Key Points
Alteplase solutions degrade steadily over time during simulated ultrasound-facilitated catheter-directed administration.
Remaining nondegraded alteplase proteins retained functional activity.
Abstract
Ultrasound-facilitated catheter-directed thrombolysis is used with low-dose alteplase to treat pulmonary embolism. This reduces the risk of bleeding that accompanies systemic administration of higher alteplase doses. Some studies suggest that alteplase given over 2 to 6 hours is safe and effective, but there are few data to support the stability of alteplase under these conditions. Therefore, we undertook this in vitro study to determine the duration of alteplase stability. Alteplase was prepared in solutions of 8 mg in 100 mL, 6 mg in 150 mL, and 8 mg in 200 mL. Solutions were administered through the EkoSonic Endovascular System (with and without ultrasound) to simulate administration over 2, 4, and 6 hours. Alteplase was assessed with reversed-phase high-performance liquid chromatography (RP-HPLC). Assays were performed at time 0 and at 30-minute intervals during simulated infusion. An enzyme-linked immunosorbent assay was used to measure alteplase concentrations at time 0 and at 15-minute intervals during simulated infusion. By using RP-HPLC in the absence of ultrasound, the alteplase concentration remained within 1% of the original concentration through 120, 240, and 360 minutes of infusion. By using RP-HPLC for measurement, alteplase in the presence of ultrasound degraded steadily over time to ∼90% of its original amount in 120 minutes, ∼80% in 240 minutes, and ∼70% in 360 minutes. The remaining alteplase was available for enzymatic activity. Alteplase solutions of 0.04 and 0.08 mg/mL degraded steadily over time during simulated ultrasound-facilitated catheter-directed administration. Alteplase that did not degrade remained available for enzymatic activity.
Introduction
Ultrasound-facilitated catheter-directed thrombolysis (USCDT) uses low-dose alteplase to treat pulmonary embolism (PE). This strategy may prevent major bleeding complications that can result from high-dose alteplase administered through a systemic vein. Previous studies have evaluated various durations of treatment for USCDT in PE. The ULTIMA trial administered alteplase into the pulmonary artery or arteries over a 15-hour period.1 The SEATTLE II trial administered alteplase over 12 hours (unilateral PE) or 24 hours (bilateral PE).2 The OPTALYSE PE trial studied 4 treatment regimens with alteplase administration ranging from 2 to 6 hours.3 In each trial, clinical improvement was defined as a reduction in the ratio of right ventricular to left ventricular (RV:LV) diameter compared with baseline.
Few data exist to support the stability of alteplase under conditions that resemble those during clinical administration. According to manufacturer’s data, reconstituted alteplase is stable for up to 8 hours, provided it is maintained at temperatures between 2°C and 30°C and at a concentration of 0.5 to 1 mg/mL.4,5 It is unclear whether the effects of ultrasound have an impact on the stability of alteplase. Reversed-phase high-performance liquid chromatography (RP-HPLC) is widely used to identify and characterize proteins and to determine the presence of impurities, and it can separate a protein molecule from its nonfunctional aggregated, degraded, or digested peptides.6,7
Enzyme-linked immunosorbent assay (ELISA) is a method for detecting and quantifying a specific protein in a complex mixture.8,9 As a tool, the ELISA provides the ability to use high-affinity antibodies to bind to and measure a specific amount of a functional protein within a compounded preparation while ignoring degraded byproducts or contaminants. In this in vitro study, our aims were to quantify alteplase concentration by using RP-HPLC and to measure functional alteplase concentrations using an ELISA in a series of alteplase solutions during simulated catheter-directed administration, with and without ultrasound.
