Key Points
Final ALCANZA data confirm more durable responses and longer PFS with brentuximab vedotin vs control in CD30+ cutaneous T-cell lymphoma.
Brentuximab vedotin extended the time to next treatment vs physician's choice, suggesting that durable responses are clinically important.
Abstract
The primary analysis of the phase 3 ALCANZA trial showed significantly improved objective responses lasting ≥4 months (ORR4; primary endpoint) and progression-free survival (PFS) with brentuximab vedotin vs physician’s choice (methotrexate or bexarotene) in CD30-expressing mycosis fungoides (MF) or primary cutaneous anaplastic large-cell lymphoma (C-ALCL). Cutaneous T-cell lymphomas often cause pruritus and pain; brentuximab vedotin improved skin symptom burden with no negative effects on quality of life. We report final data from ALCANZA (median follow-up, 45.9 months). Adults with previously treated CD30-expressing MF/C-ALCL were randomly assigned to brentuximab vedotin (n = 64) or physician’s choice (n = 64). Final data demonstrated improved responses per independent review facility with brentuximab vedotin vs physician’s choice: ORR4; 54.7% vs 12.5% (P < .001); complete response, 17.2% vs 1.6% (P = .002). Median PFS with brentuximab vedotin vs physician’s choice was 16.7 months vs 3.5 months (P < .001). Median time to the next treatment was significantly longer with brentuximab vedotin than with physician’s choice (14.2 vs 5.6 months; hazard ratio, 0.27; 95% confidence interval, 0.17-0.42; P < .001). Of 44 patients in the brentuximab vedotin arm who experienced any-grade peripheral neuropathy, (grade 3, n = 6; grade 4, n = 0), 86% (38 of 44) had complete resolution (26 of 44) or improvement to grades 1 and 2 (12 of 44). Peripheral neuropathy was ongoing in 18 patients (all grades 1-2). These final analyses confirm improved, clinically meaningful, durable responses and longer PFS with brentuximab vedotin vs physician’s choice in CD30-expressing MF or C-ALCL. This trial was registered at https://www.clinicaltrials.gov as #NCT01578499.
Introduction
Cutaneous T-cell lymphomas (CTCLs) represent a heterogeneous group of T-cell lymphomas, primarily involving the skin. Mycosis fungoides (MF) and primary cutaneous anaplastic large-cell lymphoma (C-ALCL) are 2 of the most common subtypes of CTCL.1,2 Despite being distinct malignancies, MF and C-ALCL share some clinical and pathological characteristics: they both express cluster of differentiation 30 (CD30) on their cell surface.3,4 In C-ALCL, by definition, CD30 is expressed by the majority (>75%), if not all, of tumor cells; in MF, the proportion of CD30-expressing cells is more variable (0% ≥ 75%).3-6
CTCLs usually have a chronic course and can be associated with a high symptom burden (including disfiguring lesions, debilitating pruritus, and frequent skin infections) which negatively impact a patient’s quality of life (QoL) and well-being.7-9 In early-stage CTCL, disease control often can be achieved with single or combined skin-directed therapies. For patients in the advanced stages of disease and after relapse, there are no curative options. In these settings, systemic therapies (eg, extracorporeal photopheresis, interferons, retinoids, chemotherapy, histone deacetylase inhibitors, and immunotherapies), and occasionally stem cell transplantation are often used, along with skin-directed therapies.1,2,10,11 As no regimen to date has been shown to prolong overall survival (OS) in advanced CTCL, treatment tends to focus on reducing the burden of disease, delaying progression and the need for subsequent treatment and improving QoL.7,12
The anti-CD30 antibody-drug conjugate brentuximab vedotin has been approved in the United States for adult patients with C-ALCL or CD30-expressing MF who have received prior systemic therapy and in the European Union for adult patients with CD30-expressing CTCL after at least 1 prior systemic therapy.13,14 These approvals were based on data from the ALCANZA study: an international, open-label, multicenter, randomized, phase 3 trial comparing brentuximab vedotin with physician’s choice of methotrexate chemotherapy or the retinoid bexarotene (being the most commonly used globally recommended treatment options1,11 ) in patients with previously treated MF or C-ALCL.15 With a median follow-up of 22.9 months, the primary analysis showed that brentuximab vedotin was superior to physician’s choice, demonstrating a statistically significant improvement in objective response lasting at least 4 months (ORR4; primary end point, 56% vs 13%; P < .0001), complete response (CR) rate (16% vs 2%; adjusted P = .0046), and progression-free survival (PFS; median, 16.7 vs 3.5 months; hazard ratio [HR], 0.27; 95% confidence interval [CI], 0.17-0.43; adjusted P < .0001). Patient-reported burden of symptoms, measured by the Skindex-29 questionnaire, which has not been validated for use in patients with C-ALCL or MF, showed significantly greater symptom reduction in the brentuximab vedotin group compared with the physician’s choice group (change in Skindex-29 symptom domain score −27.96 vs −8.62; adjusted P < .0001); however, these results should be interpreted with caution, as Skindex-29 is not validated for use in these diseases.
