Key Points
The modified Mayo 2004 and Mayo 2012 staging systems have prognostic value for restaging at 3 and 6 months from treatment initiation.
Migration to a higher stage than the original stage at diagnosis predicts poor prognosis.
Abstract
The utility of systemic light chain (AL) amyloidosis staging systems has been validated for newly diagnosed patients, but their role in restaging after treatment has not been explored. We designed this study to evaluate whether the currently used systems are of prognostic value at 3 and 6 months of starting first-line treatment, and whether stage migration from diagnosis impacts survival. This is a retrospective study including Mayo Clinic patients with AL amyloidosis diagnosed between 1 January 2006 and 30 June 2019; 536 and 204 patients had restaging data for at least 1 system at 3 and 6 months, respectively. Using modified Mayo 2004 staging at 3 months, median overall survival (OSs) were 11.8, 10.8, 4.6, and 1.1 years for stage I, II, IIIa, and IIIb, respectively. Using Mayo 2012, median OSs were 11.8, 9.0, 5.2, and 0.8 years for stage I, II, III, and IV, respectively. Using modified Mayo 2004 staging at 6 months, median OSs were not reached (NR), NR, 5.4, and 0.9 years for stage I, II, IIIa, and IIIb, respectively. Using Mayo 2012, OSs were NR, NR, 4.6, and 0.9 years for stage I, II, III, and IV, respectively. Worsening stage at 3 or 6 months was associated with worse survival than retaining baseline stage. In conclusion, the current staging systems can be used for restaging at 3 and 6 months from treatment initiation. Migration to higher stage predicts poor prognosis.
Introduction
Systemic light chain (AL) amyloidosis is a multisystem disease caused by the deposition of misfolded immunoglobulin light chains produced by clonal plasma cells. The clinical presentations and outcomes are dictated by which organs are involved and the degree of organ dysfunction. The severity of cardiac damage, measured by the concentrations of NT-ProBNP and cardiac troponin, is the most important factor in predicting survival and forms the basis of all AL amyloidosis staging systems.1-3 Tumor burden, estimated by the difference in serum concentrations of the involved and uninvolved light chains (dFLC), was incorporated in the Mayo 2012 staging system, which improved the ability to predict long-term survival.4 The prognostic utility of amyloidosis staging systems has been validated for newly diagnosed patients, including transplant-eligible and -ineligible patients. In addition, they have been shown to have prognostic ability when used for restaging at first relapse.5 However, the role of the currently available prognostic systems for restaging after first-line treatment has not been explored. This is particularly relevant in a disease like AL amyloidosis where the mortality rates are highest in the initial few months after diagnosis.6 Hematologic and cardiac responses with treatment, reflected by a decrease in the values of dFLC and cardiac biomarkers, respectively, have been shown to correlate with survival, with prognostic ability as early as 3 months from initiation of first-line treatment.7 However, the interpretation of those parameters may be confounded by a discrepancy between hematologic and organ responses, such as that seen in some patients treated with immunomodulatory drugs, where treatment-related increases in cardiac biomarkers are seen despite hematologic response.7 We designed this study to evaluate whether the absolute values of dFLC and cardiac biomarkers measured after the initiation of first-line treatment are of prognostic value using the currently available prognostic systems for restaging, and whether stage migration from diagnosis impacts survival. This also allows better prediction of outcomes for those patients who survive the initial few months after diagnosis.
