Key Points
FA patients given haplo-HSCT after TCRαβ+ and B-cell depletion benefit from high engraftment rate and low incidence of GVHD.
Overall and disease-free survival of 100% were achieved; only 3 patients required further interventions (ie, subsequent HSCT or HSC boost).
Abstract
We report on the outcome of 24 patients with Fanconi anemia (FA) lacking an HLA matched related or unrelated donor, given an HLA-haploidentical T-cell receptor αβ (TCRαβ+) and CD19+ cell-depleted hematopoietic stem cell transplantation (HSCT) in the context of a prospective, single-center phase 2 trial. Sustained primary engraftment was achieved in 22 (91.6%) of 24 patients, with median time to neutrophil recovery of 12 days (range, 9-15 days) and platelet recovery of 10 days (range, 7-14 days). Cumulative incidences of grade 1 to 2 acute graft-versus-host disease (GVHD) and chronic GVHD were 17.4% (95% confidence interval [CI], 5.5%-35.5%) and 5.5% (95% CI, 0.8%-33.4%), respectively. The conditioning regimen, which included fludarabine, low-dose cyclophosphamide and, in most patients, single-dose irradiation was well tolerated; no fatal transplant-related toxicity was observed. With a median follow-up of 5.2 years (range, 0.3-8.7 years), the overall and event-free survival probabilities were 100% and 86.3% (95% CI, 62.8%-95.4%), respectively (2 graft failures and 1 case of poor graft function were considered as events). The 2 patients who experienced primary graft failure underwent a subsequent successful HSCT from the other parent. This is the first report of FA patients given TCRαβ+/CD19+-depleted haplo-HSCT in the context of a prospective trial, and the largest series of T-cell–depleted haplo-HSCT in FA reported to date. This trial was registered at www.clinicaltrials.gov as #NCT01810120.
Introduction
Hematopoietic stem cell transplantation (HSCT) currently represents the only option which is potentially able to rescue bone marrow failure, as well as to prevent/treat clonal hematopoietic disorders associated with Fanconi anemia (FA). Best results have historically been reported after HSCT from HLA-matched sibling donors (MSDs),1-3 even though recent advances in donor selection, choice of conditioning regimen, pharmacologic graft-versus-host disease (GVHD) prophylaxis, and graft manipulation techniques have remarkably improved outcomes of alternative donor HSCT.4
Many FA patients lack a suitable unaffected MSD, and the length of research for a matched unrelated donor (MUD) may expose them to disease progression or clonal evolution. Moreover, a relevant proportion of patients lack a suitable MUD. HSCT from an HLA-haploidentical relative (haplo-HSCT) represents an immediately available option for virtually all patients and has been reported as an effective approach in FA.5
The infusion of megadoses of positively selected CD34+ cells aimed at overcoming the major drawbacks of haplo-HSCT (ie, severe GVHD and graft failure [GF]) results in prolonged posttransplant lymphopenia and delayed immune reconstitution, which increase the risk of transplant-related mortality due to opportunistic infections.6 To overcome this limitation, our group and others developed a graft manipulation technique based on selective depletion of T-cell receptor αβ (TCRαβ+)/CD19+ lymphocytes.7,8 The present report describes the outcomes of 24 FA patients given a TCRαβ+/CD19+-cell–depleted haplo-HSCT in the context of a prospective, single-center phase 2 trial.
Methods
This analysis involved a subset of FA patients treated in the context of a phase 2 study of allogeneic HSCT (allo-HSCT) from an HLA-partially matched family donor after negative depletion of TCRαβ+ and CD19+ cells in pediatric patients affected by hematologic disorders. Patients diagnosed with FA aged 3 months or older and younger than age 21 years, eligible for an allogeneic transplantation, lacking a related or unrelated HLA-matched donor, and with a suitable HLA-haploidentical donor, with a Lansky/Karnofsky score >40, were enrolled.
