Key Points
A novel irradiation-based conditioning regimen allows age-adapted myeloablative haploidentical transplantation for leukemia patients.
The combination of myeloablative conditioning with regulatory and conventional T-cell immunotherapy eradicates AML.
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the most effective treatment in eradicating high-risk acute myeloid leukemia (AML). Here, we present data from a novel HLA-haploidentical HSCT protocol that addressed the 2 remaining major unmet medical needs: leukemia relapse and chronic graft-versus-host disease (cGVHD). Fifty AML patients were enrolled in the study. The conditioning regimen included total body irradiation for patients up to age 50 years and total marrow/lymphoid irradiation for patients age 51 to 65 years. Irradiation was followed by thiotepa, fludarabine, and cyclophosphamide. Patients received an infusion of 2 × 106/kg donor regulatory T cells on day −4 followed by 1 × 106/kg donor conventional T cells on day −1 and a mean of 10.7 × 106 ± 3.4 × 106/kgpurified CD34+ hematopoietic progenitor cells on day 0. No pharmacological GVHD prophylaxis was administered posttransplantation. Patients achieved full donor–type engraftment. Fifteen patients developed grade ≥2 acute GVHD (aGVHD). Twelve of the 15 patients with aGVHD were alive and no longer receiving immunosuppressive therapy. Moderate/severe cGVHD occurred in only 1 patient. Nonrelapse mortality occurred in 10 patients. Only 2 patients relapsed. Consequently, at a median follow-up of 29 months, the probability of moderate/severe cGVHD/relapse-free survival was 75% (95% confidence interval, 71%-78%). A novel HLA-haploidentical HSCT strategy that combines an age-adapted myeloablative conditioning regimen with regulatory and conventional T-cell adoptive immunotherapy resulted in an unprecedented cGVHD/relapse-free survival rate in 50 AML patients with a median age of 53 years. This trial was registered with the Umbria Region Institutional Review Board Public Registry as identification code 02/14 and public registry #2384/14 and at www.clinicaltrials.gov as #NCT03977103.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the most effective treatment for high-risk acute myeloid leukemia (AML).1 Unfortunately, to date, whatever the donor (matched sibling, unrelated, or HLA haploidentical) and whatever the transplantation protocol, only up to 40% to 50% of patients become long-term survivors with no leukemia relapse and/or chronic graft-versus-host disease (cGVHD).2-8 Historically, best results were obtained in younger patients using myeloablative conditioning regimens.9 More recently, the use of reduced-intensity conditioning regimens made HSCT feasible in older patients.10 Regardless, relapse remains the major cause of transplantation failure.2-8 One may hypothesize posttransplantation immune suppressive GVHD prophylaxis weakens donor T-cell alloreactivity against leukemia and favors relapse. In pioneering HLA-haploidentical T cell–depleted HSCT in young adults,11,12 we discovered such issues could be almost totally overcome by the combination of a high-intensity conditioning regimen and natural killer (NK) cell–mediated graft-versus-leukemia (GVL) effects.13 Specific HLA class 1 mismatches triggered donor-versus-recipient NK cell alloreactions that ablated leukemia without causing GVHD.13 More recently, murine models of bone marrow transplantation and preliminary clinical studies have shown that infusion of regulatory T cells (Tregs) protected from GVHD without impairing conventional T-cell (Tcon) activity against malignant diseases.14-16 In the present HLA-haploidentical transplantation trial for AML, we combined for the first time 2 concepts: (1) together with the hematopoietic graft, the infusion of donor Tcons under the protection of previously transferred donor Tregs as the sole form of GVHD prophylaxis, and (2) a myeloablative conditioning regimen in which the irradiation strategy was adapted to the patient’s age. Patients up to age 50 years underwent total body irradiation (TBI). Patients age >50 years (up to age 65 years) or unfit for TBI underwent total marrow/lymphoid irradiation (TMLI). TMLI sculpts radiation doses to bones, lymph nodes, and spleen while reducing doses to visceral organs.17 Therefore, TMLI delivers the leukemia ablative effect of a myeloablative conditioning regimen in patients for whom TBI would have been overly toxic.18,19 Such a transplantation strategy resulted in exceptionally low leukemia relapse and cGVHD rates and consequently unprecedented event-free survival in high-risk AML patients.
Patients and methods
Patient population
The study was conducted according to the revised Helsinki Declaration and was approved and registered by the Umbria Regional Hospital Institutional Review Board. Presented data refer to HLA-haploidentical HSCT in AML patients enrolled in the study. Written informed consent was obtained from each patient. The present subgroup analysis was conducted as reported in the study protocol. All enrolled patients underwent the transplantation procedure and were evaluated for outcomes.
Donors
Donors were healthy family members who shared 1 HLA haplotype with the patient. Haploidentical donors were considered in 2 instances: either when there was no time for a donor search in the international registry because of risk of AML relapse/progression or when no acceptably matched donor was identified. Donors underwent leukaphereses for Treg and Tcon collection and granulocyte colony-stimulating factor–mobilized peripheral blood hematopoietic stem cells. Donors with HLA disparities that allowed donor-versus-recipient NK cell alloreactivity were preferred.13 In the absence of NK cell alloreactivity, maternal donors were preferred.20 For cytomegalovirus (CMV)–seronegative patients, CMV-seronegative donors were preferred.