Methods
This is a retrospective study using a prospectively maintained institutional database. Additional data were obtained by review of medical records. All patients provided informed consent for use of their medical record data for research. The study was approved by the institutional review board. We evaluated 1613 patients with biopsy-proven amyloidosis diagnosed between 1 January 2006 and 30 June 2019 and seen in Mayo Clinic within 90 days from diagnosis. We excluded patients with localized amyloidosis, and patients in whom the primary diagnosis was multiple myeloma with lytic lesions (73 patients). Of the remaining 1540 patients, 1357 (88%) had laboratory data available for staging at the time of diagnosis using at least 1 staging system. The prognostic staging systems used in this study were as follows: the Mayo 2004 staging system and its European modifications (2013 and 2015 modifications),3,8 and the Mayo 2012 staging system.2 The Mayo 2004 system was used to stratify patients into 3 groups using a cardiac Troponin T threshold of ≥0.035 µg/L (or high-sensitivity Troponin T threshold ≥50 ng/L),9 and NT-ProBNP threshold of ≥332 ng/L.1 The 2 European modifications of this system were used to further stratify stage III patients into 2 groups using the NT-ProBNP threshold of >8500 ng/L (European 2015 modification),8 or 3 groups using NT-ProBNP threshold >8500 ng/L and systolic blood pressure <100 mmHg (European 2013 modification).3 The Mayo 2012 staging system was used to stratify patients into 4 groups based on thresholds of cardiac Troponin T ≥0.025 µg/L (or high sensitivity Troponin T threshold >40 ng/L),10 NT-ProBNP ≥1800 ng/L, and dFLC ≥18 mg/dL.2 The serum free light chain concentrations were measured using the FREELITE test (The Binding Site, Birmingham, United Kingdom). The following reference ranges for normal values were used: serum κ free light chains (0.33 to 1.94 mg/dL) and serum λ free light chains (0.57 to 2.63 mg/dL).11
The modified Mayo 2004 (2015 European modification) and Mayo 2012 staging systems were then used to restage patients at 3 and 6 months from the time of initiation of first-line treatment; 669 patients had available data for at least one of the intervals. The first-line treatment in these patients included autologous stem cell transplant (ASCT) in 291 patients; among those, 195 underwent upfront ASCT, and 96 underwent ASCT postinduction chemotherapy, most commonly with cyclophosphamide, bortezomib, and dexamethasone/prednisone (61 patients). The most common non–ASCT-based regimens were cyclophosphamide, bortezomib, and dexamethasone/prednisone (n = 148), melphalan and dexamethasone/prednisone (n = 137), bortezomib and dexamethasone (n = 14), ixazomib, cyclophosphamide, and dexamethasone (n = 17), and lenalidomide, cyclophosphamide, and dexamethasone (n = 7). Cardiac response was defined as a decrease by >30% and >300 ng/L in NT-proBNP in patients who had NT-proBNP ≥ 650 ng/L at diagnosis.12 Hematologic response was defined as a reduction of >50% in the dFLC from diagnosis.12
Survival analysis at each time interval included patients who had available laboratory data for restaging. Overall survival (OS) was calculated from the time of diagnosis, and from 3 and 6 months from initiation of first-line treatment. Survival curves were plotted using the Kaplan-Meier method, and survival differences between patients in each stage were estimated using the log-rank test. We then assessed the prognostic value of the modified Mayo 2004 and Mayo 2012 staging systems, restricting the analysis to the subset of patients who had available data for staging at diagnosis, 3 months, and 6 months. For all tests, 2-sided P values <.05 were considered statistically significant. Statistical analysis was performed using the JMP pro software (SAS, Cary, NC).
