had patients with lower educational level, and comorbid patients. Receiving AHCT was associated with reduced all-cause mortality (HR = 0.58, 95% CI: 0.40-0.85). Transplantation-related mortality was low (2%). MCL patients not receiving an AHCT had an increased mortality rate, and furthermore, an undue concern about performing an AHCT in certain societal groups was seen. Improvements in supportive functions potentially increasing the likelihood of tolerating an AHCT and introduction of more tolerable treatments for these groups are needed.
Introduction
Although the prognosis in younger, intensively treated mantle cell lymphoma (MCL) patients is improving, the disease itself is still the leading cause of death in young patients,1 and survival is much worse for the elderly.2 Since 2001, treatment of MCL in the Nordic countries has included consolidation with autologous hematopoietic stem cell transplantation (AHCT) after induction chemotherapy. For younger (<65 years) patients, the Nordic MCL2 protocol (cyclophosphamide, doxorubicin hydrochloride [hydroxydaunorubicin], vincristine sulfate [Oncovin], and prednisone with rituximab alternating with high-dose cytarabine, and consolidation with AHCT) is regarded as the standard of care.3
Few studies have investigated factors predicting treatment with AHCT or not among MCL patients. One recent study reported that high comorbidity burden was associated with a low likelihood of transplantation.1 Another study from North American academic centers found that clinician’s choice (which can include many different aspects) was the main factor associated with not performing an AHCT in 67% of patients; patient preference was the reason in 18%, and other reasons (eg, mobilization failure) were cited in 3%.4 No study has yet investigated potential demographic selection mechanisms (sex, civil status, hospital size, performance status, country of birth, etc). Population-based studies describing how patient factors impact selection to treatment can increase awareness among clinicians, caregivers, and other stakeholders of medical decision making. This may lead to better supportive care and improved possibilities for equal health care. In addition, such studies could indicate which patient groups may be targeted with new drug combinations and maintenance treatment when AHCT is not possible.
The prognostic benefit of AHCT on overall survival (OS) is debated. A randomized trial in the prerituximab era showed a progression-free survival (PFS) benefit of 39 months vs 17 months for patients treated with AHCT compared with interferon maintenance.5 One retrospective study of 1029 patients showed inferior PFS, but not better OS, in AHCT-treated patients during the rituximab era,4 and an older population-based study from Sweden/Denmark, including patients in both the pre- and postrituximab eras (n = 1389) reported superior OS.6 Novel drugs, such as ibrutinib, are efficient in relapsed MCL.7 The benefits in first line and in combination with conventional chemoimmunotherapy and/or AHCT are unknown but are currently tested in trials, such as the TRIANGLE study (clinicaltrials.gov #NCT02858258).
AHCT is an intense treatment, and some patients are expected to be ineligible, mainly because of comorbidities.2 A new era with targeted drugs is emerging with promising results,8 and options that omit AHCT are presently discussed.9-11 The primary aim of the current study was to investigate associations between demographic- and disease-related factors with AHCT in a population-based cohort of young MCL patients, to characterize vulnerable groups where novel treatment concepts are particularly needed. A secondary aim was to estimate and contrast survival and mortality in patients treated with and without an AHCT.
Materials and methods
Data sources
Patients diagnosed with MCL were identified from the Swedish Lymphoma Register (coverage ∼95% compared with the Swedish Cancer Register).12 Clinical information retrieved included Ann Arbor stage, leukocyte count, lactate dehydrogenase level, Eastern Cooperative Oncology Group (ECOG) performance status, primary treatment, and AHCT. The MCL-specific international prognostic index was calculated using the algorithm defined by Hoster et al.13 In addition, hospital size and type (university, regional, or local) were identified. Using the personal identification number unique to all individuals in Sweden, the cohort was further linked to several nationwide population-based registers. Information on performed AHCT (using International Classification of Diseases codes14 ) was supplemented using the Swedish Patient Register (nationwide inpatient coverage since 1987). This register (both inpatient and specialist outpatient care) and the Swedish Cancer Register were used to classify comorbidities according to the Charlson Comorbidity Index (CCI), within 10 years prior to diagnosis. Using the national database Longitudinal Integrated Database for Health Insurance and Labor Market Studies, information was added regarding country of birth (Swedish/foreign), highest achieved educational level (≤9 years, 10 to 12 years, or >12 years of schooling), and civil status (married, never married, divorced, widower). Dates of death were retrieved from the Swedish Cause of Death Register.15
Study population
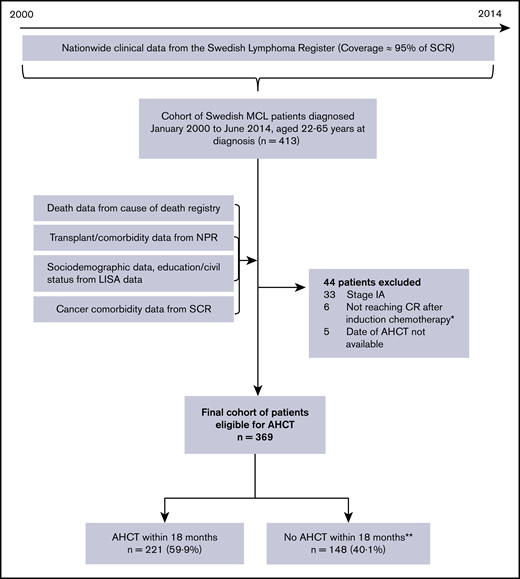
All individuals registered with a diagnosis of MCL between January 2000 and June 2014, aged 18 to 65 years, were included (n = 413) (Figure 1). Among these, patients with stage 1A disease (n = 33), patients not reaching complete or partial remission (n = 6), and patients who underwent transplantation but whose date of transplant was missing (n = 5) were excluded. The final study population comprised 369 patients.