Methods
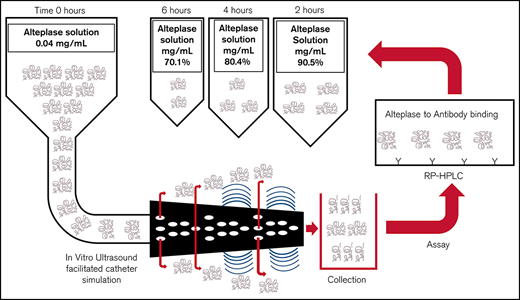
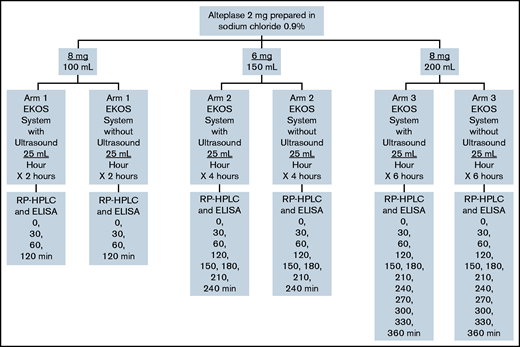
Four sets of the following admixtures of alteplase (Cathflo Activase; Genetech) in normal saline polyvinyl chloride bags were prepared for analysis: 8 mg in 100 mL (0.08 mg/mL, arm 1), 6 mg in 150 mL (0.04 mg/mL, arm 2), and 8 mg in 200 mL (0.04 mg/mL, arm 3) (Figure 1). Extemporaneous compounding was conducted by a single individual using aseptic technique in an International Organization for Standardization (ISO) Class 6 cleanroom under an ISO Class 5 laminar airflow hood, with duplicate sets generated for each admixture. All admixtures were administered through the EkoSonic Endovascular System (Boston Scientific) via smart pump technology (Alaris Pump; Becton Dickinson U.K.), with and without ultrasound, activated along the catheter treatment zone. The working length of the catheter was 106 cm and the treatment zone length was 6 cm. In arm 1, alteplase was administered at a dose of 2 mg/h (infusion rate, 25 mL/h) to simulate medication administered to a patient over 2 hours. In arm 2, alteplase was administered at a dose of 1 mg/h (infusion rate, 25 mL/h) to simulate medication administered to a patient over 4 hours. In arm 3, alteplase was administered at a dose of 1 mg/h (infusion rate, 25 mL/h) to simulate medication administered to a patient over 6 hours. The device temperature was maintained between 34°C (to reflect normal body temperature) and 43°C (maximum transducer surface temperature) with a water bath and infusion of normal saline through the coolant port of the infusion catheter. Alteplase solutions were collected after infusion through the catheter into an empty sterile infusion collection bag. Immediately after preparation (time zero) and at 30-minute intervals after simulation, 1-mL samples were removed from each collection bag and frozen using an aseptic technique. We used RP-HPLC to separate the proteins and peptides and identify the alteplase in the solution collected after infusion (Figure 2). An ELISA (Human alteplase activity assay; #IHTPAKT, Innovative Research, Novi, MI) was used to measure the concentration of functionally available alteplase. This particular ELISA uses human plasminogen activator inhibitor-1 (PAI-1) coated on a 96-well plate to capture noncomplexed human tissue plasminogen activator and washes away unbound (ie, inactive or degraded) tissue plasminogen activator. This assay does not measure enzymatic activity of the available alteplase.
Three different alteplase preparations used in this study. Concentrations of 8 mg/100 mL, 6 mg/150 mL, and 8 mg/200 mL were prepared and were administered at 2, 4, and 6 hours to simulate dose administrations in the OPTALYSE PE trial. EKOS System, EkoSonic Endovascular System.
Three different alteplase preparations used in this study. Concentrations of 8 mg/100 mL, 6 mg/150 mL, and 8 mg/200 mL were prepared and were administered at 2, 4, and 6 hours to simulate dose administrations in the OPTALYSE PE trial. EKOS System, EkoSonic Endovascular System.