Results and discussion
OS based on disease stage at diagnosis
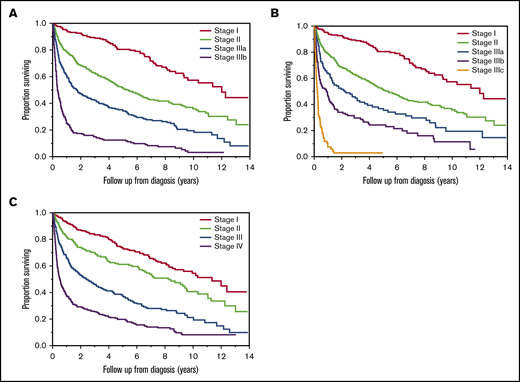
At the time of diagnosis, 1357 patients had available staging for at least 1 system. The clinical characteristics of these patients are shown in Table 1. At the time of analysis, 44% were alive. After a median follow-up of 6.1 (95% CI: 5.6-6.6) years, median OS was 4.0 (95% CI: 3.3-4.6) years. Staging data for the 2015 European modification of Mayo 2004 system were available for 1357 patients. Among those, 264 (19%), 512 (38%), 340 (25%), and 241 (18%) had stage I, II, IIIa, and IIIb, respectively. The median OSs in the 4 groups were 12.0 (95% CI: 9.3-not reached [NR]), 5.4 (95% CI: 4.4-6.4), 1.8 (95% CI: 1.3-2.5), and 0.4 (95% CI: 0.3-0.5) years, respectively (P < .001) (Figure 1A). Staging data for the 2013 European modification of the Mayo 2004 system were available for 1157 patients. Among those, 264 (23%), 512 (44%), 149 (13%), 148 (13%), and 84 (7%) had stage I, II, IIIa, IIIb, and IIIc, respectively. The OSs in the 5 groups were 12.0 (95% CI: 9.3-NR), 5.4 (95% CI: 4.4-6.4), 2.1 (95% CI: 1.3-3.7), 0.9 (95% CI: 0.4-1.2), and 0.2 (95% CI: 0.2-0.3) years, respectively (P < .001) (Figure 1B). The Mayo 2012 stage was available for 1339 patients. Among those, 332 (25%), 266 (20%), 357 (27%), and 384 (29%) patients had stage I, II, III, and IV, respectively. OSs from diagnosis in the 4 groups were 11.4 (95% CI: 8.7-NR), 8.2 (95% CI: 6.2-9.6), 2.4 (95% CI: 1.8-3.3), and 0.5 (95% CI: 0.4-0.7) years, respectively (P < .001) (Figure 1C).
Clinical characteristics at diagnosis of patients with AL amyloidosis included in the study (N = 1357)
| Parameter at diagnosis . | Median (interquartile range) or n (%) . |
|---|---|
| Age, y | 64 (58-71) |
| Sex (male) | 888 (65) |
| Cardiac involvement | 760 (74) |
| Renal involvement | 599 (58) |
| Liver involvement | 167 (16) |
| >1 organ involvement | 618 (60) |
| NT-ProBNP, ng/L | 2442 (501-6669) |
| Troponin T, µg/L | 0.03 (0.01-0.08) |
| dFLC, mg/dL | 19.7 (7.5-53.2) |
| Creatinine, mg/dL | 1.1 (0.9-1.6) |
| Alkaline phosphatase, IU/L | 99 (74-151) |
| Serum albumin, g/dL | 2.8 (2.2-3.2) |
| Serum beta2microglobulin, µg/mL | 3.4 (2.5-5.2) |
| Urine protein, g/24 h | 1.2 (0.2-5.0) |
| Bone marrow plasma cells, % | 10 (5-18) |
| Parameter at diagnosis . | Median (interquartile range) or n (%) . |
|---|---|
| Age, y | 64 (58-71) |
| Sex (male) | 888 (65) |
| Cardiac involvement | 760 (74) |
| Renal involvement | 599 (58) |
| Liver involvement | 167 (16) |
| >1 organ involvement | 618 (60) |
| NT-ProBNP, ng/L | 2442 (501-6669) |
| Troponin T, µg/L | 0.03 (0.01-0.08) |
| dFLC, mg/dL | 19.7 (7.5-53.2) |
| Creatinine, mg/dL | 1.1 (0.9-1.6) |
| Alkaline phosphatase, IU/L | 99 (74-151) |
| Serum albumin, g/dL | 2.8 (2.2-3.2) |
| Serum beta2microglobulin, µg/mL | 3.4 (2.5-5.2) |
| Urine protein, g/24 h | 1.2 (0.2-5.0) |
| Bone marrow plasma cells, % | 10 (5-18) |
OS by stage at diagnosis. The OS from diagnosis in years in AL amyloidosis patients based on 2015 European modification of Mayo 2004 staging (n = 1357) (A), 2013 modification of Mayo 2004 staging (n = 1157) (B), and Mayo 2012 staging (n = 1339) (C).
