Key Points
With sufficient resources, HSCT can safely continue in the COVID-19 pandemic if primary responsibility for COVID-19 patients is not required.
Cryopreservation of unrelated donor products correlated with slightly lower chimerism but no difference in clinical outcomes at 100 days.
Abstract
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), identified in late 2019 as the causative agent of COVID-19, was declared a pandemic by the World Health Organization on 11 March 2020. Widespread community transmission in the United States triggered a nationwide shutdown, raising major challenges for administration of hematopoietic stem cell transplant (HSCT) and chimeric antigen receptor (CAR)-T cell therapies, leading many centers to delay or cancel operations. We sought to assess the impact of the COVID-19 pandemic on operations and clinical outcomes for HSCT and CAR-T cellular therapies at the Dana-Farber Cancer Institute by reviewing administration and outcomes in 127 cell therapy patients treated during the initial COVID-19 surge: 62 adult allogeneic HSCT (allo-HSCT), 38 autologous HSCT (auto-HSCT), and 27 CAR-T patients. Outcomes were compared with 66 allo-HSCT, 43 auto-HSCT, and 33 CAR-T patients treated prior to the pandemic. A second control cohort was evaluated for HSCT groups to reflect seasonal variation in infections. Although there were changes in donor selection and screening as well as cryopreservation patterns of donor products, no differences were observed across groups in 100-day overall survival, progression-free survival, rates of non–COVID-19 infections, including hospital length of stay, neutrophil engraftment, graft failure, acute graft-versus-host disease in allo-HSCT patients, or cytokine release syndrome and neurotoxicity in CAR-T patients. No HSCT patients contracted COVID-19 between days 0 and 100. One CAR-T patient contracted COVID-19 at day +51 and died of the disease. Altogether, our data indicate that cellular therapies can be safely administered throughout the ongoing COVID-19 pandemic with appropriate safeguards.
Introduction
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which leads to the infectious respiratory disease COVID-19, emerged in the city of Wuhan, China, in late 2019, spread worldwide within 3 months, and was declared a pandemic by the World Health Organization on 11 March 2020.1 The first case of COVID-19 reported from community spread in the United States occurred in February 2020, and a nationwide lockdown was declared in the United States on 15 March 2020 to curb the rapid rise in cases. This abrupt shutdown presented major challenges in coordination and delivery of hematopoietic stem cell transplant (HSCT) and immune cell–based therapies, including chimeric antigen receptor T cells (CAR-T). Many other transplant programs were forced to reduce transplant volume, if not stop completely. Guidance from the European Society for Blood and Marrow Transplantation included deferral of nonurgent transplants when possible.2-4 The ban on international air travel and interruption of domestic routes disrupted the reliable transport of donor products. Restrictions on travel rendered evaluation difficult for prospective donors out of concern for travel-related COVID-19 exposures.
At the Dana-Farber Cancer Institute (DFCI), we made several important adjustments but continued treating patients in whom therapy was deemed urgent. In this retrospective study, we describe the impact of the COVID-19 pandemic on the operations in our program and assess the outcomes of HSCT and CAR-T therapy in patients treated in the first 3 months of the pandemic. Outcomes were compared with patients treated during the 3 months before the pandemic and over the same period of time 1 year prior to the pandemic (for HSCT patients), with particular focus on early transplant outcomes, such as engraftment, acute graft-versus-host disease (aGVHD), as well as infection rates and mortality at day 100.
Methods
Patient selection
This study was approved by the Institutional Review Board of the DFCI/Brigham and Women’s Cancer Center (BWCC). Assessment of donor workflow disruptions and data regarding unrelated donor product procurement were obtained from our donor services team, in conjunction with the National Donor Marrow Program (NMDP). Data on the impact of donor product cryopreservation were obtained from our cell manipulation core facility. We analyzed all adult patients who underwent allo-HSCT, auto-HSCT, and CAR-T therapy from December 15, 2019 to June 15, 2020 at the DFCI/BWCC. The case cohort (cohort A) consisted of patients who underwent HSCT or CAR-T therapy from 15 March to 15 June 2020, whereas the prepandemic control cohort (cohort B) included HSCT or CAR-T patients treated from 15 December 2019 to 14 March 2020. An additional cohort of patients undergoing HSCT from 15 March to 15 June 2019 (cohort C) was used as control for HSCT outcomes to eliminate the potential confounding effects of seasonality on transplant outcomes, primarily infections.
Statistical analysis
Baseline and pretransplant operational characteristics were reported descriptively and compared between cohorts using Fisher exact test, χ2 test, or Wilcoxon rank-sum test, as appropriate. Clinical outcomes of interest included progression-free survival (PFS), overall survival (OS), nonrelapse mortality (NRM), relapse, infection, engraftment, aGVHD, chimerism, and toxicity. PFS was defined as the time from stem cell or CAR-T infusion to disease relapse, progression, or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored at the time last seen alive and relapse or progression free. OS was defined as the time from cell infusion to death from any cause. Patients who were alive or lost to follow-up were censored at the time last seen alive. The Kaplan-Meier method was used to estimate PFS and OS, whereas cumulative incidence of NRM, relapse, and graft-versus-host disease (GVHD) was estimated in the context of a competing risks framework. NRM and relapse were treated as a competing event to each other, and death or relapse without developing GVHD served as a competing event for GVHD. The log-rank test and Gray test were used for group comparison of PFS and OS and cumulative incidence of NRM, relapse, and GVHD, respectively. All P values were 2-sided at a significance level of .05 and multiplicity was not considered. All calculations were performed using SAS 9.3 (SAS Institute, Inc, Cary, NC) and R version 3.3.2.
COVID-19 testing
Testing for COVID-19 in Boston began in February 2020 by the Massachusetts Department of Public Health. Initially, patients admitted to DFCI/BWCC with symptoms concerning for COVID-19 were tested by the Department of Public Health, with samples being sent to the CDC for confirmatory testing. DFCI/BWCC testing for admitted patients with symptoms began in early March. The most frequent symptom prompting COVID-19 testing was fever. Beginning 26 April 2020, all patients admitted to DFCI/BWCC were tested for COVID-19 regardless of the presence of symptoms upon admission (within 48 hours) or 48 hours prior to a planned admission (later liberalized to 72 hours prior to admission). Mask wearing and daily self-checks for medical staff were mandatory to prevent nosocomial spread of respiratory infection.