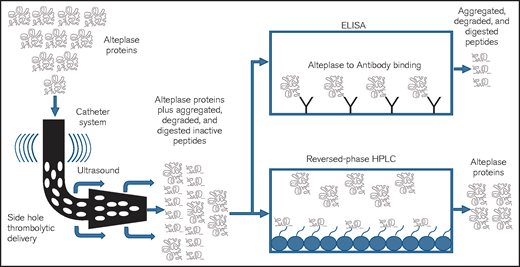
Pictorial representation of the study methodology using 2 tests: RP-HPLC and ELISA. HPLC (high-performance or high-pressure LC) is an analytic chemistry technique used to separate, identify, and quantify individual components in a mixture. A solution containing the mixture is passed through a column with adsorbent material. The different components of the mixture react with the adsorbent material leading to separation of the components as they flow out of the column. RP-HPLC uses hydrocarbon chains to generate a stronger or higher affinity for polar components, essentially the reverse of normal-phase chromatography. ELISA is a commonly used analytical biochemistry technique. It uses an enzyme immunoassay to detect the presence of a protein by directing antibodies to the protein to be measured. HPLC and ELISA have been used for biologic and pharmaceutical manufacturing, laboratory testing (of urine and blood) and in research for separating and identifying the individual components of a complex sample.
Pictorial representation of the study methodology using 2 tests: RP-HPLC and ELISA. HPLC (high-performance or high-pressure LC) is an analytic chemistry technique used to separate, identify, and quantify individual components in a mixture. A solution containing the mixture is passed through a column with adsorbent material. The different components of the mixture react with the adsorbent material leading to separation of the components as they flow out of the column. RP-HPLC uses hydrocarbon chains to generate a stronger or higher affinity for polar components, essentially the reverse of normal-phase chromatography. ELISA is a commonly used analytical biochemistry technique. It uses an enzyme immunoassay to detect the presence of a protein by directing antibodies to the protein to be measured. HPLC and ELISA have been used for biologic and pharmaceutical manufacturing, laboratory testing (of urine and blood) and in research for separating and identifying the individual components of a complex sample.
The stability of alteplase was determined using RP-HPLC to identify the percentage of the initial alteplase amount remaining at each sampling point. The average of all samples from all 3 admixture arms at each condition was used to determine the overall mean concentration at each time point. The RP-HPLC time 0 data from each arm were used to calculate the initial alteplase concentration. The ELISA was used to measure functional alteplase concentration at each sampling point as a percentage of the initial alteplase amount at time 0. Results for subsequent time points were expressed as a percentage of the initial ELISA time 0 alteplase concentration. All percentages were expressed as means with standard deviation. A sample concentration was considered stable if the alteplase concentration was >90% of the original concentration.
Results
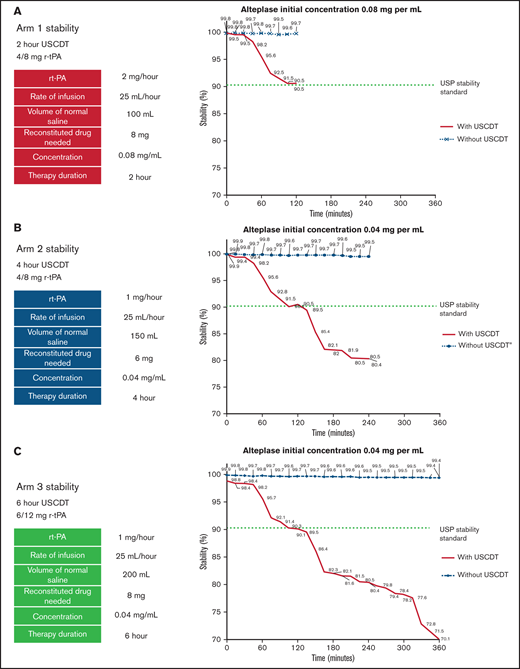
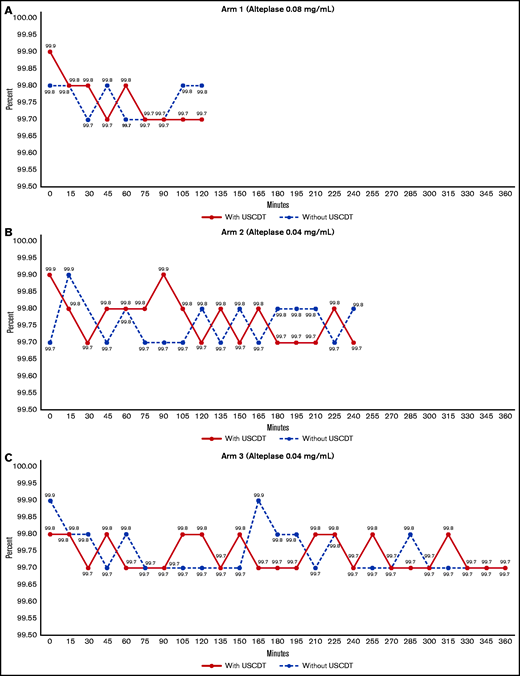