Graft processing
The Treg/Tcon selection procedure was reported in full elsewhere.15,16 Briefly, after leukapheresis, donor CD4+CD25+ Tregs were selected by immunomagnetic depletion of CD8+/CD19+ cells and subsequent positive selection of CD25+ cells (using CliniMACS device; Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany). On average, the final Treg product was composed of 71% ± 8.5% CD4+CD25+CD127−FOXP3+ cells and 27% ± 7.6% CD127+ cells. To obtain donor Tcons, CD3+ T cells were separated from the leukapheresis product by Ficoll gradient. After granulocyte colony-stimulating factor administration (10 μg/kg per day starting 4 days before the first leukapheresis), donor CD34+ cells were collected from 2 to 3 leukaphereses and positively immunoselected (CliniMACS).
Transplantation procedure
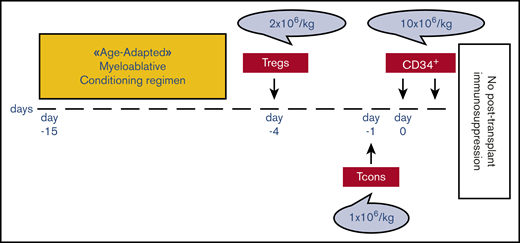
Patients up to age 50 years underwent TBI, either fractionated (9 fractions delivered twice per day for 4.5 days; total dose, 13.5 Gy) or single dose of 8 Gy with lung shielding.21 Patients age 51 to 65 years received TMLI. TMLI was administered by helical tomotherapy in 9 fractions delivered twice per day for 4.5 days. TMLI target volumes were skeletal bones for total marrow irradiation (total dose, 13.5 Gy) and major lymph node chains and spleen for total lymphoid irradiation (total dose, 11.5 Gy; supplemental Figure 1). Four patients age <50 years who were unfit for TBI underwent TMLI. TBI or TMLI were followed by thiotepa (5-10 mg/kg), fludarabine (150-200 mg/m2), and cyclophosphamide (30 mg/kg per day). Details on chemotherapy schedule and dosage are reported in supplemental Methods. Because in vitro studies had shown a 2:1 Treg/Tcon ratio to be optimal for inhibition of mixed lymphocyte reaction, patients received an infusion of 2 × 106/kg donor Tregs on day −4 followed by 1 × 106/kg Tcons on day −1.15,16 Such a target dose was achieved in all patients. A mean of 10.7 × 106 ± 3.4 × 106/kg positively purified CD34+ hematopoietic progenitor cells was infused on day 0. No pharmacological GVHD prophylaxis was administered posttransplantation (Figure 1). Pharmacological infection prophylaxes are shown in supplemental Methods. Chimerism and immune reconstitution analyses are shown in supplemental Methods.
Transplantation schema. Patients received an age-adapted myeloablative conditioning regimen based on TBI, either fractionated (9 fractions delivered twice per day for 4.5 days; total dose, 13.5 Gy) or single dose of 8 Gy with lung shielding, for those age up to 50 years or based on TMLI for those age 51 to 65 years. TMLI was administered by helical tomotherapy in 9 fractions delivered twice per day for 4.5 days. TMLI target volumes were skeletal bones for total marrow irradiation (total dose, 13.5 Gy) and major lymph node chains and spleen for total lymphoid irradiation (total dose, 11.5 Gy). TBI or TMLI were followed by thiotepa (5-10 mg/kg), fludarabine (150-200 mg/m2), and cyclophosphamide (30 mg/kg). After conditioning, all patients received an infusion of 2 × 106/kg donor Tregs on day −4 followed by 1 × 106/kg Tcons on day −1 and a megadose of positively purified CD34+ hematopoietic progenitor cells on day 0. No pharmacological GVHD prophylaxis was administered posttransplantation.
Transplantation schema. Patients received an age-adapted myeloablative conditioning regimen based on TBI, either fractionated (9 fractions delivered twice per day for 4.5 days; total dose, 13.5 Gy) or single dose of 8 Gy with lung shielding, for those age up to 50 years or based on TMLI for those age 51 to 65 years. TMLI was administered by helical tomotherapy in 9 fractions delivered twice per day for 4.5 days. TMLI target volumes were skeletal bones for total marrow irradiation (total dose, 13.5 Gy) and major lymph node chains and spleen for total lymphoid irradiation (total dose, 11.5 Gy). TBI or TMLI were followed by thiotepa (5-10 mg/kg), fludarabine (150-200 mg/m2), and cyclophosphamide (30 mg/kg). After conditioning, all patients received an infusion of 2 × 106/kg donor Tregs on day −4 followed by 1 × 106/kg Tcons on day −1 and a megadose of positively purified CD34+ hematopoietic progenitor cells on day 0. No pharmacological GVHD prophylaxis was administered posttransplantation.
End points and definitions
The primary end point was probability of 2-year GVHD/relapse-free survival (survival in the absence of relapse and moderate/severe cGVHD). Secondary end points were full donor–type engraftment and cumulative incidences of grade ≥2 acute GVHD (aGvHD), moderate/severe cGvHD,22 nonrelapse mortality (NRM; ie, death resulting from any cause in the absence of relapse), and relapse (ie, disease recurrence according to marrow morphology, flow cytometry, cytogenetics, fluorescence in situ hybridization, and/or polymerase chain reaction).