Results
Impact on clinical operations
Donor evaluation.
The donor services team at DFCI, in conjunction with directives from the NMDP, made several changes to mitigate risks in the donor evaluation process, beginning 9 March 2020. Because of the disruption and delays in air travel, the NMDP required that all unrelated donor products be cryopreserved upon arrival and that conditioning not be started on the recipient until product was received. In addition, the NMDP mandated preferential collection of peripheral blood stem cells to reduce utilization of hospital equipment and operating rooms as demanded by marrow harvests.
Prospective related donors over age 60 and/or those who lived out of state or internationally (requiring airplane travel for donation) were excluded during this time. For related donors who were deemed non–high risk, multiple procedural changes were made to reduce face-to-face contact during their evaluations. First, prior to physical examination, a Health History Screening Questionnaire was administered electronically or by phone rather than in person. Additional donor counseling was carried out via phone by advanced practice providers prior to in-person visits for physical examination. Verbal COVID-19 symptom screens on donors were conducted at 4 time points: at first contact, 1 day prior to physical examination, day of physical examination, and day prior to recipient start of conditioning. Donors deemed high risk for COVID-19 exposures were screened once more on day of collection. Related donors were advised against travel during the donation timeframe and asked to report any COVID-19 exposure or symptoms. To curtail use of public transportation, local housing was provided to donors to facilitate evaluation/collection and minimize time from physical examination to collection. Donors were educated on self-administration of granulocyte colony-stimulating factor for stem cell mobilization to avoid clinic visits and exposure risk.
In the pool of related and unrelated (for NMDP) collections facilitated by DFCI donor services between 15 March 2020 and 15 June 2020, only 2 out of 54 donors required nasopharyngeal polymerase chain reaction COVID-19 testing for symptoms after physical examination but before stem cell collection. In both these cases, the donor tested negative for COVID-19. No stem cell collections were delayed because of donor illness. One unrelated donor who was initially cleared for donation was later deferred because of being in a high COVID-19 risk group. Throughout the pandemic period, the NMDP worked very closely with DFCI donor services to ensure continued access to donors and timely acquisition of products.
We observed a notable reduction in the number of unrelated transplants during the first few months of the pandemic. In cohort A, the number of unrelated donor transplants declined from 69.2% to 57.8% (P = .21) compared with the previous 3 months (cohort B) (Table 1). There was also a shift toward using domestic unrelated donors, likely because of restrictions in international air travel. In cohort B, a majority (62.5%) of the unrelated donor transplants involved international donors. This trend reversed after the start of the pandemic, wherein the percentage of international donors declined to 37% (Table 1). However, this may have been an aberration unrelated to the pandemic, as 46.6% of donors were international in control cohort C. There was no difference in median time from donor workup request to day of transplant in the COVID-19 period compared with the pre–COVID-19 period (43 vs 42.5 days) (Table 1). In accordance with NMDP guidelines, all unrelated donor PBMC products were cryopreserved. Half of the donor products in the case cohort were fresh (n = 32). These consisted of matched related (n = 15), 7/8 mismatched related (n = 1), haploidentical (n = 11), and matched unrelated marrow (n = 5) donors.
HSCT operational characteristics
| . | Case cohort A . | Control cohort B . | Control cohort C . | P . | |
|---|---|---|---|---|---|
| . | 3/15/20 to 6/15/20 . | 12/15/19 to 3/14/20 . | 3/15/19 to 6/15/19 . | A vs B . | A vs C . |
| Allogeneic HSCT | |||||
| Patient, n | 64 | 68 | 76 | ||
| Accrual rate per month | 21.2 | 23 | 25.1 | ||
| Operational | |||||
| Referral source | |||||
| Internal | 35 (54.7) | 39 (57.4) | 35 (46.1) | ||
| Domestic | 29 (45.3) | 29 (42.6) | 41 (53.9) | ||
| International | 0 | 0 | |||
| Context, n (%) | .0008 | <.0001 | |||
| Inpatient | 46 (71.9) | 64 (94.1) | 75 (98.7) | ||
| Ambulatory | 18 (28.1) | 4 (5.9) | 1 (1.3) | ||
| Hospital length of stay, d | .24 | .001 | |||
| Median (range) | 24 (7, 75) | 23.5 (2,67) | 20 (2, 54) | ||
| Product cryopreserved, n (%) | <.0001 | <.0001 | |||
| No | 32 (50) | 64 (94.1) | 72 (94.7) | ||
| Yes | 32 (50) | 4 (5.9) | 4 (5.3) | ||
| Product procurement | |||||
| Workup to day 0, median (range), d | 43 (18, 121) | 42.5 (17, 157) | 43 (22, 190) | .79 | .33 |
| Product source, n (%) | |||||
| Marrow | 10 (21.7) | 10 (17.9) | 16 (27.6) | .63 | .65 |
| PBSC | 36 (78.3) | 46 (82.1) | 42 (72.4) | ||
| Primary/secondary* | |||||
| Primary | 44 (95.7) | 56 (100) | 57 (98.3) | .21 | .58 |
| Secondary | 2 (4.3) | 1 (1.7) | |||
| Product origin, n (%) | |||||
| Domestic | 29 (63) | 21 (37.5) | 31 (53.4) | .017 | .42 |
| International | 17 (37) | 35 (62.5) | 27 (46.6) | ||
| Autologous HSCT | |||||