Exclusion criteria included: patients given a previous allograft and experiencing grade >2 acute GVHD (aGVHD) or extensive chronic GVHD (cGVHD) at the time of inclusion, patients receiving an immunosuppressive GVHD treatment at the time of inclusion, patients with liver dysfunction (alanine aminotransferase/aspartate aminotransferase >5 times normal value or bilirubin >3 times normal value), alteration of renal function (creatinine clearance <30 mL/minute), severe cardiovascular disease (arrhythmias requiring chronic treatment, congestive heart failure, or left ventricular ejection fraction <40%), active infectious disease, serious concurrent uncontrolled medical disorder, pregnant or breastfeeding female patients, or patients lacking informed consent. The trial was approved by the local Ethical Committee of Bambino Gesù Children’s Hospital and was conducted according to the Declaration of Helsinki. All patients or parents/legal guardians provided written informed consent.
FA was confirmed with a diepoxybutane-induced chromosome breakage assay; molecular diagnosis was available for 17 patients. Indications for HSCT were severe bone marrow failure resulting in transfusion-dependent anemia or thrombocytopenia, severe neutropenia, or evidence of clonal evolution. In all patients and donors, high-resolution molecular HLA typing was performed to characterize HLA class I and II loci. Donors were first-degree relatives sharing 1 HLA haplotype with the patient. The choice of donor was based mainly on the alloreactivity of the donor toward the recipient according to the killer inhibitory receptor (KIR)-KIR-ligand model,9 cytomegalovirus serology in the donor-recipient pair, and the presence of KIR haplotype B in the donor.10,11
The conditioning regimen included intravenous fludarabine 30 mg/m2 per day and cyclophosphamide 300 mg/m2 per day for 4 days (days –6 to −3); 200 cGy single-dose total body irradiation (TBI) on day −2 was added in 21 patients. In 2 patients, parents who were concerned about the risk of secondary radiation-induced malignancies refused the use of TBI. A third patient, a 22-month-old toddler, was not given TBI considering the potential neurocognitive late effects associated with radiation in a developing central nervous system.
Pretransplantation anti-T-lymphocyte globulin (Grafalon; Neovii Biotech) 4 mg/kg per day was administered for 3 days (days –4 to −2) to control in vivo bidirectional donor-recipient alloreactivity. Rituximab (200 mg/m2) was administered on day –1 to reduce the risk of Epstein-Barr virus–related posttransplant lymphoproliferative disorders. No posttransplantation pharmacologic immunosuppressive therapy was administered. Mobilization, apheresis, and graft manipulation were performed as previously described.7,12-14 In brief, donor CD34+ hematopoietic progenitors were mobilized by subcutaneous administration of granulocyte colony-stimulating factor 12 µg/kg per day from day −5 until leukapheresis (day −1).
If the cutoff of ≥40 CD34+ cells per μL was not achieved and/or there was a predicted apheresis yield ≤12 × 106 CD34+ cells per kg of the recipient’s body weight, plerixafor (Mozobil, Genzyme) was given at a dose of 0.24 mg/kg to facilitate the collection of a megadose of hematopoietic stem and progenitor cells. Apheresis was performed with the Spectra Optia Cell Separator (Terumo BCT, Leuven, Belgium). Graft manipulation procedures were performed with the fully automated CliniMACS device (Miltenyi Biotec, Bergisch Gladbach, Germany), which was located in a clean room certified for sterile manipulations. Clinical grade reagents, disposable kits, and instrumentation were also from Miltenyi Biotec.
All patients received antiviral prophylaxis with acyclovir and antifungal prophylaxis with agents active on both yeast and molds (ie, liposomal amphotericin B or caspofungin) and prophylaxis against Pneumocystis jirovecii pneumonia with cotrimoxazole.
Posttransplantation monitoring of donor-recipient chimerism, assessed by using a quantitative polymerase chain reaction assay of short tandem repeat markers in DNA extracted from peripheral blood unfractionated cells, was performed once per week from the day of engraftment to day 100 and once per month thereafter. Bone marrow chimerism was assessed only at the time of central venous line removal (at day +100 after the allograft). Immune recovery (count of TCRαβ+, TCRγδ+, CD4+ [CD45RA+, CD45RO+], CD8+ [CD45RA+, CD45RO+], natural killer [NK, CD3–CD56+], and B cells (CD19+) was investigated at 1, 3, 6, and 12 months after transplantation. We also measured the serum levels of immunoglobulin A (IgA) and IgM at 3, 6, and 12 months after the allograft.