Statistics
Demographics and prognostic variables were compared using Fisher’s exact or χ2 test for categorical variables and Student t or Mann-Whitney U test for continuous variables. Cumulative incidences of grade ≥2 aGvHD and moderate/severe cGvHD were estimated. Cumulative incidences of relapse and NRM were estimated as competing risks. Gray test compared univariate competing risk outcomes (analyses were performed using R statistical software [version 3.4.1]). Kaplan-Meier method estimated overall survival (OS) and moderate/severe cGvHD/relapse-free survival. Log-rank test assessed the impact of conditioning regimen, genetic risk, and minimal residual disease (MRD) on graft/relapse-free survival. P values are 2 sided and considered significant at P < .05 (analyses were performed using SPSS statistical software).
Results
Patient population
Between April 2014 and November 2019, 50 consecutive AML patients with a median age of 53 years (range, 20-65 years) were enrolled and treated. Four patients were considered unfit to receive TBI because of comorbidities (supplemental Table 2).
Demographic features, disease characteristics, and disease status at transplantation are reported in Table 1 and supplemental Tables 1 and 2. Twenty patients had adverse genetic risk leukemia, 22 had intermediate risk, and 5 had favorable risk,23 and 3 patients could not be classified because of missing karyotype at diagnosis. Favorable-risk patients were underwent transplantation because they were in second complete remission or had MRD. Forty-two patients underwent transplantation while in hematological complete remission, and 8 did so with active disease. Thirty-three patients (66%) had detectable disease at transplantation by cytogenetic, immunophenotypic, and/or molecular analyses.
Characteristics of patients undergoing transplantation
| . | Conditioning regimen . | P . | Total (N = 50) . | |
|---|---|---|---|---|
| TBI based (n = 19) . | TMLI based (n = 31) . | |||
| Sex | .87 | |||
| Male | 8 | 18 | 26 | |
| Female | 11 | 13 | 24 | |
| Age, y | .01 | |||
| Median | 33 | 56 | 53 | |
| Range | 20-50 | 38-65 | 20-65 | |
| Genetic risk stratification at diagnosis | ||||
| Favorable | 2 (11) | 3 (10) | .9 | 5 (10) |
| Intermediate | 8 (42) | 14 (45) | .4 | 22 (44) |
| Adverse | 9 (47) | 11 (35) | .2 | 20 (40) |
| Missing information | 0 (0) | 3 (10) | .3 | 3 (6) |
| Other risk factors | ||||
| Secondary AML | 5 (26) | 11 (35) | .15 | 16 (32) |
| PIF | 5 (26) | 12 (39) | .1 | 17 (34) |
| Disease status at HSCT | ||||
| First CR, MRD− | 8 (42) | 9 (29) | .18 | 17 (34) |
| First CR, MRD+ | 7 (37) | 10 (32) | .4 | 17 (34) |
| ≥Second CR, MRD− | 0 (0) | 0 (0) | NA | 0 (0) |
| ≥Second CR, MRD+ | 1 (5) | 7 (23) | .1 | 8 (16) |
| Advanced | 3 (16) | 5 (16) | 1 | 8 (16) |
| DRI37 | ||||
| Low | 2 (11) | 2 (6) | .5 | 4 (8) |
| Intermediate | 6 (32) | 14 (45) | .2 | 20 (40) |
| High | 10 (53) | 8 (39) | .3 | 18 (36) |
| Very high | 1 (5) | 4 (13) | .45 | 5 (10) |
| Missing information | 0 (0) | 3 (10) | .3 | 3 (6) |
| HCT-CI risk score38 | ||||
| 0 (low) | 13 (69) | 14 (45) | .1 | 27 (54) |
| 1-2 (intermediate) | 5 (26) | 12 (39) | .2 | 17 (34) |
| ≥3 (high) | 1 (5) | 5 (16) | .2 | 6 (12) |
| . | Conditioning regimen . | P . | Total (N = 50) . | |
|---|---|---|---|---|
| TBI based (n = 19) . | TMLI based (n = 31) . | |||
| Sex | .87 | |||
| Male | 8 | 18 | 26 | |
| Female | 11 | 13 | 24 | |
| Age, y | .01 | |||
| Median | 33 | 56 | 53 | |
| Range | 20-50 | 38-65 | 20-65 | |
| Genetic risk stratification at diagnosis | ||||
| Favorable | 2 (11) | 3 (10) | .9 | 5 (10) |
| Intermediate | 8 (42) | 14 (45) | .4 | 22 (44) |
| Adverse | 9 (47) | 11 (35) | .2 | 20 (40) |
| Missing information | 0 (0) | 3 (10) | .3 | 3 (6) |
| Other risk factors | ||||
| Secondary AML | 5 (26) | 11 (35) | .15 | 16 (32) |
| PIF | 5 (26) | 12 (39) | .1 | 17 (34) |
| Disease status at HSCT | ||||
| First CR, MRD− | 8 (42) | 9 (29) | .18 | 17 (34) |
| First CR, MRD+ | 7 (37) | 10 (32) | .4 | 17 (34) |
| ≥Second CR, MRD− | 0 (0) | 0 (0) | NA | 0 (0) |
| ≥Second CR, MRD+ | 1 (5) | 7 (23) | .1 | 8 (16) |
| Advanced | 3 (16) | 5 (16) | 1 | 8 (16) |
| DRI37 | ||||
| Low | 2 (11) | 2 (6) | .5 | 4 (8) |
| Intermediate | 6 (32) | 14 (45) | .2 | 20 (40) |
| High | 10 (53) | 8 (39) | .3 | 18 (36) |
| Very high | 1 (5) | 4 (13) | .45 | 5 (10) |
| Missing information | 0 (0) | 3 (10) | .3 | 3 (6) |
| HCT-CI risk score38 | ||||
| 0 (low) | 13 (69) | 14 (45) | .1 | 27 (54) |
| 1-2 (intermediate) | 5 (26) | 12 (39) | .2 | 17 (34) |
| ≥3 (high) | 1 (5) | 5 (16) | .2 | 6 (12) |
Data presented as n (%) unless otherwise indicated. Demographics of AML patients who underwent the study protocol are reported. Patients were stratified according genetic risk at diagnosis and disease status at transplantation. Univariate analysis was performed to compare characteristics at transplantation of TBI-treated and TMLI-treated patients.