| Patients, n | 39 | 43 | 54 | ||
| Accrual rate per month | 12.9 | 14.5 | 17.9 | ||
| Operational | |||||
| Referral source, n (%) | .16 | .04 | |||
| Internal | 23 (59) | 17 (39.5) | 18 (33.3) | ||
| Domestic | 16 (41) | 25 (58.1) | 35 (64.8) | ||
| International | 0 | 1 (2.3) | 1 (1.9) | ||
| Hospital length of stay, d | .03 | .004 | |||
| Median (range) | 20 (15, 29) | 20 (15,30) | 19 (15, 41) | ||
| . | Case cohort A . | Control cohort B . | Control cohort C . | P . | |
|---|---|---|---|---|---|
| . | 3/15/20 to 6/15/20 . | 12/15/19 to 3/14/20 . | 3/15/19 to 6/15/19 . | A vs B . | A vs C . |
| Allogeneic HSCT | |||||
| Patient, n | 64 | 68 | 76 | ||
| Accrual rate per month | 21.2 | 23 | 25.1 | ||
| Operational | |||||
| Referral source | |||||
| Internal | 35 (54.7) | 39 (57.4) | 35 (46.1) | ||
| Domestic | 29 (45.3) | 29 (42.6) | 41 (53.9) | ||
| International | 0 | 0 | |||
| Context, n (%) | .0008 | <.0001 | |||
| Inpatient | 46 (71.9) | 64 (94.1) | 75 (98.7) | ||
| Ambulatory | 18 (28.1) | 4 (5.9) | 1 (1.3) | ||
| Hospital length of stay, d | .24 | .001 | |||
| Median (range) | 24 (7, 75) | 23.5 (2,67) | 20 (2, 54) | ||
| Product cryopreserved, n (%) | <.0001 | <.0001 | |||
| No | 32 (50) | 64 (94.1) | 72 (94.7) | ||
| Yes | 32 (50) | 4 (5.9) | 4 (5.3) | ||
| Product procurement | |||||
| Workup to day 0, median (range), d | 43 (18, 121) | 42.5 (17, 157) | 43 (22, 190) | .79 | .33 |
| Product source, n (%) | |||||
| Marrow | 10 (21.7) | 10 (17.9) | 16 (27.6) | .63 | .65 |
| PBSC | 36 (78.3) | 46 (82.1) | 42 (72.4) | ||
| Primary/secondary* | |||||
| Primary | 44 (95.7) | 56 (100) | 57 (98.3) | .21 | .58 |
| Secondary | 2 (4.3) | 1 (1.7) | |||
| Product origin, n (%) | |||||
| Domestic | 29 (63) | 21 (37.5) | 31 (53.4) | .017 | .42 |
| International | 17 (37) | 35 (62.5) | 27 (46.6) | ||
| Autologous HSCT | |||||
| Patients, n | 39 | 43 | 54 | ||
| Accrual rate per month | 12.9 | 14.5 | 17.9 | ||
| Operational | |||||
| Referral source, n (%) | .16 | .04 | |||
| Internal | 23 (59) | 17 (39.5) | 18 (33.3) | ||
| Domestic | 16 (41) | 25 (58.1) | 35 (64.8) | ||
| International | 0 | 1 (2.3) | 1 (1.9) | ||
| Hospital length of stay, d | .03 | .004 | |||
| Median (range) | 20 (15, 29) | 20 (15,30) | 19 (15, 41) | ||
The NMDP defines primary collection as the first donation event for a particular recipient-donor pairing. Secondary collection refers to subsequent donation of this original pairing (eg, for a stem cell boost).
Hospital impact.
DFCI inpatients are treated at the Brigham and Women’s Hospital. Our hospital system began to see a precipitous spike in positive COVID-19 tests and inpatient admissions in late March 2020. The peak in case positivity and admissions occurred on 23 April 2020. Around this time, the test positivity rate in our hospital system was 25%. At the peak, there were 171 total COVID-19 patients admitted, with ∼86 in the intensive care unit. A building of single occupancy rooms was entirely converted to makeshift intensive care units to expand capacity. Following this peak, there was a slow decrease in hospital utilization by COVID-19 patients until early June 2020, when COVID-19 admissions reached a plateau and hospital operations returned to prepandemic levels (written communication, Charles A. Morris, 5 August 2020, Brigham and Women’s Hospital internal data). Our hospital did not mandate a decrease in urgent HSCT and CAR-T therapy. However, our divisions electively reduced transplant volume partially in preparation for such a mandate and to preserve hospital resources for COVID-19 patients.
Allo-HSCT.
The characteristics of the patients, including donor type, conditioning regimen, and hematopoietic cell transplant-comorbidity index (HCT-CI) for the allo-HCT group, are shown in Table 2. Number of patients transplanted and accrual rate per month decreased during the pandemic compared with the control groups (accrual rate/month A vs B vs C: 21.2 vs 23 vs 25.1) (Table 1). No significant differences in patient age, sex, diagnosis, conditioning intensity (myeloablative vs reduced intensity), donor type, or referral patterns (ie, intra- vs extrainstitutional) were observed. During these timeframes, there were no international referrals for allo-HSCT. We made a programmatic decision to defer allo-HSCT for patients with lower-risk myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), or nonmalignant conditions in which the delay of 3 to 4 months was unlikely to negatively impact long-term outcomes. Decisions regarding which “higher-risk” patients should proceed to transplant and which could be deferred were left to the discretion of individual attending physicians. We also increased our capacity to perform outpatient reduced intensity conditioning allo-HSCT (Table 1). The use of marrow vs peripheral blood stem cells was not significantly different in our cohort (marrow product % A vs C 21.7 vs 27.6, P = .65; A vs B 21.7 vs 17.9, P = .63) (Table 1). The median hospital length of stay during the pandemic period was increased compared with the seasonal matched control from 1 year prior (A vs C 24 days vs 20 days, P = .001), but not compared with the cohort 3 months prior to the pandemic (A vs B 24 vs 23.5 days, P = .24) (Table 1). No systematic changes were implemented for care in the first 100 days posttransplant. For patients beyond day +100, use of telehealth was encouraged when feasible.