OS by stage at diagnosis. The OS from diagnosis in years in AL amyloidosis patients based on 2015 European modification of Mayo 2004 staging (n = 1357) (A), 2013 modification of Mayo 2004 staging (n = 1157) (B), and Mayo 2012 staging (n = 1339) (C).
Restaging at 3 months from treatment
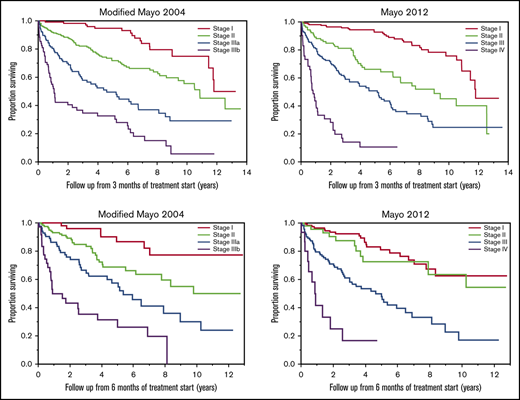
At 3 months from initiation of the first-line treatment, staging data were available for 536 patients using the modified Mayo 2004 staging, and for 528 patients using the Mayo 2012 staging; among those, 29 (5%) and 27 (5%) patients had initiated a subsequent line of treatment, respectively. Using the modified Mayo 2004 staging system, 121 (23%), 225 (42%), 117 (22%), and 73 (14%) patients had stage I, II, IIIa, and stage IIIb disease, respectively. The median OSs from 3 months in each of these 4 groups were as follows: 11.8 (95% CI: 11.4-NR), 10.8 (95% CI: 9.4-NR), 4.6 (95% CI: 2.8-6.7), and 1.1 (95% CI: 0.8-2.6) years, respectively (P < .001) (Figure 2A). Using the Mayo 2012 staging system, 208 (39%), 106 (20%), 170 (32%), and 44 (8%) patients had stage I, II, III, and stage IV disease, respectively. OSs from 3 months in these 4 groups were 11.8 (95% CI: 10.9-NR), 9.0 (95% CI: 6.2-NR), 5.2 (95% CI: 3.3-6.1), and 0.8 (95% CI: 0.6-1.1) years, respectively (P < .001) (Figure 2B).
OS by stage at 3 months. OS (in years) from 3 months of starting first-line treatment based the modified Mayo 2004 (n = 536) (A) and Mayo 2012 (n = 528) (B) stage in AL amyloidosis patients at 3 months.
OS by stage at 3 months. OS (in years) from 3 months of starting first-line treatment based the modified Mayo 2004 (n = 536) (A) and Mayo 2012 (n = 528) (B) stage in AL amyloidosis patients at 3 months.
Restaging at 6 months from treatment
At 6 months from initiation of the first-line treatment, modified Mayo 2004 staging data were available for 304 patients, and Mayo 2012 staging data were available for 299 patients. Among those, 57 (19%) and 56 (19%) patients had initiated a subsequent line of treatment, respectively. Using the modified Mayo 2004 staging system, 58 (19%), 124 (41%), 86 (28%), and 36 (12%) patients had stage I, II, IIIa, and stage IIIb disease, respectively. The median OSs from 6 months in these 4 groups were not reached (NR) (95% CI: NR-NR), NR (95% CI: 7.8-NR), 5.4 (95% CI: 3.2-9.0), and 0.9 (95% CI: 0.7-3.7) years, respectively (P < .001) (Figure 3A). Using the Mayo 2012 staging system, 120 (40%), 49 (16%), 115 (38%), and 15 (5%) patients had stage I, II, III, and stage IV disease, respectively. The OSs in each of these 4 groups were NR (95% CI: 8.4-NR), NR (95% CI: 7.9-NR), 4.6 (95% CI: 3.0-6.5), and 0.9 (95% CI: 0.2-1.8) years, respectively (P < .001) (Figure 3B).