End points for survival analyses were overall survival (OS), defined as the probability of survival from the time of HSCT to death or last follow-up, event-free survival (EFS), defined as the probability of survival from the time of HSCT to the occurrence of any event (graft failure, poor graft function, or death as a result of any cause, whichever occurred first) or the date of last follow-up, and disease-free survival (DFS), defined as the probability of survival without evidence of disease from the time of HSCT to death or last follow-up. Other end points included cumulative incidence of neutrophil and platelet engraftment; median time to neutrophil and platelet engraftment, defined as time from HSCT to the first of 3 consecutive days with an absolute neutrophil count ≥0.5 × 109/L and to the first of 7 consecutive days with an unsupported platelet count ≥20 × 109/L, respectively; cumulative incidence of aGVHD and cGVHD; and regimen-related toxicity. Lack of engraftment or transient engraftment followed by peripheral blood count decline, with pancytopenia, absence of detectable donor cells in the recipient blood, or autologous hematopoietic reconstitution was considered as graft failure.
Grade of aGVHD and cGVHD was assessed according to Glucksberg criteria15 and National Institutes of Health criteria, respectively.16 Regimen-related toxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Continuous variables were described as median values and ranges, and categorical variables were expressed as absolute values and ranges. OS and EFS were estimated by using the Kaplan-Meier method. aGVHD and cGVHD were expressed as cumulative incidences to adjust the estimations for competing risks (eg, death).17,18 Patients were censored at time of last follow-up.
Statistical analysis was performed using EZR version 1.32 (Saitama Medical Centre, Jichi Medical University),19 which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
Results and discussion
Twenty-four patients (13 males and 11 females) underwent haplo-HSCT between January 2012 and July 2020. Four patients have been previously reported.7 Details on patient, donor, and transplantation characteristics are summarized in Table 1. One patient had refractory cytopenia with multilineage dysplasia. In that patient, cytogenetic analysis of bone marrow revealed monosomy 7 and the presence of additional material of unknown origin replacing part of chromosome 11 (karyotype according to International System for Human Cytogenomic Nomenclature: 46,XX,add(11)(q14)[9]/45,idem,-7[4]/46,XX[2]. Nunc ish cen7(CEP7x1)[17/100],11q23(MLLx1) [74/100]). Array-based comparative genomic hybridization showed a deletion of chromosome 11q, a duplication of chromosome 16p, and a duplication of chromosome 21q [arr 11q14.1q25(80,368,986-134,868,407)x1, 16p13.3p12.2(1,940,455-21,285,078)x3,21q22.12q22.3(36,077,830-48,021,818)x3].
Characteristics of patients, donors, and transplantation procedures
| . | Number or median . | Percentage or range . |
|---|---|---|
| Patients | ||
| Total | 24 | 100 |
| Males | 13 | 54 |
| Females | 11 | 46 |
| Median age, y | ||
| At diagnosis | 6.2 | 0.3-20.7 |
| At HSCT | 8.6 | 1.8-20.9 |
| Median time from diagnosis to HSCT, mo | 19.5 | 1.0-84.9 |
| FANCgene alterations | ||
| FANCA | 14 | 58.3 |
| FANCG | 2 | 8.3 |
| FANCC | 1 | 4.2 |
| Unknown | 7 | 29.2 |
| Disease status before HSCT | ||
| Severe bone marrow failure* | 23 | 96 |
| Clonal evolution | 1 | 4 |