Flowchart of inclusions and exclusions to final cohort used for analyses. Stage IA patients were excluded because Swedish treatment guidelines recommend radiotherapy and no AHCT. Patients not reaching CR after induction chemotherapy were excluded because this group is not eligible for transplantation. allo, allogeneic stem cell transplantation; CR, complete remission; LISA, Longitudinal Integrated Database for Health Insurance and Labor Market Studies; NPR, National Patient Register; SCR, Swedish Cancer Register. *Among these, all patients but two died within 18 months of diagnosis. Median time to death was 8.8 months (range: 6.2 months to 4.6 years). **Among these, 27 patients (18%) had an AHCT after the predefined time period of 18 months postdiagnosis.
Flowchart of inclusions and exclusions to final cohort used for analyses. Stage IA patients were excluded because Swedish treatment guidelines recommend radiotherapy and no AHCT. Patients not reaching CR after induction chemotherapy were excluded because this group is not eligible for transplantation. allo, allogeneic stem cell transplantation; CR, complete remission; LISA, Longitudinal Integrated Database for Health Insurance and Labor Market Studies; NPR, National Patient Register; SCR, Swedish Cancer Register. *Among these, all patients but two died within 18 months of diagnosis. Median time to death was 8.8 months (range: 6.2 months to 4.6 years). **Among these, 27 patients (18%) had an AHCT after the predefined time period of 18 months postdiagnosis.
Definition of AHCT
A transplantation can be performed at different time points after the diagnosis of MCL, depending on an initial “wait-and-watch” period of no treatment and of different lengths of the induction regimen. Of all patients in our cohort who received a transplant, 89% had their transplantation within 18 months of diagnosis, leading us to select this time point in the analyses of selection mechanisms. Those undergoing an AHCT within 18 months of diagnosis were thus defined as selected for transplantation, whereas patients not experiencing an AHCT, or those who had a transplant after >18 months, were not. In a sensitivity analysis, we also investigated transplantation within 12 and 24 months. For the survival analyses, all AHCTs occurring during follow-up (n = 248) were considered.
Statistical methods
Frequencies and proportions of patient demographics and clinical characteristics recorded at time of diagnosis, including marital status, educational level, country of birth, sex, and CCI, were calculated overall and by selection to AHCT. The proportion of patients dying within 100 days of their transplantation was calculated as a measure of transplant-related mortality. To compare the likelihood of being selected to an AHCT by demographic and clinical characteristics, odds ratios (ORs) with 95% confidence intervals (CIs) were estimated from univariable and multivariable logistic regression models. The multivariable model was adjusted for calendar year of diagnosis (2000 to 2004/2005 to 2009/2010 to 2014), age at diagnosis (assuming linearity), sex, and country of birth. Variable selection was done a priori based on the assumed relationship between exposures and outcome, using a directed acyclic graph (DAG).16
Hazard ratios (HRs) with 95% CIs comparing all-cause mortality by AHCT (as a time-varying exposure) were estimated from univariable and multivariable Cox proportional hazards models with age at diagnosis as an effect modifier. Follow-up started at 6 months after diagnosis, when patients were assumed evaluated after first-line treatment, until date of death or administrative censoring (December 31, 2015). Patients dying within the first 6 months after diagnosis (n = 6) were excluded; hence, 363 patients contributed to the survival analyses. Follow-up was restricted to the first 10 years, resulting in a mean follow-up time of 5.2 years (standard deviation = 3.02).
To accurately describe the complete patient trajectory from diagnosis to death, a multistate illness-death model approach17 was taken (illustrated in supplemental Figure 1). This enabled predictions of the probability of being in each of the following states: alive without AHCT, alive with AHCT, dead before AHCT, and dead after AHCT, as functions of time since MCL diagnosis. All transition rates were modeled using flexible parametric survival models18 with 4 degrees of freedom for the baseline rate. A detailed description of the models and methods used can be found in the supplemental statistical appendix.
In all survival analyses, the underlying timescale was time since MCL diagnosis, and all results are presented stratified by age at diagnosis (≤45, 50 to 59, and 60 to 65 years of age). All analyses were based on complete cases and conducted using Stata software (StataCorp 2017; Stata Statistical Software: Release 15, StataCorp, LLC, College Station, TX).
Ethics
The study was approved by the Regional Board of the Ethical Committee in Stockholm, Sweden (2007/1335-31/4, 2010/1624-32).
Results
Demographics and selection mechanisms
Among the 369 MCL patients included, 221 patients (60%) had a transplantation within 18 months (Table 1). For the patients who underwent transplantation, induction chemotherapy was generally rituximab (R)–cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate (Oncovin), and prednisone (CHOP) alternating with R-cytarabine given according to the Nordic Lymphoma Group protocol MCL 2 or 3 (Table 2). The non–AHCT-treated patients were mostly given R-CHOP/cytarabine, R-CHOP, or R-bendamustine, whereas some were treated with chlorambucil alone (mainly prior to 2005; none after 2010).