CR, complete remission; DRI, Disease Risk Index; HCT-CI, Hematopoietic Cell Transplantation–Specific Comorbidity Index; NA, not applicable; PIF, primary induction failure.
Engraftment
One patient died at day +8 before engraftment. All other patients achieved full donor–type engraftment and reached an absolute neutrophil count >0.5 × 109/L in a median time of 13 days (range, 8-23 days). Median time to platelet recovery (platelet count >20 × 109/L) was 17 days (range, 14-72 days).
Immune reconstitution
In patients without GVHD, peripheral blood T-cell subpopulations increased rapidly. CD4+ and CD8+ cells reached 100/µL at medians of 45 days (range, 29-95 days) and 27 days (range, 19-60 days), respectively, and 200/µL at medians of 74 days (range, 39-81 days) and 50 days (range, 25-81 days), respectively (supplemental Figure 2A). Specific CD4+ and CD8+ lymphocytes against opportunistic pathogens such as Aspergillus fumigatus, Candida albicans, CMV, adenovirus, herpes simplex virus, varicella zoster virus, and Toxoplasma gondii soon emerged (supplemental Figure 2C,E). Donor B-cell reconstitution was fast (supplemental Figure 2G), producing immunoglobulin G (supplemental Figure 2I). Suspension of immune suppressive therapy in patients with aGVHD was associated with rapid increases in peripheral blood T cells and pathogen-specific CD4+ and CD8+ T-cell responses (supplemental Figure 2B,D,F, respectively), B cells, and immunoglobulin G serum concentrations (supplemental Figure 2H,J).
Conditioning regimen–related toxicity and infections
According to the Common Terminology Criteria for Adverse Events (version 5.0), a majority of conditioning regimen–related adverse events (within 30 days after transplantation) were grade 1 to 2. No patient developed cytokine release syndrome after Treg and/or Tcon infusion. No patient developed grade >3 oral or intestinal mucositis. Supplemental Table 3 reports organ-specific radiation doses in TMLI-treated patients, and Table 2 reports organ-specific toxicities in all patients. One patient developed grade 5 central nervous system toxicity and died as a result of cerebral hemorrhage. All patients experienced febrile neutropenia during the aplastic phase, which required empirical wide-spectrum antibacterial treatments. Sepsis was documented in 19 patients of a total of 65 febrile neutropenia events. Two patients died as a result of septic shock from multiresistant bacteria. CMV reactivations, defined as CMV DNA copies in whole blood >1000/mmc or any CMV detection in tissue biopsies, occurred in 22 of 50 patients (supplemental Figure 3A). Three patients had CMV localization in the gastrointestinal tract. Treatment with antiviral drugs (ganciclovir and/or foscarnet) resulted in compete resolution of infection in 20 patients. Two patients had spontaneous clearance of CMV viremia. No CMV-related deaths occurred. We did not use posttransplantation rituximab prophylaxis for Epstein-Barr virus (EBV)–seropositive patients. EBV viremias, as defined by any detection of EBV DNA in whole blood, were detected in 35 of 50 patients (supplemental Figure 3B). No patients with EBV viremia developed EBV-related symptoms. Only 2 patients experienced high EBV viral load in whole blood and received rituximab to prevent posttransplantation lymphoproliferative disease. Patient n.007TMLI had EBV clearance after rituximab treatment. However, he developed donor-derived lymphoma that tested EBV− at the pathology examination at day +860 after transplantation (supplemental Table 5). He received standard immunochemotherapy followed by T cell–depleted stem cell rescue from the same donor. He was in complete remission at day +1051 after first transplantation. Two patients developed probable invasive fungal infection. One of them died as a result of progression of the infection despite specific antifungal treatment. Autopsy confirmed disseminated multiorgan infection by A terreus. No patients developed Pneumocystis jirovecii or T gondii infection.