| Clinical phenotype | ||
| Skin manifestations | 22 | 91.6 |
| Growth retardation | 22 | 91.6 |
| Thumb or radial abnormalities | 5 | 20.8 |
| Microphthalmia | 19 | 79.2 |
| Other skeletal abnormalities | 2 | 8.3 |
| Renal and urinary tract malformations | 10 | 41.6 |
| Ear abnormalities | 3 | 12.5 |
| Congenital heart disease | 6 | 25 |
| Gastrointestinal anomalies | 1 | 4.2 |
| Previous treatment | ||
| Immunosuppressive therapy† | 2 | 8.3 |
| Androgens | 0 | 0 |
| G-CSF | 2 | 8.3 |
| Regular transfusions‡ | 20 | 83.3 |
| Donor | ||
| Father | 8 | 33 |
| Mother | 15 | 63 |
| Brother | 1 | 4 |
| Median donor age, y | 38 | 21-52 |
| Sex mismatch, donor → recipient | ||
| Female → male | 8 | 33 |
| Male → female | 4 | 17 |
| Other combinations | 12 | 50 |
| Donor/recipient HLA-match | ||
| 5/10 | 17 | 71 |
| 6/10 | 5 | 21 |
| 7/10 | 2 | 8 |
| Cytomegalovirus status for donor/recipient | ||
| Negative/negative | 3 | 12.5 |
| Positive/negative | 6 | 25.0 |
| Positive/positive | 15 | 62.5 |
| Transplantation procedure | ||
| Conditioning regimen | ||
| Fludarabine + cyclophosphamide + TBI (single 200 cGy dose) | 21 | 87.5 |
| Fludarabine + cyclophosphamide | 3 | 12.5 |
| Median cell dose infused × 106 per kg | ||
| CD34+ | 17.75 | 11.0-28.3 |
| TCRαβ+CD3+ | 0.02 | 0.00-0.098 |
| TCRγδ+CD3+ | 8.98 | 1.60-95.60 |
| NK cells | 51.86 | 10.24-248.79 |
| CD20+ | 0.03 | 0.00-0.10 |
| . | Number or median . | Percentage or range . |
|---|---|---|
| Patients | ||
| Total | 24 | 100 |
| Males | 13 | 54 |
| Females | 11 | 46 |
| Median age, y | ||
| At diagnosis | 6.2 | 0.3-20.7 |
| At HSCT | 8.6 | 1.8-20.9 |
| Median time from diagnosis to HSCT, mo | 19.5 | 1.0-84.9 |
| FANCgene alterations | ||
| FANCA | 14 | 58.3 |
| FANCG | 2 | 8.3 |
| FANCC | 1 | 4.2 |
| Unknown | 7 | 29.2 |
| Disease status before HSCT | ||
| Severe bone marrow failure* | 23 | 96 |
| Clonal evolution | 1 | 4 |
| Clinical phenotype | ||
| Skin manifestations | 22 | 91.6 |
| Growth retardation | 22 | 91.6 |
| Thumb or radial abnormalities | 5 | 20.8 |
| Microphthalmia | 19 | 79.2 |
| Other skeletal abnormalities | 2 | 8.3 |
| Renal and urinary tract malformations | 10 | 41.6 |
| Ear abnormalities | 3 | 12.5 |
| Congenital heart disease | 6 | 25 |
| Gastrointestinal anomalies | 1 | 4.2 |
| Previous treatment | ||
| Immunosuppressive therapy† | 2 | 8.3 |
| Androgens | 0 | 0 |
| G-CSF | 2 | 8.3 |
| Regular transfusions‡ | 20 | 83.3 |
| Donor | ||
| Father | 8 | 33 |
| Mother | 15 | 63 |
| Brother | 1 | 4 |
| Median donor age, y | 38 | 21-52 |
| Sex mismatch, donor → recipient | ||
| Female → male | 8 | 33 |
| Male → female | 4 | 17 |
| Other combinations | 12 | 50 |
| Donor/recipient HLA-match | ||
| 5/10 | 17 | 71 |
| 6/10 | 5 | 21 |
| 7/10 | 2 | 8 |
| Cytomegalovirus status for donor/recipient | ||
| Negative/negative | 3 | 12.5 |
| Positive/negative | 6 | 25.0 |
| Positive/positive | 15 | 62.5 |
| Transplantation procedure | ||
| Conditioning regimen | ||
| Fludarabine + cyclophosphamide + TBI (single 200 cGy dose) | 21 | 87.5 |
| Fludarabine + cyclophosphamide | 3 | 12.5 |
| Median cell dose infused × 106 per kg | ||
| CD34+ | 17.75 | 11.0-28.3 |
| TCRαβ+CD3+ | 0.02 | 0.00-0.098 |
| TCRγδ+CD3+ | 8.98 | 1.60-95.60 |
| NK cells | 51.86 | 10.24-248.79 |
| CD20+ | 0.03 | 0.00-0.10 |
G-CSF, granulocyte colony-stimulating factor.
At least 2 of the following criteria: absolute neutrophil count <0.5 × 109/L, platelet count <30 × 109/L, hemoglobin levels <8.0 g/dL.