Patient characteristics and ORs with 95% CIs of selection to AHCT within 18 mo of diagnosis among stage IB+ MCL patients up to 65 y of age diagnosed in Sweden between January 2000 and June 2014
| Variable . | n . | AHCT ≤ 18 mo . | No AHCT ≤ 18 mo . | Unadjusted OR (95% CI) . |
|---|---|---|---|---|
| Overall (row %) | 369 (100) | 221 (59.9) | 148 (40.1) | |
| Marital status at diagnosis (col %) | ||||
| Married | 216 (58.5) | 141 (63.8) | 75 (50.7) | 1.00 |
| Never married | 68 (18.4) | 39 (17.7) | 29 (19.6) | 0.72 (0.41-1.25) |
| Divorced | 73 (19.8) | 37 (16.7) | 36 (24.3) | 0.55 (0.32-0.94) |
| Widow(er) | 12 (3.3) | 4 (1.8) | 8 (5.4) | 0.27 (0.08-0.91) |
| Highest achieved education level (col %) | ||||
| Up to 9 y of schooling | 80 (21.7) | 35 (15.8) | 45 (30.4) | 0.44 (0.26-0.75) |
| 10 to 12 y of schooling | 175 (47.4) | 112 (50.7) | 63 (42.6) | 1.00 |
| >12 y of schooling | 103 (27.9) | 68 (30.8) | 35 (23.7) | 1.09 (0.66-1.82) |
| Missing | 11 (3.0) | 6 (2.7) | 5 (3.4) | · |
| Country of birth (col %) | ||||
| Sweden | 321 (87.0) | 189 (85.5) | 132 (89.2) | 1.00 |
| Foreign | 48 (13.0) | 32 (14.5) | 16 (10.8) | 1.40 (0.74-2.65) |
| Sex (col %) | ||||
| Male | 288 (78.1) | 171 (77.4) | 117 (79.1) | 1.00 |
| Female | 81 (21.9) | 50 (22.6) | 31 (20.9) | 1.10 (0.67-1.83) |
| CCI (col %) | ||||
| 0 | 277 (75.1) | 182 (82.4) | 95 (64.2) | 1.00 |
| 1 | 45 (12.2) | 23 (10.4) | 22 (14.9) | 0.55 (0.29-1.03) |
| 2+ | 47 (12.7) | 16 (7.2) | 31 (21.0) | 0.27 (0.14-0.52) |
| Year of diagnosis (col %) | ||||
| 2000 to 2004 | 114 (30.9) | 65 (29.4) | 49 (33.1) | 1.00 |
| 2005 to 2009 | 120 (32.5) | 75 (33.9) | 45 (30.4) | 1.26 (0.74-2.12) |
| 2010 to 2014 | 135 (36.6) | 81 (36.7) | 54 (36.5) | 1.13 (0.68-1.88) |
| Age at diagnosis, y (col %) | ||||
| <50 | 51 (13.8) | 39 (17.7) | 12 (8.1) | 1.00 |
| 50 to 59 | 148 (40.1) | 104 (47.1) | 44 (29.7) | 0.73 (0.35-1.52) |
| 60 to 65 | 170 (46.1) | 78 (35.3) | 92 (62.2) | 0.26 (0.13-0.53) |
| Hospital type where initial diagnosis was made (col %) | ||||
| University | 184 (53.4) | 118 (53.4) | 66 (44.6) | 1.00 |
| Regional | 127 (34.4) | 67 (30.3) | 60 (40.5) | 0.62 (0.39-0.99) |
| Local | 58 (15.7) | 36 (16.3) | 22 (14.9) | 0.92 (0.50-1.68) |
| Stage (col %) | ||||
| Ann Arbor IB and II | 37 (10.0) | 19 (8.6) | 18 (12.2) | 1.00 |
| Ann Arbor III | 49 (13.3) | 33 (14.9) | 16 (10.8) | 1.95 (0.81-4.70) |
| Ann Arbor IV | 279 (75.6) | 168 (76.0) | 111 (75.0) | 1.43 (0.72-2.85) |
| Missing | 4 (1.1) | 1 (0.5) | 3 (2.0) | |
| MCL-specific international prognostic index (col %) | ||||
| Low risk (<5.7) | 114 (30.9) | 73 (33.0) | 41 (27.7) | 1.00 |
| Intermediate risk (5.7-6.1) | 92 (24.9) | 55 (24.9) | 37 (25.0) | 0.83 (0.47-1.47) |
| High risk (>6.1) | 71 (19.2) | 38 (17.2) | 33 (22.3) | 0.65 (0.35-1.18) |
| Missing | 92 (24.9) | 55 (24.9) | 37 (25.0) | |
| Performance status (col %) | ||||
| ECOG 0 to 1 | 332 (90.0) | 207 (93.7) | 125 (84.5) | 1.00 |
| ECOG 2 to 4 | 31 (8.4) | 12 (5.4) | 19 (12.8) | 0.38 (0.18-0.81) |
| Missing | 6 (1.6) | 2 (0.9) | 4 (2.7) |