HSCT patient characteristics
| Patient characteristics . | Case cohort A . | Control cohort B . | Control cohort C . | P . | |
|---|---|---|---|---|---|
| 3/15/20 to 6/15/20 . | 12/15/19 to 3/14/20 . | 3/15/19 to 6/15/19 . | A vs B . | A vs C . | |
| Allogeneic HSCT | |||||
| Age, median (range), y | 60 (22, 77) | 62 (19, 74) | 63.5 (21, 78) | .19 | .1 |
| Sex, n (%) | .6 | .61 | |||
| Female | 30 (46.9) | 36 (52.9) | 32 (42.1) | ||
| Male | 34 (53.1) | 32 (47.1) | 44 (57.9) | ||
| Diagnosis, n (%) | .12 | .14 | |||
| Acute myeloid leukemia (AML) | 18 (28.1) | 21 (30.9) | 23 (30.3) | ||
| Acute lymphocytic leukemia | 17 (26.6) | 8 (11.8) | 13 (17.1) | ||
| Chronic myeloid leukemia | 3 (4.7) | 0 | 3 (3.9) | ||
| MDS/MPN(MDS/MPN) | 11 (17.2) | 24 (35.3) | 26 (34.2) | ||
| Therapy-related MDS/AML | 3 (4.7) | 4 (5.9) | 2 (2.6) | ||
| Other leukemia (includes chronic lymphocytic leukemia) | 2 (3.1) | 1 (1.5) | 3 (3.9) | ||
| Hodgkin lymphoma | 0 | 0 | 2 (2.6) | ||
| Non-Hodgkin lymphoma | 8 (12.5) | 8 (11.8) | 2 (2.6) | ||
| Aplastic anemia | 2 (3.1) | 1 (1.5) | 1 (1.3) | ||
| Sickle cell anemia | 0 | 1 (1.5) | 1 (1.3) | ||
| Conditioning intensity, n (%) | 1 | .3 | |||
| Myeloablative | 22 (34.4) | 23 (33.8) | 20 (26.3) | ||
| Nonmyeloablative | 41 (64.1) | 44 (64.7) | 56 (73.7) | ||
| No conditioning | 1 (1.6) | 1 (1.5) | 0 | ||
| Donor, n (%) | .37 | .88 | |||
| Matched related donor (8/8) | 15 (23.4) | 9 (13.2) | 14 (18.4) | ||
| Matched unrelated donor (8/8) | 35 (54.7) | 42 (61.8) | 44 (57.9) | ||
| 7/8 Matched unrelated donor | 2 (3.1) | 5 (7.4) | 4 (5.3) | ||
| 7/8 Matched related donor | 1 (1.6) | 0 | 1 (1.3) | ||
| Haplo | 11 (17.2) | 12 (17.6) | 12 (15.8) | ||
| Cord | 0 | 0 | 1 (1.3) | ||
| HCT-CI, median (range) | 4 (0, 10) | 4 (0, 10) | 4 (0, 12) | ||
| HCT-CI, n (%) | |||||
| 0 | 2 (3.3) | 3 (4.5) | 2 (2.7) | .21 | .09 |
| 1 | 4 (6.7) | 11 (16.4) | 10 (13.7) | ||
| 2 | 9 (15) | 9 (13.4) | 12 (16.4) | ||
| 3 | 6 (10) | 9 (13.4) | 12 (16.4) | ||
| ≥4 | 39 (65.1) | 35 (52.3) | 37 (50.6) | ||
| Autologous HSCT | |||||
| Age, median (range), y | 54 (21, 74) | 58 (19, 73) | 59.5 (19, 76) | .31 | .25 |
| Sex, n (%) | .65 | .52 | |||
| Female | 17 (43.6) | 16 (37.2) | 19 (35.2) | ||
| Male | 22 (56.4) | 27 (62.8) | 35 (64.8) | ||
| Diagnosis | .03 | .004 | |||
| Hodgkin lymphoma | 11 (28.2) | 7 (16.3) | 6 (11.1) | ||
| Non-Hodgkin lymphoma | 18 (46.2) | 17 (39.5) | 18 (33.3) | ||
| Multiple myeloma | 5 (12.8) | 18 (41.9) | 27 (50) | ||
| Solid tumors | 4 (10.3) | 1 (2.3) | 3 (5.6) | ||
| Other leukemia | 1 (2.6) | 0 | 0 | ||
| Patient characteristics . | Case cohort A . | Control cohort B . | Control cohort C . | P . | |
|---|---|---|---|---|---|
| 3/15/20 to 6/15/20 . | 12/15/19 to 3/14/20 . | 3/15/19 to 6/15/19 . | A vs B . | A vs C . | |
| Allogeneic HSCT | |||||
| Age, median (range), y | 60 (22, 77) | 62 (19, 74) | 63.5 (21, 78) | .19 | .1 |
| Sex, n (%) | .6 | .61 | |||
| Female | 30 (46.9) | 36 (52.9) | 32 (42.1) | ||
| Male | 34 (53.1) | 32 (47.1) | 44 (57.9) | ||
| Diagnosis, n (%) | .12 | .14 | |||
| Acute myeloid leukemia (AML) | 18 (28.1) | 21 (30.9) | 23 (30.3) | ||
| Acute lymphocytic leukemia | 17 (26.6) | 8 (11.8) | 13 (17.1) | ||
| Chronic myeloid leukemia | 3 (4.7) | 0 | 3 (3.9) | ||
| MDS/MPN(MDS/MPN) | 11 (17.2) | 24 (35.3) | 26 (34.2) | ||
| Therapy-related MDS/AML | 3 (4.7) | 4 (5.9) | 2 (2.6) | ||
| Other leukemia (includes chronic lymphocytic leukemia) | 2 (3.1) | 1 (1.5) | 3 (3.9) | ||
| Hodgkin lymphoma | 0 | 0 | 2 (2.6) | ||
| Non-Hodgkin lymphoma | 8 (12.5) | 8 (11.8) | 2 (2.6) | ||
| Aplastic anemia | 2 (3.1) | 1 (1.5) | 1 (1.3) | ||
| Sickle cell anemia | 0 | 1 (1.5) | 1 (1.3) | ||
| Conditioning intensity, n (%) | 1 | .3 | |||
| Myeloablative | 22 (34.4) | 23 (33.8) | 20 (26.3) | ||
| Nonmyeloablative | 41 (64.1) | 44 (64.7) | 56 (73.7) | ||
| No conditioning | 1 (1.6) | 1 (1.5) | 0 | ||
| Donor, n (%) | .37 | .88 | |||
| Matched related donor (8/8) | 15 (23.4) | 9 (13.2) | 14 (18.4) | ||
| Matched unrelated donor (8/8) | 35 (54.7) | 42 (61.8) | 44 (57.9) | ||
| 7/8 Matched unrelated donor | 2 (3.1) | 5 (7.4) | 4 (5.3) | ||
| 7/8 Matched related donor | 1 (1.6) | 0 | 1 (1.3) | ||
| Haplo | 11 (17.2) | 12 (17.6) | 12 (15.8) | ||
| Cord | 0 | 0 | 1 (1.3) | ||
| HCT-CI, median (range) | 4 (0, 10) | 4 (0, 10) | 4 (0, 12) | ||