OS by stage at 6 months. OS (in years) from 6 months of starting first-line treatment based on modified Mayo 2004 (n = 304) (A) and Mayo 2012 (n = 299) (B) stage in AL amyloidosis patients at 6 months.
OS by stage at 6 months. OS (in years) from 6 months of starting first-line treatment based on modified Mayo 2004 (n = 304) (A) and Mayo 2012 (n = 299) (B) stage in AL amyloidosis patients at 6 months.
Impact of stage migration at 3 months on OS
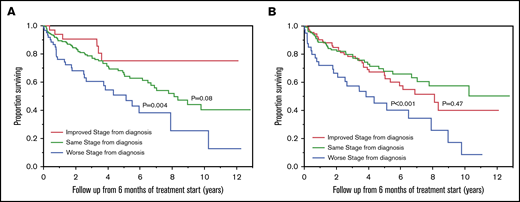
At 3 months from the start of treatment, 379 patients retained the original modified Mayo 2004 stage, 40 patients had improvement by 1 (38 patients) or more stages (2 patients), and 97 patients had worsening by 1 (77 patients) or more stages (20 patients). The OSs from 3 months were 10.8 (95% CI: 6.3-11.8) years in patients who had an improved modified 2004 stage, 10.5 (95% CI: 8.5-12.5) years in patients who retained the original modified 2004 stage, and 4.0 (95% CI: 2.3-6.1) years in patients who had a worse modified 2004 stage (Figure 4A). Using the Mayo 2012 staging system, 300 patients retained the original stage, 142 patients had improvement by 1 (120 patients) or more stages (22 patients), and 65 patients had worsening by 1 (54 patients) or more stages (11 patients). OSs were 10.5 (95% CI: 8.1-NR) years in patients who had improvement in Mayo 2012 stage, 9.8 (95% CI: 6.9-12.5) years in patients who retained the original Mayo 2012 stage, and 4.0 (95% CI: 2.3-9.4) years in patients who had worse Mayo 2012 stage (Figure 4B). When the analysis was restricted to patients who had an advanced stage (>2) at diagnosis, an improvement in stage at 3 months was associated with longer survival compared with retaining original stage, with both modified Mayo 2004 (10.8 vs 3.5 years; P < .001), and Mayo 2012 (8.1 vs 2.6 years; P < .001) staging systems (Table 2).
OS by stage migration at 3 months. OS (in years) from 3 months of starting first-line treatment in AL amyloidosis patients who had improved stage (red curve), same stage (green curve), or worse stage (blue curve) at 3 months of starting first-line treatment based on modified Mayo 2004 (A) and Mayo 2012 (B) staging systems. The P values represent the association between the groups of patients with improved stage compared with patients with the same stage as diagnosis, and patients with worse stage compared with patients with the same stage as diagnosis.
OS by stage migration at 3 months. OS (in years) from 3 months of starting first-line treatment in AL amyloidosis patients who had improved stage (red curve), same stage (green curve), or worse stage (blue curve) at 3 months of starting first-line treatment based on modified Mayo 2004 (A) and Mayo 2012 (B) staging systems. The P values represent the association between the groups of patients with improved stage compared with patients with the same stage as diagnosis, and patients with worse stage compared with patients with the same stage as diagnosis.