The primary analyses of the ALCANZA data were performed 10 months after the last patient’s end-of-treatment visit (data cutoff, 31 May 2016).15 In this article, we present the final analyses from the ALCANZA study (data cutoff, 28 September 2019), which were undertaken to confirm long-term efficacy and safety at the primary study end point (ORR4) and in other selected outcome measures, including CR rate, PFS, OS, time to next treatment (TTNT), and resolution and improvement of peripheral neuropathy (PN), a common side effect of brentuximab vedotin.13,14
Methods
Study design and patient population
The study design (including randomization procedures) and patient population for ALCANZA have been reported previously.15 In brief, patients aged ≥18 years with previously treated CD30-expressing MF or C-ALCL (after at least 1 prior systemic therapy or prior radiotherapy) were enrolled; CD30 positivity was defined as ≥10% of target lymphoid cells exhibiting a membrane, cytoplasmic, and/or Golgi staining pattern for CD30. An Eastern Cooperative Oncology Group performance status of 0 to 2 was required. Patients were randomly assigned 1:1 to receive brentuximab vedotin (1.8 mg/kg IV every 3 weeks, for up to 16 cycles) or physician’s choice (methotrexate, 5-50 mg orally once weekly or bexarotene 300 mg/m2 [target dose] orally once daily, for up to 48 weeks). Patients who had progressed on both prior methotrexate and bexarotene were excluded, as were patients with Sézary syndrome (SS; stage B2), whereas patients with stage B1 MF, indicating some blood involvement, were considered eligible. Methotrexate and bexarotene were prescribed as the standard of care, targeting the maximum tolerated effective dose. For all study drugs, dose adjustments for toxicities were applied, according to established dose-modification guidelines. Treatment continued until disease progression or unacceptable toxicity up to a maximum of 16 × 21-day cycles (48 weeks).
The ALCANZA study was conducted in accordance with Good Clinical Practice guidelines and relevant regulatory requirements. Local ethics committees or institutional review boards approved the protocol and amendments. All patients gave written informed consent. The primary and final data were gathered by the investigators, were analyzed by the sponsors, and were accessible to all authors.
Assessments
During the study, safety and efficacy assessments were undertaken every 3 weeks (before the dose on day 1 of each cycle) and at the end-of-treatment visit (30 days after the last dose of the study drug). Posttreatment, patients were followed up every 12 weeks for 2 years and then every 6 months thereafter.
Objective responses (including ORR4) and disease progression were determined per independent review facility (IRF) evaluation of global response score using International Society for Cutaneous Lymphoma/European Organization for Research and Treatment of Cancer consensus guidelines.16,17 The primary end point of ORR4 was defined as the proportion of patients achieving an objective global response lasting at least 4 months (from first to last recorded response). As previously described,15 the global response score included skin evaluation (Modified Severity-Weighted Assessment Tool) per investigator; nodal and visceral radiographic assessment per IRF; and, for patients with MF, Sézary cell count per IRF.
Other selected end points under evaluation in this final analysis included the key secondary end points of CR rate and PFS, other secondary end points of response duration and safety (specifically incidence, severity, and duration of PN), and the nonprespecified end points of ORR, TTNT, and OS. PFS was defined as the time from randomization until disease progression per IRF or death of any cause, whichever occurred first. TTNT was defined as the time from randomization to the date of the first documentation of a subsequent antineoplastic treatment or the last contact date for patients who had not received further antineoplastic treatment. Patients who died or withdrew, were lost to follow-up, or had received no antineoplastic therapy by the date of last contact were censored in the TTNT analysis. Subgroup analyses of response (ORR4, ORR, and CR) by disease subtype (MF or C-ALCL), disease stage (per investigator for patients with MF) or involvement (skin-only or extracutaneous disease per investigator for C-ALCL patients), and compartment (tumor-node-metastasis-blood stage per investigator) are also reported.