| HCT-CI, n (%) | |||||
| 0 | 2 (3.3) | 3 (4.5) | 2 (2.7) | .21 | .09 |
| 1 | 4 (6.7) | 11 (16.4) | 10 (13.7) | ||
| 2 | 9 (15) | 9 (13.4) | 12 (16.4) | ||
| 3 | 6 (10) | 9 (13.4) | 12 (16.4) | ||
| ≥4 | 39 (65.1) | 35 (52.3) | 37 (50.6) | ||
| Autologous HSCT | |||||
| Age, median (range), y | 54 (21, 74) | 58 (19, 73) | 59.5 (19, 76) | .31 | .25 |
| Sex, n (%) | .65 | .52 | |||
| Female | 17 (43.6) | 16 (37.2) | 19 (35.2) | ||
| Male | 22 (56.4) | 27 (62.8) | 35 (64.8) | ||
| Diagnosis | .03 | .004 | |||
| Hodgkin lymphoma | 11 (28.2) | 7 (16.3) | 6 (11.1) | ||
| Non-Hodgkin lymphoma | 18 (46.2) | 17 (39.5) | 18 (33.3) | ||
| Multiple myeloma | 5 (12.8) | 18 (41.9) | 27 (50) | ||
| Solid tumors | 4 (10.3) | 1 (2.3) | 3 (5.6) | ||
| Other leukemia | 1 (2.6) | 0 | 0 | ||
Auto-HSCT.
By department consensus, auto-HSCT for multiple myeloma was paused from March through May 2020, as a temporary delay in these transplants was deemed unlikely to compromise overall patient outcomes (Table 2).5 There were no operational changes for auto-HSCT enforced for other disease indications. No significant differences in patient age, sex, or conditioning intensity were observed (Table 2). There was a significantly lower proportion of referrals from external institutions during the pandemic compared with controls (% external A vs B vs C 41% vs 58.1% vs 64.8%, P = .04) (Table 1).
CAR-T.
Slightly fewer patients received CAR-T during the pandemic period compared with the control group (27 vs 33). There was no significant difference in CAR-T patient demographics between the cohorts (supplemental Table 1). CAR-T therapy patients were actively screened for infectious symptoms prior to leukapheresis, lymphodepleting chemotherapy, and cellular infusion with a low threshold to obtain COVID-19 testing. Obligatory testing for asymptomatic COVID-19 infection with nasopharyngeal polymerase chain reaction prior to lymphodepletion began on 31 March 2020 and additionally prior to CAR-T infusion starting 5 May 2020. Given a heightened risk for infectious transmission and rapid changes in international travel policy for patients and visitors, international CAR-T referrals were suspended from 18 March 2020 to 18 April 2020. There was no difference in time from leukapheresis to infusion or choice of bridging therapy (supplemental Table 1).
Impact on clinical outcomes
Allo-HSCT.
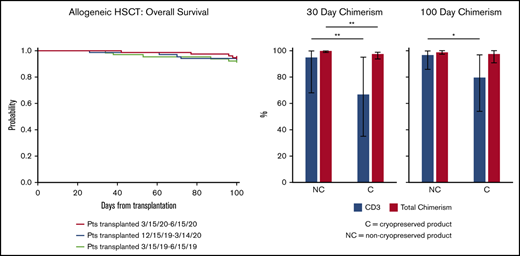
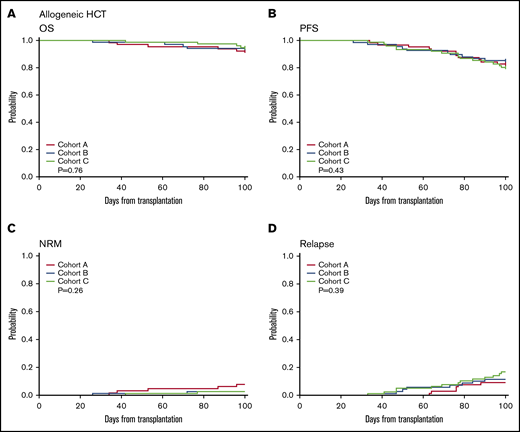
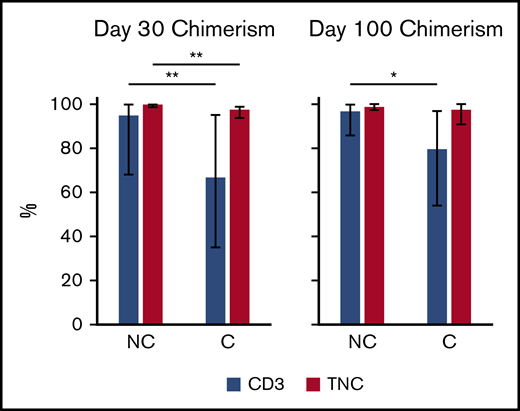
OS and PFS at day 100 were similar between cohorts (OS A vs B 92 vs 94%, P = .66, A vs C 92% vs 95%, P = .53; PFS A vs B 83% vs 85%, P = .72, A vs C 83% vs 80%, P = .68) (Table 3; Figure 1A-B). NRM was also not different in the case cohort compared with controls (A vs B 7.8% vs 2.9%, P = .16, A vs C 7.8% vs 2.6%, P = .19) (Table 3; Figure 1C-D). Despite increased cryopreservation of unrelated donor products, the incidence of graft failure (A vs C 3.1% vs 1.3%, P = .59, A vs B 3.1% vs 2.9%, P = 1) and days to neutrophil engraftment (A vs C 15 vs 15, P = .96, A vs B 15 vs 15, P = .74) did not differ (Table 4). Across groups, median day 30 and day 100 total leukocyte chimerism and CD3 chimerism were not significantly different (Table 4). However, cryopreservation of products was associated with decreased median total leukocyte chimerism at day 30 (99% vs 98%, P < .001) and median CD3 chimerism at both day 30 (95% vs 67%, P = .01) and day 100 (97% vs 80%, P = .03) (Figure 2; supplemental Table 2). The rate of early relapse at day 100 was not different (A vs C 9.4 vs 17.1%, P = .22, A vs B 9.4% vs 11.8%, P = .18) (Table 4). Longer-term outcomes and implications of lower chimerism are yet to be determined. To determine whether differences in chimerism were affected by factors other than cryopreservation, we dichotomized CD3 chimerism level into ≥80 vs <80 and performed multivariable logistic regression analysis stratified by conditioning intensity. The model included age, patient sex, diagnosis at HCT, and indicator of cryopreservation. For D30 CD3 chimerism, the odds of cryopreserved product reaching CD3 chimerism level >80 was 0.23 compared with noncryopreserved product (95% confidence interval [CI]: 0.074-0.74, P = .01). For D100 CD3 chimerism, the odds ratio was 0.22 (95% CI: 0.074-0.66, P = .0065).
