TO THE EDITOR:
Pediatric acute myeloid leukemia (AML) is a rare and profoundly heterogeneous disease at the molecular and clinical levels, with an incidence of ∼700 to 1000 cases per year.1,2 Although in recent clinical trials, the 5-year event-free survival rates for childhood AML have ranged between 49% and 64%,3,4 bone marrow relapse still occurs in up to one-third of cases, and the long-term outcomes of these patients continue to be dismal.4 Newer therapies are desperately needed.
Mutations in the FMS-like tyrosine kinase-3 receptor gene (FLT3) occur in ∼20% of children and are associated with poor prognosis.5,6 FLT3 mutations with internal tandem duplications (ITDs) in the juxtamembrane and point mutations in the tyrosine kinase domain activation loop drive proliferation by phosphorylation of downstream targets, including STAT5, SHIP, and SHP-2, and signaling through critical oncogenic pathways such as Ras/Raf/MAPK and phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin.7 Competitive inhibition of ATP-binding sites in the FLT3 receptor kinase domain with small-molecule FLT3 inhibitors represents a significant paradigm shift in AML.8-13
However, to date, FLT3 inhibition as a treatment strategy in pediatric AML has been hampered by limited potency and significant toxicity, mainly because of off-target effects. This is the case with FLT3 inhibitors that could be used for pediatric patients, such as sorafenib and midostaurin.14,15 Sorafenib, although deemed effective and more tolerable than expected in pediatric patients, has been associated with significant toxicities.14 Midostaurin, currently under more extensive evaluation, has shown limited clinical activity based on preliminary data.15 Gilteritinib, a highly potent and selective oral FLT3 inhibitor, has shown promising results in adults with relapsed/refractory (R/R) FLT3-mutated AML and frontline in combination with induction therapy.9,16 This has led to the incorporation of this agent in the new Children’s Oncology Group frontline AAML1831 trial (registered at www.clinicaltrials.gov as #NCT04293562).
Given the lack of data on this approach in children, institutional review board approval was obtained to conduct a retrospective medical record review of patients who had received at least 1 dose of gilteritinib at The University of Texas MD Anderson Cancer Center and Texas Children’s Hospital to describe the efficacy, adverse events (AEs), and toxicities of gilteritinib. The study was conducted in accordance wit the Declaration of Helsinki. Patient characteristics and treatment outcomes are summarized in Table 1. The date for data cutoff was 28 September 2020. Patients considered for the study were ≤21 years of age. Response criteria were established according to the revised recommendations of the International Working Group Response Criteria in Acute Leukemia.17 Minimal residual disease was measured institutionally by multiparameter flow cytometry with a sensitivity of 0.01% to 0.1%. FLT3 detection was assessed using polymerase chain reaction–based DNA analysis to detect ITDs and codon 835/836 point mutations in FLT3. The lower limit of detection (analytical sensitivity) of this assay is ∼1% of mutant DNA in a background of wild-type DNA. Gilteritinib toxicities were graded per the Common Terminology Criteria for Adverse Events (version 5.0)18 based on clinical documentation. Eight pediatric patients with FLT3-mutated AML were included, with ages ranging from 6 to 21 (median, 19) years. None of the patients received upfront gilteritinib, but 1 patient received it as consolidation therapy, achieved complete response, and remains in remission. The other 7 patients received it after their disease proved refractive to at least 1 line of treatment, including a median of 2 (range, 2-9) prior salvage treatments and a median of 1 (range, 1-2) prior FLT3 inhibitors. All 7 patients received gilteritinib-based combinations (Table 1). Seven patients had FLT3-ITD (median allelic frequency, 0.27; range, 0.02-0.67), and 1 had a noncanonical mutation, including the tyrosine kinase domain mutation p.N676K.