Early toxicity in patients who received TBI- or TMLI-based conditioning regimen
| . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | ||||
|---|---|---|---|---|---|---|---|---|
| TBI . | TMLI . | TBI . | TMLI . | TBI . | TMLI . | TBI . | TMLI . | |
| Oral cavity | 13 (68) | 25 (81) | 6 (32) | 6 (19) | — | — | — | — |
| CNS | — | 2 (6) | — | 2 (6) | — | — | — | 1 (3) |
| Hepatic | 1 (5) | 3 (10) | — | 1 (3) | 4 (16) | 2 (6) | — | — |
| Gastric | 18 (95) | 29 (94) | 1 (5) | 2 (6) | — | — | — | — |
| Intestinal | 11 (58) | 21 (68) | 8 (42) | 10 (32) | — | — | — | — |
| Renal | — | — | — | — | — | — | — | — |
| Pulmonary | 6 (32) | 1 (35) | 1 (5) | — | — | — | — | — |
| Bladder | — | 2 (6) | 1 (5) | — | — | — | — | — |
| Cardiac | 6 (32) | 3 (10) | 1 (5) | — | — | — | — | — |
| . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . | ||||
|---|---|---|---|---|---|---|---|---|
| TBI . | TMLI . | TBI . | TMLI . | TBI . | TMLI . | TBI . | TMLI . | |
| Oral cavity | 13 (68) | 25 (81) | 6 (32) | 6 (19) | — | — | — | — |
| CNS | — | 2 (6) | — | 2 (6) | — | — | — | 1 (3) |
| Hepatic | 1 (5) | 3 (10) | — | 1 (3) | 4 (16) | 2 (6) | — | — |
| Gastric | 18 (95) | 29 (94) | 1 (5) | 2 (6) | — | — | — | — |
| Intestinal | 11 (58) | 21 (68) | 8 (42) | 10 (32) | — | — | — | — |
| Renal | — | — | — | — | — | — | — | — |
| Pulmonary | 6 (32) | 1 (35) | 1 (5) | — | — | — | — | — |
| Bladder | — | 2 (6) | 1 (5) | — | — | — | — | — |
| Cardiac | 6 (32) | 3 (10) | 1 (5) | — | — | — | — | — |
Data presented as n (%). Organ-specific toxicity that occurred within first 30 d after transplantation reported according Common Terminology Criteria for Adverse Events (version 5.0).
CNS, central nervous system.
GVHD
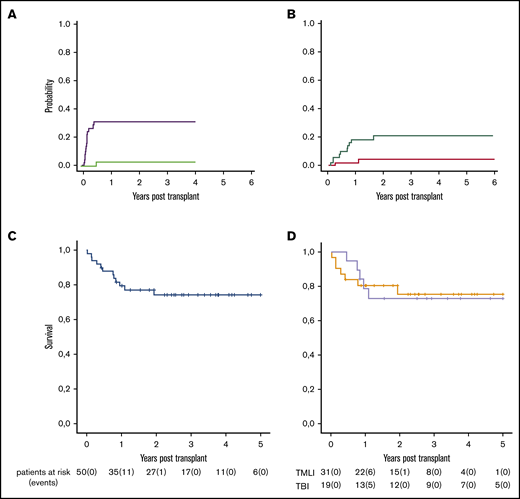
Fifteen patients developed grade ≥2 aGVHD (cumulative incidence, 33%; 95% confidence interval [CI] 30%-35%), and 12 of 15 developed grade 3 to 4 aGVHD. Median time to onset was 41 days (range, 23-69 days; Figure 2A). At the time of diagnosis, skin was involved in 12 patients, intestine in 13, and liver in only 4. All patients have been treated with first-line steroids. aGVHD was steroid refractory in only 5 patients, and 3 of them had full resolution of the disease after second-line treatment. Twelve of 15 patients with aGVHD were alive and no longer receiving immunosuppressive therapy. Of the remaining 3 patients, 2 died as a result of aGVHD and 1 of leukemia relapse. Interestingly, we found aGVHD occurred in 3 of the 19 patients who underwent TBI conditioning and 12 of the 31 patients who underwent TMLI conditioning (TBI: cumulative incidence, 16%; 95% CI, 13%-18% vs TMLI: cumulative incidence, 41%; 95% CI, 39%-43%; P = .05; supplemental Figure 4A). None of the aGVHD patients progressed to moderate/severe cGVHD and required prolonged immune suppressive therapy. Four patients developed mild cGVHD (xerophtalmia or xerostomia) that required topical therapy and fully resolved over time. No patient developed moderate cGVHD. One patient developed severe cGVHD and died (cumulative incidence of moderate/severe cGVHD, 2%; 95% CI, 1%-3%; Figure 2A).
Outcomes of the whole cohort of patients. (A) Cumulative incidences of grade ≥2 aGvHD (purple line) and moderate/severe cGVHD (green line). (B) Cumulative incidences of relapse (red line) and NRM (green line). (C) Moderate/severe cGVHD/relapse-free survival by Kaplan-Meier curve after a median follow-up of 29 months (range, 8 days to 6 years). (D) Moderate/severe cGVHD/relapse-free survival according to type of conditioning regimen (gray line, TBI-based conditioning regimen; orange line, TMLI-based conditioning regimen).
Outcomes of the whole cohort of patients. (A) Cumulative incidences of grade ≥2 aGvHD (purple line) and moderate/severe cGVHD (green line). (B) Cumulative incidences of relapse (red line) and NRM (green line). (C) Moderate/severe cGVHD/relapse-free survival by Kaplan-Meier curve after a median follow-up of 29 months (range, 8 days to 6 years). (D) Moderate/severe cGVHD/relapse-free survival according to type of conditioning regimen (gray line, TBI-based conditioning regimen; orange line, TMLI-based conditioning regimen).
NRM and causes of death
NRM occurred in 10 patients (cumulative incidence, 21%; 95% CI, 20%-23%; Figure 2B). Causes were venoocclusive disease (n = 2), aGVHD (n = 2), cGVHD (n = 1), not otherwise specified pneumonia (n = 1), septic shock (n = 2), invasive aspergillosis (n = 1), and cerebral hemorrhage (n = 1). No differences in NRM were observed among patients conditioned with TBI vs TMLI (supplemental Figure 4B). Adverse genetic risk at diagnosis had no impact on NRM (Figure 3A). Patients who were MRD+ or had active disease at transplantation tended (P = .06) to have an increased risk of NRM (Figure 3D).