100-day clinical outcomes
| . | . | . | . | P* . | |
|---|---|---|---|---|---|
| . | Case cohort A . | Control cohort B . | Control cohort C . | A vs B . | A vs C . |
| Allo-HSCT, % | |||||
| OS (95% CI) | 92 (82, 97) | 94 (85, 98) | 95 (87, 98) | 66 | 53 |
| PFS (95% CI) | 83 (71, 90) | 85 (74, 92) | 80 (69, 88) | 72 | 68 |
| NRM (95% CI) | 7.8 (2.9, 16) | 2.9 (0.5, 9.2) | 2.6 (0.5, 8.3) | 16 | 19 |
| Relapse (95% CI) | 9.4 (3.8, 18) | 11.8 (5.5, 21) | 17 (9.6, 26) | 9 | 38 |
| Auto-HSCT, % | |||||
| OS (95% CI) | 100 (100, 100) | 98 (85, 100) | 98 (88, 100) | 39 | 5 |
| PFS (95% CI) | 97 (83, 100) | 91 (77, 96) | 94 (84, 98) | 2 | 61 |
| IEC, % | |||||
| OS (95% CI) | 92.6 (74, 98) | 91 (74, 97) | † | 78 | † |
| PFS (95% CI) | 70 (49, 84) | 58 (39, 72) | † | 29 | † |
| . | . | . | . | P* . | |
|---|---|---|---|---|---|
| . | Case cohort A . | Control cohort B . | Control cohort C . | A vs B . | A vs C . |
| Allo-HSCT, % | |||||
| OS (95% CI) | 92 (82, 97) | 94 (85, 98) | 95 (87, 98) | 66 | 53 |
| PFS (95% CI) | 83 (71, 90) | 85 (74, 92) | 80 (69, 88) | 72 | 68 |
| NRM (95% CI) | 7.8 (2.9, 16) | 2.9 (0.5, 9.2) | 2.6 (0.5, 8.3) | 16 | 19 |
| Relapse (95% CI) | 9.4 (3.8, 18) | 11.8 (5.5, 21) | 17 (9.6, 26) | 9 | 38 |
| Auto-HSCT, % | |||||
| OS (95% CI) | 100 (100, 100) | 98 (85, 100) | 98 (88, 100) | 39 | 5 |
| PFS (95% CI) | 97 (83, 100) | 91 (77, 96) | 94 (84, 98) | 2 | 61 |
| IEC, % | |||||
| OS (95% CI) | 92.6 (74, 98) | 91 (74, 97) | † | 78 | † |
| PFS (95% CI) | 70 (49, 84) | 58 (39, 72) | † | 29 | † |
IEC, immune effector cell.
Log-rank test for comparison of OS and PFS and Gray test for comparison of cumulative incidences of NRM and relapse.
No 2019 control cohort available for CAR-T patients.
One hundred-day allo-HSCT outcomes. One hundred-day OS and PFS for allo-HSCT (A-B), as well as NRM (C) and relapse (D) in the case cohort (red) compared with control cohorts (blue and light green).
One hundred-day allo-HSCT outcomes. One hundred-day OS and PFS for allo-HSCT (A-B), as well as NRM (C) and relapse (D) in the case cohort (red) compared with control cohorts (blue and light green).
Allo-HSCT 100-day outcomes
| . | . | . | . | P . | |
|---|---|---|---|---|---|
| . | Case cohort A . | Control cohort B . | Control cohort C . | A vs B . | A vs C . |
| Graft failure, n (%) | 2 (3.1) | 2 (2.9) | 1 (1.3) | 1 | .59 |
| Days to engraftment, median (range) | 15 (6, 36) | 15 (6, 29) | 15 (4, 25) | .74 | .96 |
| Grade 2 to 4 aGVHD, n (%) | 7 (10.9) | 11 (16.2) | 7 (9.2) | .45 | .78 |
| Grade 3 to 4 aGVHD, n (%) | 4 (6.2) | 1 (1.5) | 1 (1.3) | .2 | .18 |
| Day 30% donor chimerism, median | |||||
| CD3 | 86.5 | 92 | 83.5 | .25 | .64 |
| Total | 99 | 99 | 98 | .66 | .29 |
| Day 100% donor chimerism, median | |||||
| CD3 | 91 | 97 | 96 | .36 | .26 |
| Total | 99 | 99 | 99 | .42 | .79 |
| Relapse, n (%) | 6 (9.4) | 8 (11.8) | 13 (17.1) | .18 | .22 |
| . | . | . | . | P . | |
|---|---|---|---|---|---|
| . | Case cohort A . | Control cohort B . | Control cohort C . | A vs B . | A vs C . |
| Graft failure, n (%) | 2 (3.1) | 2 (2.9) | 1 (1.3) | 1 | .59 |
| Days to engraftment, median (range) | 15 (6, 36) | 15 (6, 29) | 15 (4, 25) | .74 | .96 |
| Grade 2 to 4 aGVHD, n (%) | 7 (10.9) | 11 (16.2) | 7 (9.2) | .45 | .78 |
| Grade 3 to 4 aGVHD, n (%) | 4 (6.2) | 1 (1.5) | 1 (1.3) | .2 | .18 |
| Day 30% donor chimerism, median | |||||
| CD3 | 86.5 | 92 | 83.5 | .25 | .64 |
| Total | 99 | 99 | 98 | .66 | .29 |
| Day 100% donor chimerism, median | |||||
| CD3 | 91 | 97 | 96 | .36 | .26 |
| Total | 99 | 99 | 99 | .42 | .79 |
| Relapse, n (%) | 6 (9.4) | 8 (11.8) | 13 (17.1) | .18 | .22 |
Chimerism and cryopreservation. Total chimerism (red bars) and CD3 chimerism (blue bars) at days 30 and 100 for cryopreserved (C) or noncryopreserved (NC) products. *P ≤ .05, **P ≤ .01.