| Variable . | n . | AHCT ≤ 18 mo . | No AHCT ≤ 18 mo . | Unadjusted OR (95% CI) . |
|---|---|---|---|---|
| Overall (row %) | 369 (100) | 221 (59.9) | 148 (40.1) | |
| Marital status at diagnosis (col %) | ||||
| Married | 216 (58.5) | 141 (63.8) | 75 (50.7) | 1.00 |
| Never married | 68 (18.4) | 39 (17.7) | 29 (19.6) | 0.72 (0.41-1.25) |
| Divorced | 73 (19.8) | 37 (16.7) | 36 (24.3) | 0.55 (0.32-0.94) |
| Widow(er) | 12 (3.3) | 4 (1.8) | 8 (5.4) | 0.27 (0.08-0.91) |
| Highest achieved education level (col %) | ||||
| Up to 9 y of schooling | 80 (21.7) | 35 (15.8) | 45 (30.4) | 0.44 (0.26-0.75) |
| 10 to 12 y of schooling | 175 (47.4) | 112 (50.7) | 63 (42.6) | 1.00 |
| >12 y of schooling | 103 (27.9) | 68 (30.8) | 35 (23.7) | 1.09 (0.66-1.82) |
| Missing | 11 (3.0) | 6 (2.7) | 5 (3.4) | · |
| Country of birth (col %) | ||||
| Sweden | 321 (87.0) | 189 (85.5) | 132 (89.2) | 1.00 |
| Foreign | 48 (13.0) | 32 (14.5) | 16 (10.8) | 1.40 (0.74-2.65) |
| Sex (col %) | ||||
| Male | 288 (78.1) | 171 (77.4) | 117 (79.1) | 1.00 |
| Female | 81 (21.9) | 50 (22.6) | 31 (20.9) | 1.10 (0.67-1.83) |
| CCI (col %) | ||||
| 0 | 277 (75.1) | 182 (82.4) | 95 (64.2) | 1.00 |
| 1 | 45 (12.2) | 23 (10.4) | 22 (14.9) | 0.55 (0.29-1.03) |
| 2+ | 47 (12.7) | 16 (7.2) | 31 (21.0) | 0.27 (0.14-0.52) |
| Year of diagnosis (col %) | ||||
| 2000 to 2004 | 114 (30.9) | 65 (29.4) | 49 (33.1) | 1.00 |
| 2005 to 2009 | 120 (32.5) | 75 (33.9) | 45 (30.4) | 1.26 (0.74-2.12) |
| 2010 to 2014 | 135 (36.6) | 81 (36.7) | 54 (36.5) | 1.13 (0.68-1.88) |
| Age at diagnosis, y (col %) | ||||
| <50 | 51 (13.8) | 39 (17.7) | 12 (8.1) | 1.00 |
| 50 to 59 | 148 (40.1) | 104 (47.1) | 44 (29.7) | 0.73 (0.35-1.52) |
| 60 to 65 | 170 (46.1) | 78 (35.3) | 92 (62.2) | 0.26 (0.13-0.53) |
| Hospital type where initial diagnosis was made (col %) | ||||
| University | 184 (53.4) | 118 (53.4) | 66 (44.6) | 1.00 |
| Regional | 127 (34.4) | 67 (30.3) | 60 (40.5) | 0.62 (0.39-0.99) |
| Local | 58 (15.7) | 36 (16.3) | 22 (14.9) | 0.92 (0.50-1.68) |
| Stage (col %) | ||||
| Ann Arbor IB and II | 37 (10.0) | 19 (8.6) | 18 (12.2) | 1.00 |
| Ann Arbor III | 49 (13.3) | 33 (14.9) | 16 (10.8) | 1.95 (0.81-4.70) |
| Ann Arbor IV | 279 (75.6) | 168 (76.0) | 111 (75.0) | 1.43 (0.72-2.85) |
| Missing | 4 (1.1) | 1 (0.5) | 3 (2.0) | |
| MCL-specific international prognostic index (col %) | ||||
| Low risk (<5.7) | 114 (30.9) | 73 (33.0) | 41 (27.7) | 1.00 |
| Intermediate risk (5.7-6.1) | 92 (24.9) | 55 (24.9) | 37 (25.0) | 0.83 (0.47-1.47) |
| High risk (>6.1) | 71 (19.2) | 38 (17.2) | 33 (22.3) | 0.65 (0.35-1.18) |
| Missing | 92 (24.9) | 55 (24.9) | 37 (25.0) | |
| Performance status (col %) | ||||
| ECOG 0 to 1 | 332 (90.0) | 207 (93.7) | 125 (84.5) | 1.00 |
| ECOG 2 to 4 | 31 (8.4) | 12 (5.4) | 19 (12.8) | 0.38 (0.18-0.81) |
| Missing | 6 (1.6) | 2 (0.9) | 4 (2.7) |
Due to rounding, not all percentages add up to 100. Bold indicates significant exposures associated with a lower chance of being treated with AHCT.
col, column.