PN (defined using the standardized Medical Dictionary for Regulatory Activities [MedDRA] query [SMQ] PN), which incorporates peripheral motor neuropathy and peripheral sensory neuropathy) was evaluated and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03.
Statistical analysis
Sample size calculations and adjustments for multiplicity (for the primary and key secondary end points) are reported in the primary publication.15 In this final analysis, differences in response end points were analyzed using a Cochran-Mantel-Haenszel χ2 test stratified by diagnosis (C-ALCL or MF). Kaplan-Meier analyses were used to estimate survival distributions for all time-to-event outcomes. Stratified Cox regression models were then used to estimate HRs and associated 95% CIs for the time-to-event outcomes, with stratified log-rank tests employed to evaluate statistically significant differences (P < .05) between the treatment arms. The subgroup analyses are reported using descriptive statistics only.
Efficacy analyses were performed in the intent-to-treat (ITT) population and safety analyses were undertaken in all patients who received at least 1 dose of study treatment (safety population). The analyses were performed with SAS, version 9.3. ALCANZA is registered with ClinicalTrials.gov (NCT01578499).
Results
From August 2012 through 31 July 2015, 131 patients with previously treated, CD30-expressing MF or C-ALCL were enrolled and randomly assigned 1:1 to receive study treatment (brentuximab vedotin or physician’s choice) at 52 sites in Australia, European Union, Switzerland, Brazil and the United States. Of these patients, 128 (64 in each arm) were included in the ITT population (MF, n = 97; C-ALCL, n = 31); 3 patients with MF were excluded because of insufficient CD30 expression. A total of 128 patients received study treatment and were included in the safety population (brentuximab vedotin arm, n = 66; physician’s choice arm, n = 62). Patient flow through the study is presented in supplemental Figure 1.15 Patient baseline characteristics for the ITT population, which have been published in full previously,15 are summarized in supplemental Table 1.
Responses
The final analysis, at a median follow-up of 45.9 months (95% CI, 41.0-49.4), demonstrated significantly improved responses to brentuximab vedotin compared with physician’s choice (Table 1). In the brentuximab vedotin arm vs physician’s choice arm, the ORR4 per IRF (primary end point) was 54.7% vs 12.5% (P < .001), the ORR per IRF was 65.6% vs 20.3% (P < .001), and the CR rate per IRF (key secondary end point) was 17.2% vs 1.6% (P = .002).
Summary of efficacy in the ITT population
| . | Brentuximab vedotin (n = 64) . | Physician’s choice (n = 64) . | P . |
|---|---|---|---|
| ORR4 per IRF, n (%) | 35 (54.7)* | 8 (12.5) | <.001 |
| Best response per IRF, n (%) | |||
| ORR (CR+PR) | 42 (65.6) | 13 (20.3) | <.001 |
| CR | 11 (17.2) | 1 (1.6) | .002 |
| PR | 31 (48.4) | 12 (18.8) | |
| SD | 10 (15.6) | 18 (28.1) | |
| PD | 5 (7.8) | 22 (34.4) | |
| Median PFS per IRF, months (95% CI)† | 16.7 (15.4-21.6) | 3.5 (2.4-4.6) | |
| HR for PFS (95% CI) | 0.38 (0.25-0.58) | <.001 | |
| 3-y OS rate, % (95% CI) | 64.4 (50.7-75.2) | 61.9 (47.3-73.6)‡ | |
| HR for OS (95% CI) | 0.75 (0.42-1.32) | .310 | |
| . | Brentuximab vedotin (n = 64) . | Physician’s choice (n = 64) . | P . |
|---|---|---|---|
| ORR4 per IRF, n (%) | 35 (54.7)* | 8 (12.5) | <.001 |
| Best response per IRF, n (%) | |||
| ORR (CR+PR) | 42 (65.6) | 13 (20.3) | <.001 |
| CR | 11 (17.2) | 1 (1.6) | .002 |
| PR | 31 (48.4) | 12 (18.8) | |
| SD | 10 (15.6) | 18 (28.1) | |
| PD | 5 (7.8) | 22 (34.4) | |
| Median PFS per IRF, months (95% CI)† | 16.7 (15.4-21.6) | 3.5 (2.4-4.6) | |
| HR for PFS (95% CI) | 0.38 (0.25-0.58) | <.001 | |
| 3-y OS rate, % (95% CI) | 64.4 (50.7-75.2) | 61.9 (47.3-73.6)‡ | |
| HR for OS (95% CI) | 0.75 (0.42-1.32) | .310 | |
PD, progressive disease; PR, partial response; SD, stable disease.