Patient characteristics
| Age/sex . | FLT3 mutation . | Other mutations . | CNS status . | Upfront chemotherapy regimen . | Time from diagnosis to first relapse, mo . | Gilteritinib regimen (line of therapy) . | Gilteritinib dosing schedule . | Cycles . | Response to gilteritinib and cycle of MRD . | Toxicity . | Status . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6/M | FLT3 non-ITD p.N676K missense variant of TKD | RUNX1, GATA2, CREBBP | CNS3 | ADE + GO | Refractory | FLAG-Ida + gilteritinib | 40 mg (2 mg/kg) daily d 1-14 | 1 | NR | Alanine aminotransferase increased, febrile neutropenia, creatinine elevation, seizure, anemia, thrombocytopenia | Alive | 7 |
| 21/F | FLT3 ITD, ITD ratio of 0.27 | NPM1 | CNS1 | CLIA + VEN | NA | CLIA + gilteritinib consolidation | 80 mg daily | 4 | CR; MRD− first cycle | Febrile neutropenia, constipation, abdominal pain, dysuria, bacteremia, acidosis, anemia, thrombocytopenia | Alive | 10 |
| 21/F | FLT3-ITD, ITD ratio of 0.10 | NPM1 | CNS1 | 7 + 3 and midostaurin | Refractory | CLIA + VEN + gilteritinib | 120 mg daily ×14 d/cycle ×2 cycles; 40 mg daily post-SCT maintenance ×3 mo and then increased to 80 mg daily | 3, then post-SCT maintenance | CR; MRD− third cycle | Febrile neutropenia, anemia, thrombocytopenia, bacteremia, colitis, HTN | Alive | 14 |
| 17/F | FLT3 ITD, ITD ratio of 0.02 | Negative | CNS1 | ADE + sorafenib | Refractory | Cytarabine + GO + gilteritinib | 120 mg daily ×1 cycle; post-SCT maintenance 40, 80, 120 mg daily (adjusted because of AEs) | 5, then post-SCT maintenance | CR; MRD− after SCT | Febrile neutropenia, nausea, hypomagnesemia, neuropathy, thrombocytopenia, anemia, paresthesia, rhabdomyolysis, lung infection | Alive | 18 |
| 19/M | FLT3 ITD and D835 point mutation; ITD ratio of 0.625 and D835 ratio of 0.062 | Mosaic germ line GATA2 deletion, KIT | CNS3 | ADE | Refractory | (1) Mitoxantrone + cytarabine; (2) AZA, fludarabine, cytarabine; (3) DAC; (4) AMG330 (BiTE CD33/CD3); (5) CAR T-cell infusion; (6) Ox40 mAb; (7) S64315 (MCL-1 inhibitor); (8) DAC + VEN + midostaurin; (9) DCLL9718S (anti-CLL1) + AZA; (10) DAC + VEN + sorafenib; (10) DAC + VEN + gilteritinib + ponatinib; (11) fludarabine + cytarabine + vorinostat + ponatinib + gilteritinib | 120 mg daily | 7 | NR | Alanine aminotransferase increased, febrile neutropenia, fungal pneumonia, bacteremia, intracranial hemorrhage, acidosis | Dead | 8 |
| 20/F | FLT3-ITD then FLT3-TKD (ratios unknown) | CUX1, WT1 | CNS1 | ADE + sorafenib | 27 mo; day +700 from SCT | (1) FLAG + midostaurin; (2) cytarabine + mitoxantrone + midostaurin; (3) second SCT; (4) DAC + vorinostat + sunitinib; (5) DAC + VEN + sunitinib; (6) DAC + sunitinib + AZA + gilteritinib + VEN | 120 mg daily continuous | 1 | NR | Febrile neutropenia, anemia, thrombocytopenia | Dead | 2 |