Distribution of first-line chemotherapy regimens among stage IB+ MCL patients up to 65 y of age at diagnosis in Sweden between January 2000 and June 2014, by selection to treatment with AHCT within 18 mo of diagnosis
| Treatment . | n (col %) . | AHCT ≤18 mo (col %) . | No AHCT ≤18 mo (col %) . |
|---|---|---|---|
| NLG-MCL2/MCL3 protocol | 193 (52.3) | 165 (74.7) | 28 (18.9) |
| R-CHOP alternating with R-cytarabine or R-cytarabine single* | 16 (4.3) | 4 (1.8) | 12 (8.1) |
| R-CHOP/CHOEP/COP* | 38 (10.3) | 5 (2.3) | 33 (22.3) |
| Chlorambucil | 12 (3.3) | 0 (0.0) | 12 (8.1) |
| R-fludarabine/cyclophosphamide* | 4 (1.1) | 0 (0.0) | 4 (1.1) |
| R-bendamustine* | 4 (1.1) | 0 (0.0) | 4 (1.1) |
| Wait and watch | 10 (2.7) | 0 (0.0) | 10 (6.8) |
| Radiotherapy only | 7 (1.9) | 0 (0.0) | 7 (4.7) |
| Other treatment† | 4 (1.1) | — | — |
| Missing | 81 (22.0) | 46 (20.8) | 35 (23.7) |
| Total | 369 (100) | 221 (100) | 148 (100) |
| Treatment . | n (col %) . | AHCT ≤18 mo (col %) . | No AHCT ≤18 mo (col %) . |
|---|---|---|---|
| NLG-MCL2/MCL3 protocol | 193 (52.3) | 165 (74.7) | 28 (18.9) |
| R-CHOP alternating with R-cytarabine or R-cytarabine single* | 16 (4.3) | 4 (1.8) | 12 (8.1) |
| R-CHOP/CHOEP/COP* | 38 (10.3) | 5 (2.3) | 33 (22.3) |
| Chlorambucil | 12 (3.3) | 0 (0.0) | 12 (8.1) |
| R-fludarabine/cyclophosphamide* | 4 (1.1) | 0 (0.0) | 4 (1.1) |
| R-bendamustine* | 4 (1.1) | 0 (0.0) | 4 (1.1) |
| Wait and watch | 10 (2.7) | 0 (0.0) | 10 (6.8) |
| Radiotherapy only | 7 (1.9) | 0 (0.0) | 7 (4.7) |
| Other treatment† | 4 (1.1) | — | — |
| Missing | 81 (22.0) | 46 (20.8) | 35 (23.7) |
| Total | 369 (100) | 221 (100) | 148 (100) |
Due to rounding, not all percentages add up to 100.
CHO(E)P, cyclophosphamide, vincristine, doxorubicin (etoposide), and prednisone; NLG-MCL2/MCL3 protocol Nordic lymphoma group: mantle cell lymphoma 2 and 3 protocol containing dose-intensified cyclophosphamide, vincristine, doxorubicin, and prednisone in combination with rituximab alternating high-dose cytarabine.
12 out of 16 patients received rituximab in the CHOP/cytarabine group; 23 out of 38 in the CHOP/CHOEP/COP group; 1 out of 4 in the fludarabine/cyclophosphamide group, and all patients in the bendamustine group.
Either R-bendamustine alternating with R-cytarabine, or unspecified chemotherapy; detailed frequencies not shown in subgroups due to cell count <4.
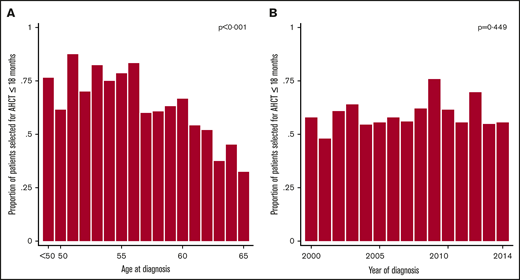
There was no significant increasing trend in the likelihood of being selected to AHCT over calendar year overall (Table 1; Figure 2) nor within age groups (supplemental Figure 2). Based on univariable analyses, a reduced chance of receiving AHCT was seen for divorced patients, widows/widowers, individuals with lower educational level, higher comorbidity burden, old age (Figure 2), diagnosed at a regional hospital, and having a higher ECOG performance status (Table 1).
Probability of being selected for an AHCT within 18 months of diagnosis. Results are presented as a function of age (A) and calendar year (B) among stage IB+ MCL patients up to 65 years of age diagnosed in Sweden between January 2000 and June 2014. Patients aged <50 years at diagnosis were collapsed into 1 age group. P values from test of linear trend.
Probability of being selected for an AHCT within 18 months of diagnosis. Results are presented as a function of age (A) and calendar year (B) among stage IB+ MCL patients up to 65 years of age diagnosed in Sweden between January 2000 and June 2014. Patients aged <50 years at diagnosis were collapsed into 1 age group. P values from test of linear trend.
From multivariable analyses, never married patients and divorced patients had a lower chance of receiving an AHCT than married patients (Table 3). This was also seen for patients with 9 years or less of schooling (compared with 10 to 12 years), and comorbid patients (compared with patients with CCI = 0). Redefining the outcome to AHCT within 12 or 24 months did not alter these conclusions (data not shown).
ORs with 95% CIs of selection to AHCT within 18 mo after diagnosis among stage IB+ MCL patients up to 65 y of age at diagnosis in Sweden between January 2000 and June 2014, by marital status, highest achieved educational level, and comorbidity
| . | Adjusted OR* (95% CI) . |
|---|---|
| Marital status at diagnosis | |
| Married | 1.00 |
| Never married | 0.48 (0.26-0.88) |
| Divorced | 0.50 (0.28-0.89) |
| Widow(er) | 0.33 (0.09-1.16) |
| Highest achieved education level | |
| Up to 9 y of schooling | 0.53 (0.30-0.93) |
| 10 to 12 y of schooling | 1.00 |
| >12 y of schooling | 1.11 (0.65-1.90) |
| Hospital size | |
| University hospital | 1.00 |
| Regional hospital | 0.71 (0.44-1.16) |
| Local hospital | 0.99 (0·52-1.88) |
| CCI | |
| 0 | 1.00 |
| 1 | 0.61 (0.31-1.18) |
| 2+ | 0.35 (0.18-0.70) |
| . | Adjusted OR* (95% CI) . |
|---|---|
| Marital status at diagnosis | |
| Married | 1.00 |
| Never married | 0.48 (0.26-0.88) |
| Divorced | 0.50 (0.28-0.89) |
| Widow(er) | 0.33 (0.09-1.16) |
| Highest achieved education level | |
| Up to 9 y of schooling | 0.53 (0.30-0.93) |
| 10 to 12 y of schooling | 1.00 |
| >12 y of schooling | 1.11 (0.65-1.90) |
| Hospital size | |
| University hospital | 1.00 |
| Regional hospital | 0.71 (0.44-1.16) |
| Local hospital | 0.99 (0·52-1.88) |
| CCI | |
| 0 | 1.00 |
| 1 | 0.61 (0.31-1.18) |
| 2+ | 0.35 (0.18-0.70) |
From separate logistic regression models, adjusted for calendar year of diagnosis (categorized), age at diagnosis (linear), sex, and country of birth.