Outcomes of patients according to genetic risk at diagnosis and disease status at transplantation. (A) Cumulative incidence of NRM according to genetic risk at diagnosis (green line, favorable/intermediate genetic risk; red line, adverse genetic risk at diagnosis). (B) Cumulative incidence of relapse according to genetic risk at diagnosis (orange line, favorable/intermediate genetic risk; gray line, adverse genetic risk at diagnosis). (C) Moderate/severe cGVHD/relapse-free survival (by Kaplan-Meier curve) according to genetic risk at diagnosis (purple line, favorable/intermediate genetic risk [RISK−]; blue line, adverse genetic risk at diagnosis [RISK+]). (D) Cumulative incidence of NRM according to disease status at transplantation (red line, MRD+/active disease; green line, MRD−). (E) Cumulative incidence of relapse according to disease status at transplantation (lavender line, MRD+/active disease; orange line, MRD−). (F) Moderate/severe cGVHD/relapse-free survival according to disease status at transplantation (green line, MRD+/active disease; blue line, MRD−).
Outcomes of patients according to genetic risk at diagnosis and disease status at transplantation. (A) Cumulative incidence of NRM according to genetic risk at diagnosis (green line, favorable/intermediate genetic risk; red line, adverse genetic risk at diagnosis). (B) Cumulative incidence of relapse according to genetic risk at diagnosis (orange line, favorable/intermediate genetic risk; gray line, adverse genetic risk at diagnosis). (C) Moderate/severe cGVHD/relapse-free survival (by Kaplan-Meier curve) according to genetic risk at diagnosis (purple line, favorable/intermediate genetic risk [RISK−]; blue line, adverse genetic risk at diagnosis [RISK+]). (D) Cumulative incidence of NRM according to disease status at transplantation (red line, MRD+/active disease; green line, MRD−). (E) Cumulative incidence of relapse according to disease status at transplantation (lavender line, MRD+/active disease; orange line, MRD−). (F) Moderate/severe cGVHD/relapse-free survival according to disease status at transplantation (green line, MRD+/active disease; blue line, MRD−).
Relapse and survival
Two patients relapsed (cumulative incidence, 4%; 95% CI, 3%-6%) at a median follow-up of 34 months (range, 5-72 months; Figure 2B). Both were MRD+ and had received transplants from non-NK alloreactive donors. One patient died. The other underwent successful retransplantation after a chemotherapy-based (treosulfan, thiotepa, fludarabine, and cyclophosphamide) conditioning regimen from an NK alloreactive donor with the addition of Treg/Tcon immunotherapy. Currently, at 3-year follow-up, she was alive and well and cGVHD/leukemia free. Because of the relatively high median age of the entire cohort, mothers could be chosen as donors in only 5 patients. Therefore, it was not possible to evaluate their effect on relapse.
At a median follow-up of 29 months (range, 8 days to 6 years), probability of OS was 77% (95% CI, 74%-80%; supplemental Figure 5A), GVHD/relapse-free survival was 48% (95% CI, 44%-51%; supplemental Figure 5B), and moderate/severe cGVHD/relapse-free survival was 75% (95% CI, 71%-78%). Thirty-eight of 50 patients were alive and free from moderate/severe cGVHD and leukemia (Figure 2C). Survival was not affected by irradiation protocol (TBI vs TMLI; Figure 2D). Adverse genetic risk at diagnosis did not have an impact on incidence of relapse or survival (Figure 3B-C). Patients who were MRD+ or had active disease at transplantation had significantly worse moderate/severe cGVHD/relapse-free survival (hazard ratio, 1.3; 64% vs 89%; P = .02), mainly because of increased NRM (Figure 3D-F). Detailed outcomes are reported in supplemental Tables 4 and 5.
Discussion
The present HLA-haploidentical transplantation protocol combines an age-adapted myeloablative conditioning regimen with Treg/Tcon adoptive immunotherapy. It resulted in an impressive 75% moderate/severe cGVHD/relapse-free survival rate in AML patients, many of whom were at high risk of relapse. With a median follow-up of 29 months, the high number of survivors with no evidence of leukemia relapse and/or cGVHD seems superior to that achieved with popular myeloablative HLA-haploidentical transplantation protocols for AML (eg, unmanipulated grafts followed by posttransplantation cyclophosphamide).4,5 Such studies showed 14% to 28% NRM, 21% to 44% leukemia relapse, and 14% to 30% cGVHD rates. Although not always specified, graft/relapse-free survival seemed to be in the range of 40% to 60%.4-6 Furthermore, results of the present protocol compare favorably even with those of matched sibling and matched unrelated donor transplantations.2,3,7 As we previously demonstrated, Treg infusions largely prevented the potential lethality of infusing 1 million Tcons in the absence of any pharmacological GVHD prophylaxis.15,16 In fact, only 2 patients died as a result of aGVHD. However, Tregs did not fully control Tcon alloreactivity, because the incidence of aGVHD was quite high (33%). Nonetheless, even patients with grade 3 to 4 aGVHD promptly responded to immune suppression. This allowed early withdrawal of aGVHD therapy (at a median time after transplantation, 115 days; range, 60-240 days) and resulted in no progression to cGVHD, immune recovery with low infectious mortality, and low impact on NRM. We observed a higher incidence of aGVHD in patients who had been conditioned with TMLI. Such a finding suggests the older age of this group of patients might have played a role. Indeed, older age has been reported to increase the risk of aGVHD.24 The extremely low incidence of cGVHD was probably related to Treg infusion.25 Indeed, it has been demonstrated that cGVHD is ameliorated by Treg infusion or administration of low-dose interleukin-2 to boost/expand Tregs.26 NRM occurred in 10 of 50 patients. Therefore, the current transplantation