| 19/F | FLT3 ITD, ITD ratio of 0.46 | NPM1, TP53, WT1 | CNS1 | ADE + sorafenib | 10 mo | (1) CPX-351 + FLAG; (2) cytarabine + PEG + midostaurin; (2) DAC + VEN + sorafenib; (3) SCT + post-SCT maintenance with sorafenib; (4) azacitidine + VEN + gilteritinib; (5) second SCT + post-SCT maintenance AZA + gilteritinib | 80 mg daily continuous | 3, then post-SCT maintenance | CR; MRD− third cycle | Febrile neutropenia, anemia, bacteremia, neuropathy, thrombocytopenia, hypomagnesemia, thromboembolic event, paresthesia, alanine aminotransferase increased, creatinine increase | Alive | 12 |
| 14/M | FLT3-ITD allelic ratio of 0.67 (N609_L610ins) | NUP98-NSD1, WT1 S118fs*81 | CNS1 | ADE | Refractory | AZA-FLA + gilteritinib ×2 cycles, HSCT, post-HSCT gilteritinib | AZA-FLA + gilteritinib ×2 cycles, HSCT, post-HSCT gilteritinib | 2, then post-SCT maintenance | CR; MRD− first cycle | Tooth discoloration, febrile neutropenia with S mitis bacteremia, anemia, thrombocytopenia | Alive | 7 |
| Age/sex . | FLT3 mutation . | Other mutations . | CNS status . | Upfront chemotherapy regimen . | Time from diagnosis to first relapse, mo . | Gilteritinib regimen (line of therapy) . | Gilteritinib dosing schedule . | Cycles . | Response to gilteritinib and cycle of MRD . | Toxicity . | Status . | Follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6/M | FLT3 non-ITD p.N676K missense variant of TKD | RUNX1, GATA2, CREBBP | CNS3 | ADE + GO | Refractory | FLAG-Ida + gilteritinib | 40 mg (2 mg/kg) daily d 1-14 | 1 | NR | Alanine aminotransferase increased, febrile neutropenia, creatinine elevation, seizure, anemia, thrombocytopenia | Alive | 7 |
| 21/F | FLT3 ITD, ITD ratio of 0.27 | NPM1 | CNS1 | CLIA + VEN | NA | CLIA + gilteritinib consolidation | 80 mg daily | 4 | CR; MRD− first cycle | Febrile neutropenia, constipation, abdominal pain, dysuria, bacteremia, acidosis, anemia, thrombocytopenia | Alive | 10 |
| 21/F | FLT3-ITD, ITD ratio of 0.10 | NPM1 | CNS1 | 7 + 3 and midostaurin | Refractory | CLIA + VEN + gilteritinib | 120 mg daily ×14 d/cycle ×2 cycles; 40 mg daily post-SCT maintenance ×3 mo and then increased to 80 mg daily | 3, then post-SCT maintenance | CR; MRD− third cycle | Febrile neutropenia, anemia, thrombocytopenia, bacteremia, colitis, HTN | Alive | 14 |
| 17/F | FLT3 ITD, ITD ratio of 0.02 | Negative | CNS1 | ADE + sorafenib | Refractory | Cytarabine + GO + gilteritinib | 120 mg daily ×1 cycle; post-SCT maintenance 40, 80, 120 mg daily (adjusted because of AEs) | 5, then post-SCT maintenance | CR; MRD− after SCT | Febrile neutropenia, nausea, hypomagnesemia, neuropathy, thrombocytopenia, anemia, paresthesia, rhabdomyolysis, lung infection | Alive | 18 |