Survival for patients aged 18 to 65 years who underwent AHCT vs those who did not
In all, 5 patients (2.0%) out of the 221 patients receiving an AHCT, at any time point after MCL diagnosis, died within 100 days (reflecting a low transplantation-related mortality). A total of 145 patients died during the full follow-up, of whom 113 (78%) died because of lymphoma (81%, 82%, and 75% in the age groups <50, 50 to 59, and 60 to 65, respectively). From multivariable Cox regression analyses, being selected for an AHCT was associated with a reduction in all-cause mortality (HRall = 0.58; 95% CI: 0.40-0.85). There was no evidence of effect modification by age (P > .05), indicating that the effect of AHCT was similar between the different age groups (Table 4). In an unadjusted analysis, receiving cytarabine significantly reduced the all-cause mortality rate by 47%. However, the protective effect seen for AHCT on all-cause mortality was not altered when adding cytarabine (yes/no) to the fully adjusted multivariable model in Table 4. Moreover, based on that model, cytarabine no longer had a significant effect on mortality (HR = 1.01; 95% CI: 0.56-1.80). When excluding patients treated with chlorambucil, watch and wait, and radiotherapy alone from the adjusted model in Table 4 (n = 29 patients excluded), the results for all ages combined were HR = 0.48 (95% CI: 0.32-0.71) (ie, a stronger protective effect of AHCT on OS also when patients treated with intensive regimens only were included in the non-AHCT group). This indicated that AHCT additionally improved prognosis when compared with patients treated with intensive induction regimens but without an AHCT.
HRs with 95% CIs comparing all-cause mortality between AHCT and non-AHCT treated stage IB+ MCL patients diagnosed between January 2000 and June 2014 aged up to 65 y at diagnosis, who were alive 6 mo after diagnosis when follow-up started (n = 363)
| . | . | HR* (95% CI) . | HR† (95% CI) . |
|---|---|---|---|
| Age at diagnosis . | AHCT . | (n = 363) . | (n = 342) . |
| All ages‡ | Yes | 0.45 (0.32-0.63) | 0.58 (0.40-0.85) |
| No | 1.00 | 1.00 | |
| ≤49 y | Yes | 0.43 (0.14-1.35) | 0.38 (0.12-1.19) |
| No | 1.00 | 1.00 | |
| 50 to 59 y | Yes | 0.47 (0.26-0.86) | 0.55 (0.29-1.02) |
| No | 1.00 | 1.00 | |
| 60 to 65 y | Yes | 0.53 (0.34-0.84) | 0.70 (0.43-1.14) |
| No | 1.00 | 1.00 |
| . | . | HR* (95% CI) . | HR† (95% CI) . |
|---|---|---|---|
| Age at diagnosis . | AHCT . | (n = 363) . | (n = 342) . |
| All ages‡ | Yes | 0.45 (0.32-0.63) | 0.58 (0.40-0.85) |
| No | 1.00 | 1.00 | |
| ≤49 y | Yes | 0.43 (0.14-1.35) | 0.38 (0.12-1.19) |
| No | 1.00 | 1.00 | |
| 50 to 59 y | Yes | 0.47 (0.26-0.86) | 0.55 (0.29-1.02) |
| No | 1.00 | 1.00 | |
| 60 to 65 y | Yes | 0.53 (0.34-0.84) | 0.70 (0.43-1.14) |
| No | 1.00 | 1.00 |
Follow-up was restricted to the first 10 y after diagnosis.
From a Cox proportional hazards model treating AHCT as a time-varying exposure (for age-stratified results, an interaction between AHCT and age at diagnosis was included).
From a Cox proportional hazards model as above, adjusted for calendar year of diagnosis (as a restricted cubic spline), sex, civil status, educational level, CCI, stage, and performance status.
A nonsignificant interaction with AHCT and age was seen, indicating that the association between AHCT and all-cause mortality was similar between age groups.
The OS experienced by patients who underwent transplantation as a function of time since transplantation is shown in supplemental Figure 3. Further elaborating on death and selection in the illness-death model showed that the proportion of patients dying before an AHCT was performed increased with increasing age (Figure 3). Among patients having undergone an AHCT, the proportion of patients dying after AHCT was similar across age groups. The 10-year OS in each age group (illustrated by the black dashed line in Figure 3) was 57%, 52%, and 32% aged ≤49, 50 to 59, and 60 to 65 years at diagnosis, respectively.
The probability of being alive after MCL diagnosis. The probability of being alive without having had an AHCT (blue field), alive and having had an AHCT (red field), dead without previous AHCT (light green field), and dead after AHCT (dark green field). The results are stratified by age, as a function of time since diagnosis, among stage IB+ MCL patients diagnosed between January 2000 and June 2014 aged up to 65 years at diagnosis, who were alive 6 months after diagnosis when follow-up started (n = 363). Follow-up was restricted to the first 10 years after diagnosis. The overall survival in each age group is the sum of the blue and red fields (indicated by the black dashed line). The 10-year overall survival was 57% among patients ≤49 years, 52% in those 50 to 59 years, and 32% in those 60 to 65 years at diagnosis. Pointwise probabilities, with 95% CIs, of being in each of the states are shown in supplemental Table 1.