protocol was associated with lower NRM as compared with the ∼40% NRM rate of our previous T cell–depleted haploidentical transplantation protocols.11,12 The immune regulation exerted by Tregs on GVHD did not result in clinically significant inhibition of pathogen-specific responses. Viral (eg, EBV and CMV) reactivations did occur, but they cleared spontaneously in most cases, and no CMV-related deaths were observed. Therefore, Treg/Tcon adoptive immunotherapy allowed for the development of immune competence and reduced infectious mortality. In addition, the present conditioning regimen was less intensive/toxic than our previous ones, because for most patients, irradiation was fractionated instead of single dose, and for all patients, cyclophosphamide doses were lower than previously used.15,16 Nine of the 10 patients who died as a result of NRM were MRD+ or had active disease at the time of transplantation. TMLI in place of TBI in older patients allowed for the delivery of equal-to-TBI irradiation doses to the bone marrow for leukemia ablation and to lymph nodes for immunosuppression while sparing toxicity to major organs.17-19 Therefore, TMLI was instrumental in delivering a myeloablative conditioning regimen in this age group without any increase in NRM when compared with younger patients. The incidence of leukemia relapse was exceptionally low. Only 2 of the 50 patients relapsed at a median follow-up of 34 months. Both were MRD+ at the time of transplantation (supplemental Tables 1 and 2). MRD is a well-recognized risk factor for adverse outcomes in AML patients.27 The risk of relapse in MRD+ patients approaches 65% to 70%, and OS is estimated to be ∼25%.28 In the present study, the cumulative incidence of relapse in the 33 patients who were MRD+ or had active disease at transplantation was 7%, with an impressive 64% moderate/severe cGVHD/relapse-free survival. Adverse genetics at diagnosis comprise another strong predictor for increased risk of relapse.29,30 In a large, retrospective series of patients with complex karyotype (≥3 abnormalities) AML, Ciurea et al30 reported the cumulative incidence of relapse to be ∼50% after matched sibling or unrelated donor HSCT. In the present study, none of the 20 patients with adverse genetics (most of them with complex karyotype or monosomy/deletion of chromosome 7 or 5) relapsed, and their probability of moderate/severe cGVHD/relapse-free survival was 72% (Figure 3C). Therefore, our transplantation strategy abrogated the high risk of relapse of AML with adverse genetic risk factors at diagnosis. The use of high-dose irradiation in the conditioning regimen was justified by its recognized leukemia killing effect.31 The potential antileukemic effect of TMLI17-19 was comparable to that of TBI, because they both delivered equal irradiation doses to the bone marrow. In fact, leukemia relapse rates were the same in the 2 groups (supplemental Figure 4C).
In addition to the myeloablative strength of the high-intensity irradiation-based conditioning, powerful immunological mechanisms played major roles in curing leukemia. Our previous T cell–depleted HSCT protocol, which also used high-dose TBI (without Treg/Tcon immunotherapy), was associated with a leukemia relapse rate of ∼35% (when performed in the absence of NK cell alloreactivity).13 Subsequently, our pioneering observations on donor-versus-recipient NK cell alloreactivity (a reaction that can occur in 30%-50% of donor/recipient pairs) unequivocally proved the principle that donor-versus-recipient immune reactions exert potent GVL effects when: (1) they are devoid of GVHD potential (eg, NK cell alloreactivity), and (2) they are exerted in the absence of posttransplantation immune suppression.13 Here, as in other recent studies by our group, the addition of Treg/Tcon immunotherapy provided a further extremely powerful GVL effect. In the current protocol, leukemia relapse was remarkably low (4%), regardless of presence vs absence of donor-versus-recipient NK cell alloreactivity, which was present in 14 of 50 patients in this cohort. Leukemia relapse was low, even in patients who received immune suppression (eg, steroids, antithymocyte globulins, or cyclosporine) because of grade ≥2 aGVHD. This suggests that the antileukemic effect of T-cell immunotherapy occurred early after transplantation and before the need for immune suppressive treatment for aGVHD.
In an attempt to understand how such a powerful GVL effect may take place despite possible counteraction by the infusion of Tregs, we performed experiments in murine xenogeneic transplantation models. We found human Tregs expressed low levels of CXCR4 bone marrow homing receptor and could not home to the bone marrow; they did home to the periphery, where they regulated human Tcons and prevented GVHD. In contrast, Tcons possessed CXCR4 and homed to the bone marrow, where they exerted an unopposed (by Tregs) GVL effect.32 Indeed, in a previous study by our group, we observed that mice that received primary AML cells with or without Tregs died as a result of leukemia. Mice that received leukemia cells in combination with Tcons died as a result of aGVHD. However, mice that received leukemia cells plus Tregs and Tcons survived with no evidence of leukemia or aGVHD.16
In conclusion, a haploidentical transplantation protocol that combined an age-adapted myeloablative conditioning regimen with Treg/Tcon adoptive immunotherapy provided remarkably low leukemia relapse and cGVHD rates. This resulted in an unprecedented 75% moderate/severe cGVHD/relapse-free survival rate in 50 AML patients, despite the fact that 31 of 50 were age 50 to 65 years. These data show T cell–depleted haploidentical HSCT with TMLI-based conditioning and Treg/Tcon immunotherapy may be applied in patients who are not eligible for standard myeloablative conditioning regimens because of age or unfitness.