| 19/M | FLT3 ITD and D835 point mutation; ITD ratio of 0.625 and D835 ratio of 0.062 | Mosaic germ line GATA2 deletion, KIT | CNS3 | ADE | Refractory | (1) Mitoxantrone + cytarabine; (2) AZA, fludarabine, cytarabine; (3) DAC; (4) AMG330 (BiTE CD33/CD3); (5) CAR T-cell infusion; (6) Ox40 mAb; (7) S64315 (MCL-1 inhibitor); (8) DAC + VEN + midostaurin; (9) DCLL9718S (anti-CLL1) + AZA; (10) DAC + VEN + sorafenib; (10) DAC + VEN + gilteritinib + ponatinib; (11) fludarabine + cytarabine + vorinostat + ponatinib + gilteritinib | 120 mg daily | 7 | NR | Alanine aminotransferase increased, febrile neutropenia, fungal pneumonia, bacteremia, intracranial hemorrhage, acidosis | Dead | 8 |
| 20/F | FLT3-ITD then FLT3-TKD (ratios unknown) | CUX1, WT1 | CNS1 | ADE + sorafenib | 27 mo; day +700 from SCT | (1) FLAG + midostaurin; (2) cytarabine + mitoxantrone + midostaurin; (3) second SCT; (4) DAC + vorinostat + sunitinib; (5) DAC + VEN + sunitinib; (6) DAC + sunitinib + AZA + gilteritinib + VEN | 120 mg daily continuous | 1 | NR | Febrile neutropenia, anemia, thrombocytopenia | Dead | 2 |
| 19/F | FLT3 ITD, ITD ratio of 0.46 | NPM1, TP53, WT1 | CNS1 | ADE + sorafenib | 10 mo | (1) CPX-351 + FLAG; (2) cytarabine + PEG + midostaurin; (2) DAC + VEN + sorafenib; (3) SCT + post-SCT maintenance with sorafenib; (4) azacitidine + VEN + gilteritinib; (5) second SCT + post-SCT maintenance AZA + gilteritinib | 80 mg daily continuous | 3, then post-SCT maintenance | CR; MRD− third cycle | Febrile neutropenia, anemia, bacteremia, neuropathy, thrombocytopenia, hypomagnesemia, thromboembolic event, paresthesia, alanine aminotransferase increased, creatinine increase | Alive | 12 |
| 14/M | FLT3-ITD allelic ratio of 0.67 (N609_L610ins) | NUP98-NSD1, WT1 S118fs*81 | CNS1 | ADE | Refractory | AZA-FLA + gilteritinib ×2 cycles, HSCT, post-HSCT gilteritinib | AZA-FLA + gilteritinib ×2 cycles, HSCT, post-HSCT gilteritinib | 2, then post-SCT maintenance | CR; MRD− first cycle | Tooth discoloration, febrile neutropenia with S mitis bacteremia, anemia, thrombocytopenia | Alive | 7 |
ADE, cytarabine, daunorubicin, etoposide; AZA, azacitidine; BiTE, bispecific T-cell engager; CAR, chimeric antigen receptor; CLIA, cladribine, idarubicin, cytarabine; CNS, central nervous system; CR, complete response; DAC, decitabine; FLAG, fludarabine, cytarabine, granulocyte colony-stimulating factor; GO, gemtuzumab ozogamicin; HSCT, hematopoietic stem cell transplantation; HTN, hypertension; Ida, idarubicin; mAb, monoclonal antibody; MRD, minimal residual disease; NA, not applicable; NR, no response; PEG, pegaspargase; SCT, stem cell transplantation; TKD, tyrosine kinase domain; VEN, venetoclax.
For the older pediatric patients (age >17 years), flat dosing was used, starting at 120 mg and then reducing to 80 mg for maintenance as needed for myelosuppression or toxicity. Weight-based dosing was 2 mg/kg for the 6-year-old patient and 1.73 mg/kg for the 14-year-old patient given the available tablet size of 80 mg.