The probability of being alive after MCL diagnosis. The probability of being alive without having had an AHCT (blue field), alive and having had an AHCT (red field), dead without previous AHCT (light green field), and dead after AHCT (dark green field). The results are stratified by age, as a function of time since diagnosis, among stage IB+ MCL patients diagnosed between January 2000 and June 2014 aged up to 65 years at diagnosis, who were alive 6 months after diagnosis when follow-up started (n = 363). Follow-up was restricted to the first 10 years after diagnosis. The overall survival in each age group is the sum of the blue and red fields (indicated by the black dashed line). The 10-year overall survival was 57% among patients ≤49 years, 52% in those 50 to 59 years, and 32% in those 60 to 65 years at diagnosis. Pointwise probabilities, with 95% CIs, of being in each of the states are shown in supplemental Table 1.
The probability of being selected to an AHCT and remaining alive was highest, both short and long term, in the 2 youngest age groups (≤49 years: 1-year probability = 0.44, 95% CI: 0.28-0.60, 10-year probability = 0.47, 95% CI: 0.28-0.62, and 50 to 59 years: 1-year probability = 0.46, 95% CI: 0.36-0.57, 10-year probability = 0.43, 95% CI: 0.32-0.53). For older patients, the corresponding probabilities remained low over follow-up time (Figure 3; supplemental Table 1).
Survival for patients aged 66 to 70 years who underwent AHCT vs those who did not
For completeness, an additional 216 patients aged 66 to 70 are presented in supplemental Table 2. Among these, only 38 (17.6%) received a transplant. Among this highly selected group of elderly patients, no one died within 100 days of the AHCT. The all-cause mortality rate was lower among those undergoing an AHCT compared with non–AHCT-treated patients.
Survival by civil status and educational level
Based on unadjusted models on all patients, being a widow(er) increased the all-cause mortality rate (HR = 2.76, P = .01) compared with married as did having a lower education level (HR = 1.57, P = .018). We performed the same analyses on only the 248 patients who underwent transplantation (with risk time starting at time of AHCT), showing similar, albeit slightly weaker and not significant, results: widow(er) HR = 2.76 (P = .43) and lower educational level HR = 1.16 (P = .28).
Discussion
A surprisingly high proportion of younger (<65 years) MCL patients (40%) were not treated with AHCT as part of their primary treatment. Factors associated with a lower likelihood of being selected to an AHCT included higher age, being never married or divorced, having any comorbidity, and lower socioeconomic status. We also noted survival disadvantages for non–AHCT-treated patients, implying that these selection mechanisms may have prognostic implications. This calls for the need of a more informed decision making in relation to not only the patients’ health prior to AHCT but also their sociodemographic situation. In case an AHCT is deemed impossible, integration of novel treatment concepts (eg, tyrosine kinase inhibitors and bcl-2 inhibitors) instead or in combination with standard treatment is needed.
Although several review articles have discussed which MCL patients should be treated with transplantation, these have mostly focused on comorbidity burden and tumor biological characteristics.9-11 Studies directly evaluating other types of selection mechanisms to AHCT are rare. One study found clinical decisions to be the most common reason for not transplanting (67%), but which factors the clinicians based their decision on were not reported.4 It is possible that social factors could have influenced the decisions, as well as comorbidity burden. Marital status is likely an indication of the extent of social support. We can only speculate that less social support may lead to both an unwillingness from the patients and/or a fear from the treating physicians to administer very demanding treatment. Taken together, selecting a treatment regimen not including an AHCT for a frail patient or a patient with comorbidities may indeed be the most appropriate management, whereas other factors may not be as well motivated. These groups of patients should be considered for an AHCT (given that they do not present with comorbidities hindering a transplantation) or be offered other potentially novel treatment alternatives.
In Sweden, all health care is governmentally funded, and thus, lower educational level and lower income should not lead to a lower access to specialized care. Instead, our finding of an association between lower educational level and less likelihood of being selected to a transplant could imply that there is room for improvement in the communication and information around treatment risks across all societal groups. Patient organizations in Sweden also advocate for more patient involvement in treatment decisions (www.blodcancerforbundet.se).
Retrospective studies and clinical trials investigating the prognosis for patients given a consolidative AHCT compared with those not receiving AHCT4,19-23 show better PFS in patients receiving AHCT but no clear OS benefit. This study provides support for a benefit in OS, at least up to the age of 65 years. One randomized trial has been performed,5 in the prerituximab era, showing a better PFS in AHCT-treated patients. We are eagerly awaiting the results of the currently ongoing randomized trial TRIANGLE, designed for patients <65 years, with the aim of evaluating omission of AHCT by adding novel agents to standard induction treatment. In the age span 60 to 65 years, we observe that approximately half of patients died without going through an AHCT after 10 years. To not select patients in this age span to CHOP/cytarabine and AHCT up front might make them too old/frail for a transplantation if a relapse occurs a few years later. The induction treatments in the nontransplant group were very diverse, and to give a less intense treatment, only temporarily decreasing the tumor burden, might not be beneficial in the long run. However, the survival advantages with an AHCT remained also when adjusting for the presence or absence of cytarabine treatment, and when all less intensively treated patients in the comparison groups were excluded, indicating that an AHCT additionally improves survival also when compared with patients treated with intensive induction regimens but without AHCT. The type of induction chemotherapy prior to the transplantation has been shown not to influence survival,24 potentially strengthening the reasoning for the benefit of the transplantation itself. The conditioning regimens has in most cases in this cohort been with BCNU, etoposide, cytarabine, and melphalan (BEAM)/BCNU, etoposide, cytarabine, and cyclophosphamide (BEAC). Total body irradiation as conditioning regimen has lately been associated with superior prognosis.25 The improvement of the conditioning regimen prior to an AHCT further strengthens the results with an OS benefit with an AHCT (being even more efficient today than when the patients in this cohort were treated).