Chimerism and cryopreservation. Total chimerism (red bars) and CD3 chimerism (blue bars) at days 30 and 100 for cryopreserved (C) or noncryopreserved (NC) products. *P ≤ .05, **P ≤ .01.
We observed no differences in the incidence of grade II to IV aGVHD or grade III-IV aGVHD between cohorts (Table 4). The overall incidence of non–COVID-19 infections between day 0 and day 100 was similar (supplemental Table 3). There was an increased incidence of febrile neutropenia as a proportion of total infections during the pandemic period compared with the seasonally matched control cohort of the prior year (A vs C 46.9% vs 28.9%, P = .036). There were no allo-HSCT patients diagnosed with COVID-19 between day 0 and day 100.
One patient with “stable” MDS whose transplant in March 2020 was deferred because of the COVID-19 pandemic subsequently transformed to AML despite hypomethylation therapy. This patient underwent induction therapy, achieved remission, and has now undergone allo-HSCT. Two allo-HSCT patients were deferred because of patient preference, one of whom responded to medical therapy but had clinical decline for unclear reasons and subsequently died. The other returned to their local provider and has not followed up at DFCI. One CD34+ cell boost was delayed because of donor collection issues owing to COVID-19. That patient died of sepsis before boost could be scheduled. Five allo-HSCT patients had transplants delayed because of donor issues related to COVID-19. One was converted to a haploidentical donor and is now doing well. The second ultimately underwent transplant from the original donor 3 months later than intended but had early relapse and died. The third and fourth patients received transplants from the original donor a few months later than planned and are now doing well. The last patient has only 1 available donor identified worldwide and thus far has been unable to undergo transplant because of travel restrictions. However, this patient is clinically well and is planned for a transplant in early 2021.
Auto-HSCT.
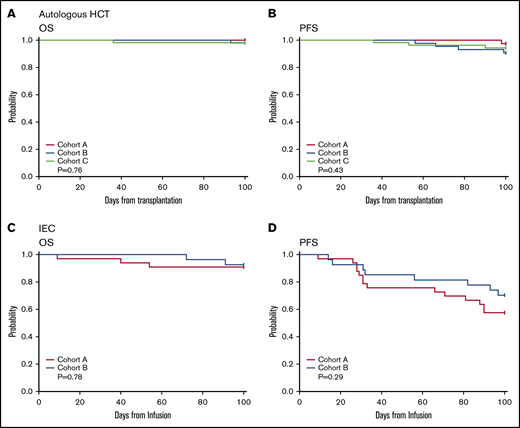
There was no significant difference in OS (A vs C 100% vs 98%, P = .5, A vs B 100% vs 98%, P = .39) or PFS (A vs C 97% vs 94%, P = .61, A vs B 97 vs 91%, P = .2) at day 100 in the case cohort compared with control group B or C (Figure 3A-B; Table 3). No patients were diagnosed with COVID-19 from day 0 to 100 posttransplant. No auto-HSCTs were delayed because of a patient testing positive for COVID-19 on the preadmission screen. There was no difference in incidence of non–COVID-19 infections, including incidence of febrile neutropenia (supplemental Table 3).
Auto-HSCT and CAR-T one hundred-day outcomes. One hundred-day OS and PFS for auto-HSCT (A-B), and CIEC/CAR-T therapy (C-D) in the case cohort (red) compared with the control cohort (blue and light green).
Auto-HSCT and CAR-T one hundred-day outcomes. One hundred-day OS and PFS for auto-HSCT (A-B), and CIEC/CAR-T therapy (C-D) in the case cohort (red) compared with the control cohort (blue and light green).
Two multiple myeloma patients who were deferred subsequently had disease progression. One has since undergone auto-HSCT with disease control; the other has been unable to proceed to auto-HSCT because of recurrent infections. Several lymphoma patients were deferred because of patient preference. All these patients underwent auto-HSCT within 5 months after the original plan for HSCT.
CAR-T.
OS and PFS at day 100 did not differ between the CAR-T cohorts (Figure 3C-D; Table 3). No difference was observed in non–COVID-19 infections (supplemental Table 3). One patient with diffuse large B-cell lymphoma who received tisagenlecleucel tested positive for SARS-CoV-2 51 days after infusion and ultimately succumbed to COVID-19–related complications on day 121 postinfusion. The rate of any grade cytokine release syndrome and any grade immune effector cell-associated neurotoxicity syndrome was similar (supplemental Table 4). The use of tocilizumab did not vary between groups; however, there was a reduction in steroid use for cytokine release syndrome in the COVID-19 cohort (16 vs 6, P = .02) (supplemental Table 4). Two CAR-T patients deferred therapy because of patient preference. One continues to do well, and the other had disease progression and is receiving local therapy.