All 8 patients developed grade 3 febrile neutropenia, grade 3 anemia, and grade 4 thrombocytopenia. The median time to onset was 15 days (range, 2 days to 1 month). One patient had grade 4 acidosis and intracranial hemorrhage at the end of life. One patient with leptomeningeal disease had grade 3 seizures. He was found to have increased opening pressure on lumbar puncture, and although there was concern for posterior reversible encephalopathy syndrome given its association with gilteritinib, his clinical team attributed this AE to leptomeningeal disease. Grade 3 or 4 AEs considered at least possibly related to gilteritinib included febrile neutropenia and thrombocytopenia in all patients. Other reported grade 1 to 2 AEs included creatine elevation (n = 2), constipation, abdominal pain, dysuria, bacteremia (n = 3), acidosis, hypertension, colitis, nausea, hypomagnesemia (n = 2), peripheral neuropathy (n = 2), paresthesia (n = 2), rhabdomyolysis, lung infection (n = 2), thromboembolic event, alanine aminotransferase increase (n = 3), headache, and tooth discoloration. Of these, creatine elevation, abdominal pain, constipation, bacteremia, nausea, peripheral neuropathy, paresthesias, rhabdomyolysis, lung infection, alanine aminotransferase increase, headache, and tooth discoloration were considered possibly related gilteritinib. Notable grade 1 to 2 AEs occurring after stem cell transplantation included paresthesia and creatine kinase elevation. Both cases resolved with dose reduction. There was no reported association with or worsening of graft-versus-host disease. No sudden death, arrhythmia, or persistent seizures were recorded.
Among the 8 patients treated with gilteritinib, 5 (63%) had a CR, and 4 of these had refractory disease to prior therapies. Of the 7 patients with R/R disease, 4 (57%) had a CR after initiation of gilteritinib. Four patients underwent allotransplantation directly after combination treatment with gilteritinib; all are in CR, and all are continuing to use gilteritinib (at 40, 80, or 120 mg once daily) as stem cell transplantation maintenance. The maintenance dosing varies based on tolerability.
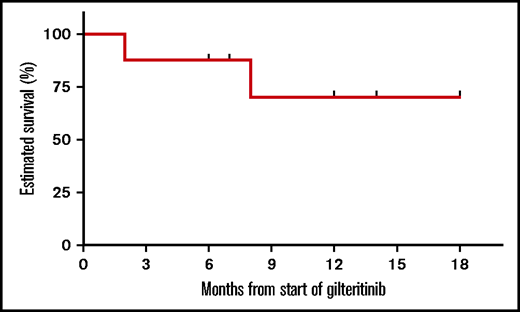
With a median follow-up of 9 (range, 2-18) months from the start of gilteritinib, as depicted in the Kaplan-Meier curve in Figure 1, 6-month overall survival (OS) was 87.5% and 1-year OS was 70%. Among responders (n = 5), the median number of gilteritinib cycles to best response was 1 (range, 1-2). None of the 5 responders subsequently relapsed. It is worth noting, however, that 3 of the responders had NPM1 mutations with a low FLT3-ITD allelic ratio, which has been found to be a favorable mutation in some patients.19,20 Six patients (including 5 with R/R disease and 1 receiving consolidation with gilteritinib) remain alive.
This case series, the largest to date in pediatrics using gilteritinib in AML, involves 2 of the largest pediatric cancer institutes in the United States. This review, although limited in patient number and length of follow-up, illustrates a 62% remission rate (5 of 8), with 6 of 8 patients alive at a median follow-up of 9 months. It is important to note the responders are all continuing to receive gilteritinib. Historically, FLT3 mutations confer an OS of 50% to 60%.5 We acknowledge the limitation of this retrospective review, but despite the small sample size and limited follow-up, the survival in pediatric patients receiving gilteritinib is encouraging.
This case series demonstrates that gilteritinib is safe to consider for use in pediatric and young adult patients with FLT3-mutated AML. Pediatric studies (registered at www.clinicaltrials.gov as #NCT03730012, along with Children’s Oncology Group AAML1831) are underway to evaluate the use of gilteritinib in patients with relapsed disease and as upfront therapy.
Authorship
Contribution: All authors contributed significantly to the body of work; D.M. and B.C. designed research, performed research, contributed vital analytical tools, analyzed data, and wrote the paper; L.T. and J.S.Y. performed research, contributed vital analytical tools, and analyzed data; M.R., K.M.M., C.N., N.J.S., N.D., T.M.K., and C.D. contributed vital analytical tools and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David McCall, Department of Pediatrics, Unit 87, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: dmccall1@mdanderson.org.
References
Author notes
Requests for data sharing may be submitted to David McCall (dmccall1@mdanderson.org).