The design of an age-adapted myeloablative transplantation protocol based on the combination of TMLI conditioning plus Treg/Tcon immunotherapy is certainly a challenging goal. However, it did achieve outstanding results. We recognize that such a transplantation protocol may seem complex, because it involves expertise on tomotherapy on the one hand and cell selection on the other. However, cell selection is based on the use of automated systems and is straightforward and reproducible, because it uses commercially available kits. In any event, we believe the unprecedented clinical results obtained in the younger age group undergoing transplantation with conventional TBI plus Treg/Tcon adoptive immunotherapy (transplantation that is obviously less complex than transplantation using TMLI) might facilitate more widespread use of this transplantation protocol outside the Perugia center. Furthermore, the present data need to be challenged in a larger cohort of patients and, it is hoped, in a multicentric study.
Finally, the exceptionally low incidence of leukemia relapse in patients undergoing transplantation in hematological remission makes it realistic to take up the challenge of attempting to cure patients with chemotherapy-resistant leukemia at transplantation. Such a challenge seems even more feasible in view of the possibility of increasing the TMLI dose up to 20 Gy,33 and the current availability of in vitro experiments demonstrating Treg function can be boosted by activation with interleukin-2 and tumor necrosis factor α,34 in vitro expansion,35 and T-cell FOXP3 engineering.36 Consequently, more Tcons could be infused safely for a greater GVL function.
An individual-level deidentified data set containing the variables analyzed in the primary results paper can be expected to be available upon request. Requests should be sent to antonio.pierini@unipg.it. Data are available to researchers whose proposed analysis is found by the Hematopoietic Stem Cell Transplantation Program in Perugia to be feasible and of scientific merit and who agree to the terms and conditions of use. Data will be made available starting 3 months after publication and for no longer than 2 years.
Acknowledgments
The authors thank Geraldine Anne Boyd for editorial assistance, the nonprofit charity associations Comitato per la Vita Daniele Chianelli and Associazione Umbra per lo Studio e la Terapia di Leucemie e Linfomi, and the residents and nurses of the hematopoietic stem cell transplantation program. The authors also thank the patients and their caregivers for participating in this study.
This study was supported by grants from the Associazione Italiana per la Ricerca sul Cancro, START-UP grant 20456 (A.P.), individual grants 23604 (B.F.) and IG18481 (A.V.), European Research Council advanced grant 740230 (B.F.), and European ERA-NET on Translational Cancer Research grant I95G12001330001 (A.V.).
Authorship
Contribution: A.P. provided clinical care, performed research, and wrote the manuscript; L.R. performed research and wrote the manuscript; A.C., A.T., and S.T. provided clinical care; F.F., T.Z., and R.I.O. processed the graft; S.S., C.Z., and G.I. designed and performed radiation treatment; A.M., S.P., S.C., M.D.I., and C.M. performed research; M.M., S.B., and R.L. collected clinical data; R.T. performed HLA typing; O.M. evaluated suitability of donors; M.P.M. and B.F. treated patients from diagnosis to transplantation; and M.F.M., C.A., and A.V. designed the study, provided overall guidance, and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Pierini, Piazzale Menghini 1, 06132 Perugia, Italy; e-mail: antonio.pierini@unipg.it.
References
Author notes
A.P. and L.R. contributed equally to this work.
The full-text version of this article contains a data supplement.


![Outcomes of patients according to genetic risk at diagnosis and disease status at transplantation. (A) Cumulative incidence of NRM according to genetic risk at diagnosis (green line, favorable/intermediate genetic risk; red line, adverse genetic risk at diagnosis). (B) Cumulative incidence of relapse according to genetic risk at diagnosis (orange line, favorable/intermediate genetic risk; gray line, adverse genetic risk at diagnosis). (C) Moderate/severe cGVHD/relapse-free survival (by Kaplan-Meier curve) according to genetic risk at diagnosis (purple line, favorable/intermediate genetic risk [RISK−]; blue line, adverse genetic risk at diagnosis [RISK+]). (D) Cumulative incidence of NRM according to disease status at transplantation (red line, MRD+/active disease; green line, MRD−). (E) Cumulative incidence of relapse according to disease status at transplantation (lavender line, MRD+/active disease; orange line, MRD−). (F) Moderate/severe cGVHD/relapse-free survival according to disease status at transplantation (green line, MRD+/active disease; blue line, MRD−).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/5/5/10.1182_bloodadvances.2020003739/2/m_advancesadv2020003739f3.png?Expires=1768012382&Signature=D-N1bv~1MGV1YPivJ-bQgZw8KD~VMkW9n4N87InksIucO3sPMFC5SuVpMwhDa-fy9t1jk9dwGVeFm6jiKJX2F0VOEzAf2q75Rw2Ia8woX8E0yO5t-MecjOU~5DKtqTUw8MBzu8FVTwaSV0nnuhAOP2xbI9pD4L7GKn1Gk8GSL-hmdUd2n3Iwa5eGA7tZVJ~gEPnmSzj9szfEINAykNQjG2VsD7N8UQxcOjdMcq4E0EAxKRmQZiv-BchmMdBFQiuMqu39P5J3opRO7rQzsWOFpZGD3Cq4vWpbLSFj9jAil8JiQo6VZ1QR5G6wm1zVO4jx2KqD01SB97iB~MLSBj5Tpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)