OS based on stage migration
| . | Modified Mayo 2004 stage . | Mayo 2012 stage . | ||
|---|---|---|---|---|
| OS (years) if stage changed from: . | Same vs worsened . | Same vs improved . | Same vs worsened . | Same vs improved . |
| Baseline to 3 mo | 10.5 vs 4.0; P < .001 | 10.5 vs 10.8; P = .27 | 9.8 vs 4.0; P = .01 | 9.8 vs 10.5; P = .21 |
| Baseline to 6 mo | 8.1 vs 5.1; P = .004 | 8.1 vs NR; P = .08 | NR vs 3.8; P < .001 | NR vs 8.1; P = .47 |
| Baseline to 3 mo restricting to > stage 2 patients | Not assessed | 3.5 vs 10.8; P < .001 | Not assessed | 2.6 vs 8.1; P < .001 |
| Baseline to 6 mo restricting to > stage 2 patients | Not assessed | 5.0 vs NR; P = .02 | Not assessed | 3.7 vs 5.9; P = .12 |
| . | Modified Mayo 2004 stage . | Mayo 2012 stage . | ||
|---|---|---|---|---|
| OS (years) if stage changed from: . | Same vs worsened . | Same vs improved . | Same vs worsened . | Same vs improved . |
| Baseline to 3 mo | 10.5 vs 4.0; P < .001 | 10.5 vs 10.8; P = .27 | 9.8 vs 4.0; P = .01 | 9.8 vs 10.5; P = .21 |
| Baseline to 6 mo | 8.1 vs 5.1; P = .004 | 8.1 vs NR; P = .08 | NR vs 3.8; P < .001 | NR vs 8.1; P = .47 |
| Baseline to 3 mo restricting to > stage 2 patients | Not assessed | 3.5 vs 10.8; P < .001 | Not assessed | 2.6 vs 8.1; P < .001 |
| Baseline to 6 mo restricting to > stage 2 patients | Not assessed | 5.0 vs NR; P = .02 | Not assessed | 3.7 vs 5.9; P = .12 |
The impact of improving, worsening, or retaining original stage at 3 and 6 mo in the entire cohort, and in patients who had advanced stage at diagnosis.
Impact of stage migration at 6 months on OS
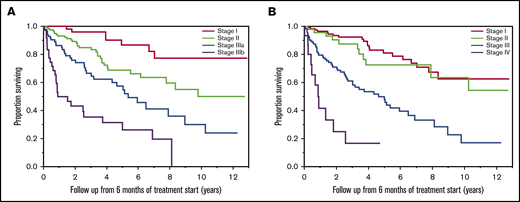
At 6 months from the start of treatment, 190 patients retained the original modified Mayo 2004 stage, 33 patients had improvement by 1 (31 patients) or more stages (2 patients), and 61 patients had worsening by 1 (51 patients) or more stages (10 patients). OSs were NR (95% CI: NR-NR), 8.1 (95% CI: 6.5-NR), and 5.1 (95% CI: 2.5-7.9) years in patients who had improved, same, or worse modified Mayo 2004 stage, respectively (Figure 5A). Using the Mayo 2012 staging system, 127 patients retained the original stage, 110 patients had improvement by 1 (86 patients) or more stages (24 patients), and 41 patients worsening by 1 (37 patients) or more stages (4 patients). OSs were 8.1 (95% CI: 5.0-NR), NR (95% CI: 7.0-NR), 3.8 (95% CI: 1.8-7.9) years in patients who had better, same, or worse Mayo 2012 stage at 6 months compared with diagnosis, respectively (Figure 5B). When the analysis was restricted to patients who had an advanced stage at diagnosis, improvement in stage was associated with better survival than retaining original stage when the modified Mayo 2004 staging system was used (NR vs 5.0 years; P = .02), but it was not statistically significant when the Mayo 2012 staging system was used (5.9 vs 3.7 years; P = .12) (Table 2).
OS by stage migration at 6 months. OS (in years) from 6 months of starting first-line treatment in AL amyloidosis patients who had improved stage (red curve), same stage (green curve), or worse stage (blue curve) at 6 months of starting first-line treatment based on modified Mayo 2004 (A) and Mayo 2012 (B) staging systems. The P values represent the association between the groups of patients with improved stage compared with patients with the same stage as diagnosis, and patients with worse stage compared with patients with the same stage as diagnosis.
OS by stage migration at 6 months. OS (in years) from 6 months of starting first-line treatment in AL amyloidosis patients who had improved stage (red curve), same stage (green curve), or worse stage (blue curve) at 6 months of starting first-line treatment based on modified Mayo 2004 (A) and Mayo 2012 (B) staging systems. The P values represent the association between the groups of patients with improved stage compared with patients with the same stage as diagnosis, and patients with worse stage compared with patients with the same stage as diagnosis.