Immunosuppressive therapy: antithymocyte globulin, cyclosporine, and steroids.
Regular transfusions aimed at keeping the platelet count above the threshold of 20 × 109/L and the hemoglobin level above 9 g/dL.
The girl was induced into remission with a sequential strategy that included pretransplant chemotherapy (fludarabine 30 mg/m2 per day plus cytarabine 1 g/m2 twice per day for 5 days) followed by HSCT, as previously described.20 In all patients, the conditioning regimen was well tolerated, with no cases of grade 4 toxicity. Grade 3 extrahematologic toxicity was observed in 18 patients (75%) who experienced 19 events. The most common toxicities included oral mucositis in 15 patients, gastrointestinal toxicity in 2 patients, and hepatotoxicity characterized by an increase in serum transaminase levels in 2 patients.
Engraftment was achieved in 22 patients (91.6%), with median time for neutrophil and platelet recovery of 12 days (range, 9-15 days) and 10 days (range, 7-14 days), respectively. Monitoring of donor-recipient chimerism confirmed stable engraftment of donor hematopoiesis in all 22 patients. One patient, who was not given TBI during conditioning, experienced poor graft function 5 months after HSCT, which resolved after donor CD34+-selected HSC boost. Apparently, no factor triggered the development of poor graft function. No preparation was administered to this patient before the HSC boost infusion.
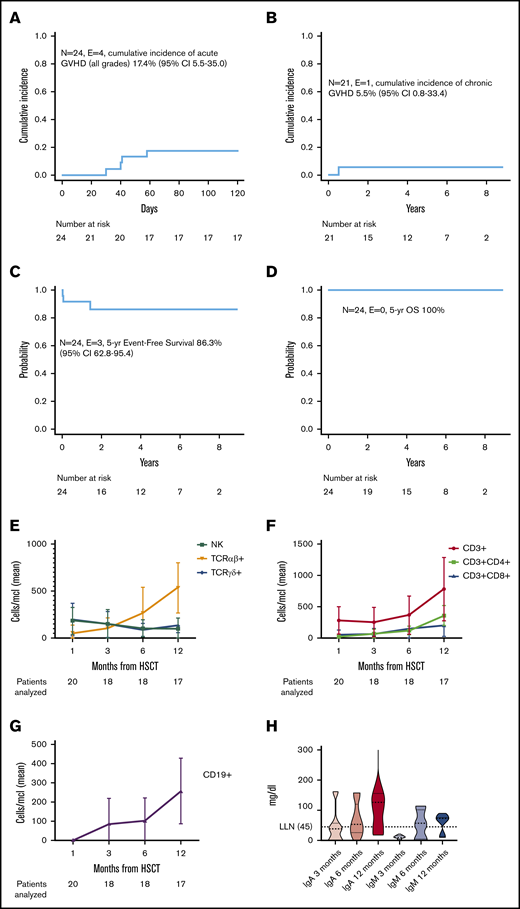
Primary GF occurred in 1 patient on day 11 and in another patient on day 18 after HSCT. The number of CD34+ cells infused in these 2 patients was 22.74 × 106 per kg and 18.74 × 106 per kg of recipient body weight, respectively. Neither the cell dose infused nor the number of pretransplant transfusions differed between these 2 children and those of other patients who had sustained engraftment of donor cells. Both patients were rescued through a second allograft from the other haploidentical parent after a conditioning regimen that included low-dose cyclophosphamide, fludarabine, and cytarabine. Four patients experienced grade 1 to 2 aGVHD (3 patients with stage I to II skin-only aGVHD, one with stage I lower gastrointestinal aGVHD) at a median time of 41 days (range, 30-58 days) from HSCT. The patient with aGVHD and gastrointestinal involvement underwent upper gastrointestinal endoscopy and colonoscopy with multiple biopsies that confirmed the clinical diagnosis. Of the 22 patients at risk, only 1 developed mild (skin-only) cGVHD preceded by aGVHD. The cumulative incidence for aGVHD and cGVHD was 17.4% (95% confidence interval [CI], 5.5%-35.5%) and 5.5% (95% CI, 0.8%-33.4%), respectively (Figure 1A-B).