In arms 1, 2, and 3, the concentration of infused alteplase without ultrasound remained within 1% of the originally prepared concentration through 120, 240, and 360 minutes, respectively (Figure 3). The application of ultrasound to the catheter during alteplase infusion induced a rapid decrease in alteplase concentration beginning at 30 minutes in arms 1, 2, and 3 (Figure 3). The percentage of original concentration in the infused solutions at 120, 240, and 360 minutes was ∼90%, ∼80%, and ∼70%, respectively, compared with the United States Pharmacopeia (USP) Standard for Stability (90%). The rate of degradation seemed to be similar across all 3 alteplase preparations in the presence of ultrasound for the first 120 minutes. Between 120 and 240 minutes, alteplase degradation in the presence of ultrasound proceeded at a similar rate in arms 2 and 3. Beyond 240 minutes, alteplase concentration further declined with no apparent nadir through 360 minutes.
Stability of alteplase for arms 1, 2, and 3. Alteplase concentrations are measured by RP-HPLC over 2, 4, and 6 hours. (A) When exposed to ultrasound alteplase degrades to approximately 90% of its original concentration in 2 hours. (B) When exposed to ultrasound alteplase degrades to approximately 80% of its original concentration in 4 hours. (C) When exposed to ultrasound alteplase degrades to approximately 70% of its original concentration in 6 hours.
Stability of alteplase for arms 1, 2, and 3. Alteplase concentrations are measured by RP-HPLC over 2, 4, and 6 hours. (A) When exposed to ultrasound alteplase degrades to approximately 90% of its original concentration in 2 hours. (B) When exposed to ultrasound alteplase degrades to approximately 80% of its original concentration in 4 hours. (C) When exposed to ultrasound alteplase degrades to approximately 70% of its original concentration in 6 hours.
The functionally active alteplase remaining, as measured by concentration bound to PAI-1 as a percentage of time 0, was stable in the absence of ultrasound across all 3 arms and throughout the 120-, 240- and 360-minute infusions, respectively (Figure 4). The application of ultrasound to different concentrations and for increasing durations in arms 1, 2, and 3 had no effect on functionally active alteplase, which was >99% of baseline across all conditions (Figure 4).
Alteplase concentrations measured by ELISA over 2, 4, and 6 hours. (A) Intact alteplase present, with or without ultrasound exposure, was functionally active (approximately 99%) at 2 hours. (B) Intact alteplase present, with or without ultrasound exposure, was functionally active (approximately 99%) at 4 hours. (C) Intact alteplase present, with or without ultrasound exposure, was functionally active (approximately 99%) at 6 hours.
Alteplase concentrations measured by ELISA over 2, 4, and 6 hours. (A) Intact alteplase present, with or without ultrasound exposure, was functionally active (approximately 99%) at 2 hours. (B) Intact alteplase present, with or without ultrasound exposure, was functionally active (approximately 99%) at 4 hours. (C) Intact alteplase present, with or without ultrasound exposure, was functionally active (approximately 99%) at 6 hours.
RP-HPLC (Figure 3) showed that alteplase concentration degraded steadily during in vitro ultrasound-facilitated catheter-directed administration. However, the ELISA (Figure 4) showed that the percent of alteplase that remained was functionally active through its covalent binding with biotinylated human PAI-1 on the microtiter plate.