On an individual level, we do not know whether the patients in this cohort who did not receive a transplant could have been safely selected for an AHCT. It is, however, known that the majority of MCL patients die of their lymphoma,1 underscoring that MCL itself is in general more life-threatening over time than the comorbidity burden, and prompting more efficient treatment options. The transplantation-related mortality was low (2.0%), highlighting the safety of this treatment at a population level among those selected to it.
A French study of non-Hodgkin lymphoma patients (≥70 years, range: 70 to 80, n = 81, including 15 with MCL) showed that a transplantation was acceptable in the absence of comorbidity (73% of included patients had no comorbidities),26 and 1 other group reported similar findings.27 In the early 2000s, guidelines for MCL in Sweden recommended an upper age limit of 65 years for AHCT consolidation, which was abandoned gradually. During later years, patients >65 years of age could be given a transplantation. Among the 38 patients aged 66 to 70 in our data who received an AHCT, both overall mortality rate and treatment-related mortality were low, indicating the safety of this regimen. Altogether, this raises the question if there is undue concern about the toxicity of AHCT in MCL patients.
One strength of our study is the generalizability of the national population-based findings, compared with selected cohorts in randomized trials. Although randomized trials have clear advantages when evaluating different treatments, our results imply that many patients would be deemed ineligible for inclusion in trials having transplantation in 1 study arm (such as TRIANGLE), because an inclusion criterion will always be “fit for transplantation.” Thus, when investigating selection mechanisms as we have done, all patients within a population (also those being unfit and ineligible for trials) need to be included. We did not have information on failure of mobilization of stem cells. This is rare and usually is overcome with novel drugs/techniques. Although the register-based setting allows for a comparison in an unselected group of patients, information on patients’ opinion for not receiving a transplant was not available, and we could not capture milder comorbidities in nonspecialized outpatient care.15 All comparisons between treatments based on observational data should be interpreted as a combination of the selection process and the efficacy of treatment. With this in mind, real-world evidence on the selection to treatment and outcome thereafter can still provide insight and knowledge that would never be accomplished in a randomized trial setting.
The lack of TP53 mutational status and Ki67 expression of included patients prevented us from stratification by biological characteristics. However, the cohort covered the era prior to broad introduction of novel drugs, such as BTK inhibitors, and prior to introduction of novel disease-monitoring tools outside of clinical trials and hence should not have affected the results. Biomarkers should be brought into decision making in the future; for example, TP53 mutations identify younger MCL patients who do not benefit from intensive chemoimmunotherapy.28 In addition, minimal residual disease evaluations after induction chemotherapy and prior to transplantation impact survival,29 as PET status does,30 and will likely be brought into decision making. Our study shows that we also should not overlook the patient’s demographics in our decision-making process.
Conclusions
We were able to identify groups of patients (those who were never married, those with fewer years of schooling, and those with higher comorbidity burden) where an AHCT was seldom provided. This had prognostic implications, and it calls for improvements in both supportive functions and clinicians' awareness about their decisions to potentially make more patients eligible for an AHCT. For frail patients, our results also call for introduction of alternative, more tolerable treatments for a large fraction of MCL patients, which in turn may improve survival.
Presented in abstract form at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7-10 December 2019.
The data are a result of linkages of several nationwide registers as described in “Materials and methods.” The data are available upon reasonable request from the corresponding author, Ingrid Glimelius, at ingrid.glimelius@igp.uu.se.
Acknowledgments
This study was supported by the Swedish Cancer Society CAN (2012/774 and 19 0109 SCIA) and a public-private real-world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals NV (contract 5-63/2015), The Swedish Cancer Society (19 0123 Pj 01 H) and the Swedish Society of Medicine (I.G.), and the United Kingdom’s Medical Research Council Methodology Research Panel (MR/P015433/1) (M.C.).
Janssen Pharmaceuticals NV approved submission of the final version of the manuscript.
The different sponsors had no role in data collection, analysis, and interpretation of data or writing of the report.
Authorship
Contribution: I.G., K.E.S., A.A.-L., M.J.C., S.E., M.J., and C.E.W. designed the study; C.E.W. and M.J.C. provided the statistical analysis and figures; I.G. drafted the manuscript; I.G., K.E.S., and M.J. collected the data and verified the underlying data; and I.G., K.E.S., A.A.-L., M.J.C., S.E., M.J., and C.E.W. critically reviewed the manuscript.
Conflict-of-interest disclosure: M.J. has received honoraria from Janssen, Gilead, Celgene, Roche, and Acerta and research support from Janssen, Roche, Celgene, AbbVie, and Gilead. K.E.S. has received honoraria from Celgene and research support from Janssen. I.G. has received honoraria from Janssen. S.E. has an ongoing role as project manager in a public-private real-world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals NV. The remaining authors declare no competing financial interests.
Correspondence: Ingrid Glimelius, Uppsala University Hospital, Oncology Clinic, Entrance 78, 751 85 Uppsala, Sweden; e-mail: ingrid.glimelius@igp.uu.se.
References
Author notes
The full-text version of this article contains a data supplement.