OS by stage in patients with staging data at all intervals (n = 193). OS (in years) from 3 months of starting first-line treatment based on the modified Mayo 2004 (A) and Mayo 2012 (B) stage in AL amyloidosis patients at 3 months. OS (in years) from 6 months of starting first-line treatment based on the modified Mayo 2004 (C) and Mayo 2012 (D) stage in AL amyloidosis patients at 6 months.
OS by stage in patients with staging data at all intervals (n = 193). OS (in years) from 3 months of starting first-line treatment based on the modified Mayo 2004 (A) and Mayo 2012 (B) stage in AL amyloidosis patients at 3 months. OS (in years) from 6 months of starting first-line treatment based on the modified Mayo 2004 (C) and Mayo 2012 (D) stage in AL amyloidosis patients at 6 months.
Survival analysis among subjects with available staging data for all intervals
Among all patients included in the study, 193 patients had staging data available at diagnosis, 3 months, and 6 months from starting first-line treatment using at least 1 staging system (modified Mayo 2204 and/or Mayo 2012). At the time of diagnosis, OSs were NR (95% CI: 6.2-NR), 9.2 (95% CI: 6.5-NR), 5.6 (95% CI: 3.1-NR), and 1.4 (95%Ci: 0.9-7.5) years in patients with stage I, II, IIIa, and IIIb disease, respectively, using modified Mayo 2004 staging. OSs were NR (95% CI: 8.2-NR), NR (95% CI: 5.7-NR), 4.9 (95% CI: 3.7-NR), and 5.6 (95% CI: 2.3-7.5) years in patients with stage I, II, III, and IV disease, respectively, using Mayo 2012 staging. At 3 months from starting first-line treatment, OSs were NR (95% CI: 6.9-NR), 8.6 (95% CI: 5.8-NR), 4.6 (95% CI: 2.8-NR), and 1.1 (95% CI: 0.7-5.2) years in patients with stage I, II, IIIa, and IIIb disease, respectively, using modified Mayo 2004 staging (Figure 6A). OSs were NR (95% CI: 8.2-NR), 6.7 (95% CI: 3.9-NR), 4.6 (95% CI: 2.9-7.1), and 1.1 (95% CI: 0.5-2.8) years in patients with stage I, II, III, and IV disease, respectively, using Mayo 2012 staging (Figure 6B). At 6 months, OSs were NR (95% CI: NR-NR), NR (95% CI: NR-5.5), 5.9 (95% CI: 4.3-NR), and 0.8 (95% CI: 0.4-2.5) in patients with stage I, II, IIIa, and IIIb disease, respectively, using modified Mayo 2004 staging (Figure 6C). OSs were NR (95% CI: 7.8-NR), NR (95% CI: 7.9-NR), 4.3 (95% CI: 2.5-5.9), and 0.8 (95% CI: 0.8-0.2-2.6), in patients with stage I, II, III, and IV disease, respectively, using Mayo 2012 staging (Figure 6D).
Since the introduction of amyloidosis staging systems, there has been significant improvement in survival outcomes attributed to earlier diagnosis, deeper responses achieved with novel treatments, and decreased transplant-related mortality.6,13,14 Despite this, the currently available staging systems, based on the concentration of cardiac biomarkers and dFLC, retain their ability to predict survival at the time of diagnosis. This was demonstrated in this study where the Mayo 2012 and the European modification of the Mayo 2004 staging system had the ability to discriminate 4 groups of patients with different survival outcomes.