Discussion
We measured the presence of alteplase and functional alteplase under 6 catheter-directed administration regimens: 2 mg/h for 2 hours with and without ultrasound, 1 mg/h for 4 hours with and without ultrasound, and 1 mg/h for 6 hours with and without ultrasound. In the absence of ultrasound, the alteplase concentration remained stable with little to no degradation. In contrast, when we used RP-HPLC, we found that alteplase degraded over time during simulated ultrasound-facilitated catheter-directed administration. While alteplase was degrading, the alteplase that remained was functionally active, maintaining >99% in measurements at all time points across all 3 regimens. Our study suggests that during USCDT simulated administration, alteplase stability exceeded 90% for up to 2 hours. Thereafter, alteplase stability failed to meet USP standards for the 1-mg/h 4-hour regimen and the 1-mg/h 6-hour regimen. However, in the absence of ultrasound, functional alteplase did not degrade at 4 hours or at 6 hours.
Three trials have evaluated alteplase regimens using USCDT to treat PE. The ULTIMA trial compared USCDT with alteplase plus intravenous unfractionated heparin (UFH) vs UFH alone in 59 patients with intermediate-risk PE.1 Alteplase was administered at 1 mg/h per catheter for 5 hours then reduced to 0.5 mg/h per catheter for 10 hours. The maximum alteplase dose was 20 mg for patients with bilateral catheters and 10 mg for patients with a unilateral catheter. Bilateral catheters were placed in 87% of the USCDT patients. The primary end point, the echocardiographic difference in the RV:LV diastolic diameter ratio at baseline compared with 24 hours, was 0.30 ± 0.20 standard deviation in the alteplase arm vs 0.03 ± 0.16 (P < .001) in the UFH arm. There were no major bleeding complications in either group. SEATTLE II, a single-arm study, used 1 mg/h for 24 hours with a unilateral catheter or 1 mg/h per catheter for 12 hours with bilateral catheters in 150 patients with massive and submassive PE.2 Bilateral catheters were placed in 57% of the USCDT patients. The mean RV:LV diastolic diameter ratio decreased from 1.55 at baseline to 1.13 at 48 hours (P < .0001) after the procedure. There was 1 major bleeding episode.
The OPTALYSE PE trial studied lower doses of alteplase and shorter durations of therapy by using 4 study arms in 100 intermediate-risk patients with acute PE (all in 1 vs 2 lungs): arm 1: USCDT for 2 hours with alteplase infused at 2 mg/h per catheter (range, 4-8 mg); arm 2: USCDT for 4 hours with alteplase infused at 1 mg/h per catheter (range, 4-8 mg); arm 3: USCDT for 6 hours with alteplase infused at 1 mg/h per catheter (range, 6-12 mg); arm 4: USCDT for 6 hours with alteplase infused at 2 mg/h per catheter (range, 12-24 mg).3 Bilateral catheters were placed in 86% of the USCDT patients. The RV:LV diastolic diameter ratio improved in all treatment arms at 48 hours. This outcome did not differ on the basis of either the dose or the duration of USCDT. The absence of a dose-response curve may be explained by the rapid degradation of alteplase during USCDT. In addition, rates of major bleeding (overall 4%) did not differ as alteplase dosing increased. This suggests that in the presence of ultrasound, very little functional alteplase remains after the initial 2-hour infusion.