Outcome and immune reconstitution of patients enrolled in the study. Cumulative incidence of (A) aGVHD and (B) cGVHD in the study population. Probability of (C) EFS and (D) OS. (E) Reconstitution kinetics of αβ+, γδ+ T cells and NK cells in patients receiving TCRαβ+/CD19+-depleted haplo-HSCT. A rapid recovery of γδ+ T and NK cells was observed within the first few weeks posttransplant followed by a progressive increase of αβ+ T and B lymphocytes over time. Reconstitution kinetics of (F) CD4+ and CD8+ T cells and (G) B lymphocytes. (H) Kinetics of immunoglobulin serum level recovery: median (dashed thick line), 25th and 75th percentiles (dotted thin lines), and ranges are represented in violin plots. Immunoglobulin recovery was observed in parallel to B-cell reconstitution. Peripheral blood samples were obtained 1, 3, 6, and 12 months after HSCT. Absolute numbers of each cell subset are shown together with standard error of the mean. E, events; LNN, lower limit of normal; N, number; yr, year.
Outcome and immune reconstitution of patients enrolled in the study. Cumulative incidence of (A) aGVHD and (B) cGVHD in the study population. Probability of (C) EFS and (D) OS. (E) Reconstitution kinetics of αβ+, γδ+ T cells and NK cells in patients receiving TCRαβ+/CD19+-depleted haplo-HSCT. A rapid recovery of γδ+ T and NK cells was observed within the first few weeks posttransplant followed by a progressive increase of αβ+ T and B lymphocytes over time. Reconstitution kinetics of (F) CD4+ and CD8+ T cells and (G) B lymphocytes. (H) Kinetics of immunoglobulin serum level recovery: median (dashed thick line), 25th and 75th percentiles (dotted thin lines), and ranges are represented in violin plots. Immunoglobulin recovery was observed in parallel to B-cell reconstitution. Peripheral blood samples were obtained 1, 3, 6, and 12 months after HSCT. Absolute numbers of each cell subset are shown together with standard error of the mean. E, events; LNN, lower limit of normal; N, number; yr, year.
All patients experiencing aGVHD responded to either topical or systemic steroids, and the child with cGVHD was successfully treated with extracorporeal photochemotherapy. Eleven patients experienced 1 or more viral reactivations or infections (6 cytomegalovirus, 4 herpesvirus-6, 2 adenovirus) at a median time of 30 days (range, 20-69 days) from HSCT, the cumulative incidence for this complication being 45.8% (95% CI, 21.7%-62.5%). All infectious episodes resolved with the appropriate antiviral treatment (ganciclovir for cytomegalovirus and herpesvirus-6 and cidofovir for adenoviral infections) without clinical complications. Neither fungal infections nor Epstein-Barr virus–related posttransplant lymphoproliferative disorders were recorded.
The patient who received a transplant after being treated for refractory cytopenia with multilineage dysplasia, a girl carrying a homozygous FANCA mutation, developed a grade 2 vulvar intraepithelial neoplasia 20 months after HSCT but was successfully treated. No other posttransplant malignancies were observed.
With a median follow-up of 5.2 years (range, 0.3-8.7 years), all patients are alive. The Kaplan-Meier estimate of EFS was 86.3% (95% CI, 62.8%-95.4%) (2 GFs and 1 poor graft function were considered as events) (Figure 1C-D). The 2 patients who experienced GF underwent a subsequent successful haplo-HSCT, leading to 100% DFS. Of the lymphoid subsets investigated (Figure 1E-G), NK and γδ+T cells show prompt recovery in the early posttransplant period, followed by progressive emergence of αβ+ T lymphocytes. This is the first report of FA patients given TCRαβ+/CD19+-depleted haplo-HSCT in the context of a prospective trial, and the largest series of T-cell–depleted haplo-HSCT in FA reported to date.
Several considerations emerge from our data. First, as older age, high transfusion burden, previous androgen exposure, and the development of clonal evolution have been associated with lower survival rates in FA patients,3,21 the timing of HSCT is critical to outcome. Although the main advantage of haplo-HSCT lies in immediate and nearly universal donor availability, the strong bidirectional alloreactivity may result in high incidences of GF and severe GVHD. Our experience suggests that haplo-HSCT after selective removal of TCRαβ+ and CD19+ lymphocytes is able to guarantee high engraftment rates with low incidence of both aGVHD and cGVHD.