Despite improvement in survival, amyloidosis remains a disease of high early mortality.6 Thus, the ability to predict prognosis after diagnosis and at different stages of disease is of clinical importance. It is known that hematologic and cardiac responses after first-line treatment are associated with improved survival.7 However, some patients may have a discrepancy in hematologic and cardiac responses, which confounds the interpretation of treatment efficacy.15 The absolute value of the dFLC posttreatment was shown to impact survival irrespective of the baseline dFLC value, which led to the introduction of the very good partial response category, defined by dFLC <40 mg/L, to identify a subset of patients with survival intermediate between partial and complete response.7 This suggests that the absolute value of the amyloidogenic free light chain, and not only the relative reduction, is of prognostic value after treatment. In a previous study in Mayo Clinic, the modified Mayo 2004 and Mayo 2012 staging systems had prognostic value when applied at the time of first relapse.5 In our study, both Mayo 2004/European staging system and Mayo 2012 systems at 3 months from initiating first-line treatment discriminated 4 groups with different survival. At 6 months from initiation of first-line treatment, Mayo 2004/European staging system also discriminated 4 groups with different survival. When the Mayo 2012 staging system was used, there was no significant difference in OS between stage I and II (P = .44), but both stages were associated with improved survival compared with stage III and IV. This may be secondary to lack of adequate power.
In this study, we also observed that migration to a higher stage as early as 3 months after treatment identified a subset of patients with worse prognosis using either staging system. An improvement in stage was associated with significantly improved OS compared with retaining the original stage in the subset of patients with advanced disease at diagnosis. Because of the nature of the study design, these findings are only applicable to patients who survived at least 3 months after treatment start. In this study, the sample size was insufficient to assess separately the impact of migration between each stage pair, and thus, further evaluation is needed to determine whether migration from stage II to stage I has prognostic significance.
This study is limited by its retrospective nature, heterogeneous treatment regimens, and the lack of results for 1 or more parameters of staging systems in a subset of patients, which may create selection bias. However, when the analysis was restricted to patients with available data for staging at diagnosis, and 3 months and 6 months from treatment start, the findings were consistent with those in the original cohort, although this analysis is limited by small sample size.
Despite these limitations, our study, based on a large cohort with long follow-up, presents novel findings supporting the utility of the currently available staging systems for prognostication at 3 and 6 months from treatment initiation; this is increasingly important as survival outcomes continue to improve, and especially when the hematologic and organ responses to treatment are conflicting or not available.
In conclusion, the current restaging systems retain their prognostic ability in the era of modern treatment, and in addition, have prognostic ability when used for restaging at 3 and 6 months from initiation of first-line treatment. Migration to a higher disease stage predicts decreased survival, whereas improvement in stage predicts longer survival in the subset of patients with advanced stage at diagnosis.
Data from this paper may be acquired by contacting the corresponding author, Shaji K. Kumar, e-mail: kumar.shaji@mayo.edu.
Authorship
Contribution: N.A. and S.K.K. collected and analyzed the data, wrote the first draft, and approved the final version of the manuscript; and A.D., E.M., F.K.B., P.K., M.Q.L., Y.L.H., A.F., M.A.H., S.R.H., N.L., D.D., J.A.L., R.S.G., Y.L., W.I.G., T.K., R.W., R.A.K., S.V.R., and M.A.G. managed patients, revised the manuscript critically, and approved the final version of the manuscript.
Conflict-of-interest disclosure: P.K. received research funding from Takeda Pharmaceuticals, Celgene, and Amgen. A.D. received research funding from Celgene, Millennium Pharmaceuticals, Pfizer, and Janssen and received a travel grant from Pfizer. D.D. serves as a consultant for Alexion, Apellis, GlaxoSmithKline, Janssen, Millenium/Takeda, and Rigel and received research funding from Juno Therapeutics and Karyopharm Therapeutics. M.A.G. served as a consultant for Millennium Pharmaceuticals and received honoraria from Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Novartis, GlaxoSmithKline, Prothena, Ionis Pharmaceuticals, and Amgen. M.Q.L. received research funding from Celgene. N.L. serves on an advisory board for Takeda Pharmaceuticals. S.K.K. served as a consultant for Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Janssen, and Bristol-Myers Squibb and received research funding from Celgene, Millennium Pharmaceuticals, Novartis, Onyx Pharmaceuticals, AbbVie, Janssen, and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Shaji K. Kumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.