Although this catheter system has approval from the US Food and Drug Administration for selective infusion of physician-specified fluids (including thrombolytics), there are no specific recommendations for alteplase dilutions or alteplase stability from the device or alteplase manufacturers. The manufacturer recommends that alteplase in 2-mg, 50-mg, and 100-mg vials be administered within 8 hours of reconstitution when stored at 2° to 30°C.4,5 Alteplase formulations (2 mg, 50-vials) have been studied without ultrasound in dilutions of 0.01 mg/mL in normal saline. Bioactivity measured by clot lysis assay and protein concentration measured by ELISA remained unchanged at time points 0, 4, 8, and 24 hours at ambient room temperature.10
Alteplase stability has been further evaluated in the setting of acoustic cavitation.11 Alteplase 1-mg/mL samples, combined with contrast, were exposed to 60 minutes of 2 MHz ultrasound at various acoustical pressures and compared with controls. The enzymatic activity was comparable in the 2 groups. Alteplase bioactivity and stability have been studied during in vitro exposure from ultrasound energy as part of a novel intravascular wire.12 Alteplase solutions exposed to low-power ultrasound energy for up to 6 minutes remained active and stable as determined by protein assays. Alteplase stability has also been studied during emergency medical transport by helicopter. When alteplase concentrations during air ambulance transfer were compared with control samples from routine emergency department care, there were no significant differences in alteplase activity.13 Others have used alteplase solutions ranging from 0.03 to 0.2 mg per mL analyzed by liquid chromatography-mass spectrometry (LC-MS), and they showed degradation ranging from 0% to 36% in peptide concentrations at 24 hours.14
Our study has some limitations. This was a single-center study. Practices and devices are likely to vary at other interventional centers. The catheter is commercially available in lengths of 106 and 135 cm with ultrasound (treatment) zones of 6, 12, 18, 24, 30, 40, and 50 cm. Because we tested only one size of catheter, we did not evaluate ultrasound exposure duration as a factor in alteplase degradation. The alteplase flow rate and longer exposure to the ultrasound treatment zone may have had an impact on alteplase degradation. We did not measure enzymatic bioactivity of alteplase by using a clot lysis assay. Therefore, we cannot be certain whether the remaining alteplase retained its thrombolytic characteristics. Future studies should link alteplase stability to the timing of administration and to therapeutic outcomes.
Our study also has many strengths. We evaluated preparations with alteplase 2-mg vials, which is the most likely formulation to be used in USCDT, rather than alteplase 50-mg or 100-mg vials used in previous studies.10,13 We also performed our assays to correspond to the administration durations in the OPTALYSE PE trial, extending to 2, 4, and 6 hours rather than limiting the analysis to 0, 6, or 60 minutes.11,12,14
Our study has operational and clinical implications. Other investigators have shown that alteplase mixed in a diluent is stable for 24 hours and should not raise concerns with solutions compounded by pharmacists.10 However, once a compounded alteplase solution begins to be administered via USCDT, the delivered alteplase dose may be dependent on infusion rate, ultrasound treatment zone exposure, and solution concentration. For example, the alteplase dose delivered to a thrombus with a compounded concentration of 0.04 mg/mL over a treatment zone of 6 cm for 12 hours may differ from a compounded concentration of 0.08 mg/mL over a treatment zone of 50 cm for 24 hours. Thermal effects, localized shear forces, and aggregation may have an impact on the intact alteplase delivered locally. The extent to which intact (nondegraded) alteplase contributes to efficacy is unknown, and this may have contributed to the challenges of identifying an optimum dose. A conservative strategy in the setting of USCDT would be to administer a compounded alteplase solution over a 2-hour interval.
Neither the alteplase manufacturer nor the catheter manufacturer are required to provide in vitro or in vivo drug stability information. Assays for drug stability that require unique laboratory expertise are expensive to perform and often difficult to interpret. This testing burden has fallen to the clinician. A comprehensive assay series that includes tandem mass spectrometry, ELISA, RP-HPLC, and clot lysis (functional assays) that are tied to clinical outcomes over various times of alteplase administration at the time of regulatory device or drug approval should be performed.15
In summary, we found that alteplase solutions degrade rapidly during simulated USCDT administration but remain stable in the absence of ultrasound-facilitated thrombolysis. In this latter setting, alteplase solutions of 0.04 and 0.08 mg/mL were stable for only 2 hours when referenced to USP standards. Future clinical studies that administer alteplase for longer than 2 hours in the setting of ultrasound-facilitated thrombolysis should measure whether the alteplase remains functionally active.
Acknowledgment
The authors acknowledge the donation of an EkoSonic Endovascular System (Boston Scientific) for the completion of this study.
Authorship
Contribution: J.F., K. Marquis, L.B., L.K.T., K. McLaughlin, and A.J.G. identified and developed the methods for the testing; K. Marquis completed the laboratory assays; U.C., G.P., J.M.C., and S.Z.G. helped analyze and interpret the data; J.F. drafted the manuscript; and all authors reviewed drafts and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Fanikos, Brigham and Women’s Hospital, 75 Francis St, L2, Boston, MA 02115; e-mail: jfanikos@bwh.harvard.edu; jfanikos@partners.org.