Second, FA patients represent a population prone to developing severe aGVHD because of the underlying DNA repair defect and deregulation of the apoptotic process.22,23 Therefore, we suggest that these patients may benefit more from GVHD prophylaxis based on ex vivo removal of TCRαβ+ cells (the lymphocyte subset responsible for GVHD) than from the posttransplant use of methotrexate and cyclosporine, which has been associated with a delayed onset of aGVHD rather than decreased incidence of aGVHD.23 Moreover, as in children, aGVHD has been shown to predict the development of cGVHD,24 a major risk factor for late posttransplantation malignancies in FA patients,25 the reduction of GVHD incidence may also translate into better long-term outcome.
The development of second malignancies in FA patients has also been associated with the use of irradiation,26 although this correlation was not confirmed in a larger retrospective study.3 Even though a 5-year follow-up does not allow drawing any firm conclusions regarding the potential risk of developing secondary tumors, 1 case of malignancy was observed in our cohort. Several studies explored the use of radiation-free conditioning regimens for alternative donor HSCT,27 with good engraftment rates.28 However, as these preparative regimens included alkylating agents such as busulfan, which is potentially responsible for excessive regimen-related toxicity, the optimal conditioning strategy remains to be determined. Recently, an alternative haplo-platform with infusion of T-cell–replete grafts followed by posttransplantation cyclophosphamide as an in vivo T-cell depletion strategy has been explored in FA patients, with favorable outcomes.29,30 Engraftment rate was high, but the cumulative incidence of grade 3 to 4 aGVHD and cGVHD was a major concern.30 Moreover, in FA patients, the potential risk of increased toxicity associated with hypersensitivity to DNA-crosslinking agents should be considered.
As far as immune reconstitution is concerned, 11 patients in our study developed post-HSCT viral reactivations or infections, but none of them died as a result of transplant-related complications. This finding compares favorably with other haplo-HSCT platforms and may be speculatively attributed to anti-infectious activity of mature NK and γδ+ T cells infused with the graft and to a faster reconstitution of adaptive immunity.31-34 Both NK and γδ+ T cells have been shown to be capable of exerting a protective effect against life-threatening opportunistic infections, including cytomegalovirus.33,34
Rio et al35 recently reported the results of lentiviral-mediated hematopoietic gene therapy in non-conditioned patients with FANCA FA. Although the approach is fascinating from a biological point of view, it still needs to be optimized and carries the intrinsic limitations of being influenced by the patient’s hematopoietic reservoir and need to prepare a gene defect–specific construct.
In summary, our data indicate that TCRαβ+/CD19+-depleted haplo-HSCT is a valuable strategy for FA patients, ensuring a good engraftment rate and low incidence of post-HSCT complications, thus resulting in excellent survival.
To request data, please e-mail Luisa Strocchio (luisa.strocchio@opbg.net).
Acknowledgments
This work was partly supported by grants from the Associazione Italiana Ricerca sul Cancro, (Investigator Grant ID 21724) (F.L.), Ministero della Salute (Ricerca Finalizzata) (F.L.), and Ministero dell’Istruzione, Università e Ricerca (Project PRIN 2017 ID 2017WC8499) (F.L.).
Authorship
Contribution: F.L. designed the study and supervised the project; L.S., D.P., and P.M. collected the data; P.M. and F.L. analyzed and interpreted the data; G.L.P. performed graft manipulation and graft characterization; L.S., D.P., M. Algeri, F.R., G.L., R.M.P., K.G., S.G., F.d.B., P.M., and F.L. were involved in the clinical management of patients; V.B. performed immune monitoring; G.L. performed donor apheresis; M. Andreani performed HLA typing; E.A. and A.N. performed genetic analysis; L.G. performed radiotherapy; L.S., D.P., P.M., and F.L. wrote and edited the manuscript; and all authors had access to primary clinical trial data, contributed to the intellectual content of this article, and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luisa Strocchio, Department of Pediatric Hemato-Oncology and Cell and Gene Therapy, IRCCS Bambino Gesù Children’s Hospital, Piazza Sant’Onofrio 4, 00165 Rome, Italy; e-mail: luisa.strocchio@opbg.net.
References
Author notes
P.M. and F.L. contributed equally to this work as last coauthors.