Discussion
The emergence of the SARS-CoV-2 virus and rapid worldwide outbreak leading to a global pandemic of the respiratory illness COVID-19 represent a singular event in modern medicine. Although periodic influenza pandemics have occurred in the 20th century, an outbreak of this magnitude is unprecedented in the era of HSCT. The threat of easily transmissible infectious diseases, primarily respiratory viruses, has loomed as potential global pandemics. The epidemics of SARS-CoV-1 in 2003, H5N1 avian influenza in 2004, MERS-CoV in 2012, and the 2009 H1N1 pandemic were all sufficiently contained without the global devastation wreaked by COVID-19.6 By the end of 2020, >81 million people worldwide were infected with COVID-19 and >1.75 million have died.7
For the administration of HSCT and CAR-T immune effector cell therapy, the pandemic posed major challenges, both in care coordination and in safe delivery of therapies in this vulnerable patient population that is significantly immunocompromised and yet requires expeditious therapy for their underlying hematologic cancers.8-14 The threat of such a pandemic was not wholly unpredicted; indeed, more than a decade ago, steps for preparation and response actions for HSCT patients in the event of a pandemic infection were discussed, given the recognition that a global pandemic on this scale was nearly inevitable.15,16 Indeed, annual influenza and other respiratory pathogens continue to pose a significant threat to immunocompromised patients, including HSCT and CAR-T patients each year.17-21 Perhaps, then, the field of hematologic malignancies is well poised to lead the charge in implementing precautions and safeguards to ensure that these and other high-risk patients continue to receive high-quality care throughout the ongoing pandemic.22-27
Our data show that at our tertiary care center with appropriate patient care resources, careful donor selection, increased screening of donors and patients, and appropriate use of personal protective equipment and symptoms screens by medical staff, HSCT and CAR-T therapies may be safely carried out in the era of COVID-19. Reassuringly, the fact that we did not observe any nosocomial COVID-19 infections in HSCT or CAR-T patients demonstrates that these therapies can be safely administered during this period. In addition, the lack of significant delays in time from workup to transplant during the pandemic despite challenges imposed by travel restrictions is a testament to the dedication of our transplant coordination staff, donor services team, and the NMDP. Moreover, the example of our MDS patient whose transplant was deferred by the pandemic whose disease later transformed to AML highlights the risk associated with delaying treatment of hematologic malignancies and the benefit of proceeding with definitive therapy during the pandemic, as long as appropriate safeguards are implemented.
Despite structural changes implemented during the COVID-19 pandemic, we found no difference in OS or PFS in our cohorts. For allo-HSCT patients in particular, cryopreservation of unrelated donor products does not appear to negatively affect early clinical outcomes. Prior studies have suggested that cryopreservation of peripheral blood stem cells could impair cellular function and engraftment, and that cryopreservation is associated with significant variability in CD34+ cell recovery.28-30 With increased cryopreservation of products, we found no differences in primary graft failure. However, we did observe lower median CD3 and total chimerism, suggesting that cryopreservation may modestly impair graft integrity. The current analysis is limited to outcomes in only the first 100 days after transplant, so the longer-term implications of lower chimerism are not yet clear.31 Longer follow-up of our cohort is needed to assess whether product cryopreservation and lower chimerism at days 30 and 100 could be associated with longer-term deleterious effects, such as late relapse, or higher incidence of chronic GVHD, if immunosuppression is tapered in an effort to boost chimerism. Furthermore, we observed no differences in other potential adverse outcomes for allo-HSCT patients, such as incidence of grade II to IV or grade III to IV aGVHD.
We also observed no differences in the incidence of non–COVID-19 infectious processes during the pandemic period. On one hand, perhaps this is not so surprising, as most infections in HSCT patients are bloodstream (because of gut translocation or line-associated infections), which are not expected to be affected by the presence of a viral respiratory pandemic, where none of the patients were infected with COVID-19. Nevertheless, it is somewhat unexpected that there appears to be no difference in the rate of viral infections observed during this period, compared with either the 3 months prior to the pandemic or the seasonally matched cohort 1 year prior. It is important to note that HSCT and CAR-T patients are commonly advised to engage in strict mask adherence after treatment, when they are significantly immunosuppressed.22 Although we did not observe a difference in the rate of infections overall or in bloodstream infections, we did observe a significant difference in the incidence of febrile neutropenia in all-HSCT between the pandemic case cohort compared with the seasonally matched control cohort 1 year prior. There were no clear structural or practice variations between these 2 time points that might explain this observation. We do not expect that undetected COVID-19 infection would explain this difference, as COVID-19 testing was available for all patients who were febrile during this timeframe.
CAR-T therapy has demonstrated the potential to induce unprecedented response rates in various intractable B-cell malignancies, including aggressive B-cell lymphoma and pediatric/young adult acute lymphoblastic leukemia,32-34 leading to greater demand for access to CAR-T therapies. Thus, the 2020 COVID-19 pandemic posed concerns for safe delivery of therapy.
Despite the promise of effective vaccines on the horizon, the many regions of the United States and other countries are experiencing severe second and third waves of infection. As such, it is imperative to devise and effectively implement mechanisms for donor selection and screening, timely and safe delivery of cellular products, both allogeneic and autologous, and safe hospital operations to allow continued stem cell transplantation and CAR-T therapy.
Requests for data may be made by contacting the corresponding author, Vincent T. Ho, at vincent_ho@dfci.harvard.edu.
Acknowledgments
The authors acknowledge the NMDP for their efforts to maintain access to donors throughout the ongoing pandemic, to the DFCI Donor Services Program for their ongoing work on screening and recruiting donors, and to the DFCI/BWCC patient care teams for providing clinical care for these patients.
Authorship
Contribution: K.M., U.A., C.J.W., R.J.S., C.J., and V.T.H. conceived the project; K.M., A.S., U.A., C.C.M., J.P., and V.T.H. designed the study; C.A. provided operational and donor-related information; K.M. and A.S. collected clinical data; H.T.K. designed and performed the statistical analysis; U.A., C.C.M, C.C., J.H.A., J.K., M.G., R.R., S.N., C.J., and V.T.H. participated in identification, collection, and interpretation of patient information; and all authors participated in manuscript writing and review and provided final approval of the manuscript.
Conflict-of-interest disclosure: C.J.W. holds equity in BioNTech, Inc, and receives research funding from Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Vincent T. Ho, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215; e-mail: vincent_ho@dfci.harvard.edu.
References
Author notes
The full-text version of this article contains a data supplement.