Based on additional information provided to the IRF after the 31 May 2016 data cutoff, the IRF determined that 1 patient had not achieved ORR4 as was originally reported; the change in status was determined through a standard IRF adjudication process.
Median follow-up for OS in the brentuximab vedotin arm was 48.4 mo.
Median follow-up for OS in the physician’s choice arm was 42.9 mo.
Survival end points
PFS.
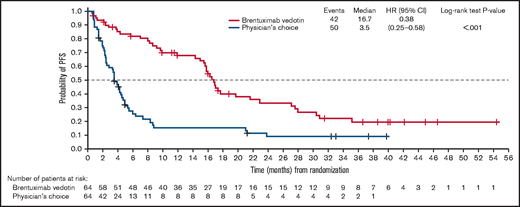
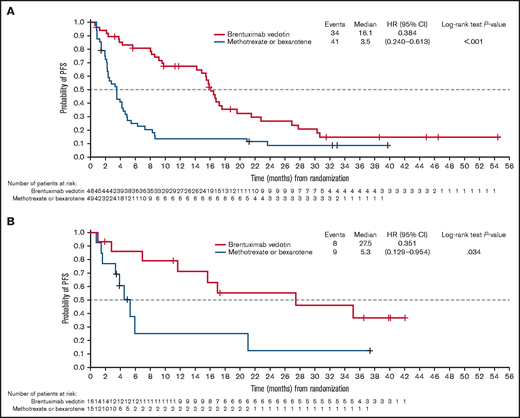
With a median follow-up of 36.8 months, the key secondary end point of PFS per IRF was significantly longer in the brentuximab vedotin arm than in the physician’s choice arm (median PFS 16.7 vs 3.5 months; HR, 0.38; 95% CI, 0.25-0.58; P < .001; Figure 1; Table 1). Median PFS for cycles 1 to 5, 6 to 12, and 13 to 16 of brentuximab vedotin was 3.8, 15.4, and 21.6 months, respectively; Kaplan-Meier estimates of PFS by treatment cycle 12, 18, and 24 months after the first dose are detailed in Table 2. In the patients with MF, median PFS per IRF was 16.1 months in the brentuximab vedotin arm vs 3.5 months in the physician’s choice arm (HR, 0.38; 95% CI, 0.24-0.61) (Figure 2A). In patients with MF with early (stages IA-IIA) and advanced disease (stages IIB-IVB), respective median PFS values with brentuximab vedotin and physician’s choice were 17.2 vs 3.9 months and 16.1 vs 2.8 months (supplemental Figure 2), respectively. Median PFS per IRF in patients with C-ALCL was 27.5 vs 5.3 months in patients who received brentuximab vedotin vs physician’s choice (HR, 0.35; 95% CI, 0.13-0.95; Figure 2B).
PFS per IRF in the ITT population. PFS was defined as the time from randomization until disease progression per IRF or death of any cause, whichever occurred first. Patients who were lost to follow-up, withdrew consent, or discontinued treatment because of undocumented disease progression after the last adequate disease assessment were censored at the last disease assessment.
PFS per IRF in the ITT population. PFS was defined as the time from randomization until disease progression per IRF or death of any cause, whichever occurred first. Patients who were lost to follow-up, withdrew consent, or discontinued treatment because of undocumented disease progression after the last adequate disease assessment were censored at the last disease assessment.
PFS per IRF by number of cycles of brentuximab vedotin received in the ITT population
| . | Treatment cycles, n . | ||
|---|---|---|---|
| 1-5 (n = 19) . | 6-12 (n = 17) . | 13-16 (n = 28) . | |
| Median PFS, months | 3.8 | 15.4 | 21.6 |
| PFS for extended follow-up, %* | |||
| 12 mo | 27.3 | 58.8 | 96.0 |
| 18 mo | 18.2 | 32.7 | 57.3 |
| 24 mo | 18.2 | 26.1 | 46.9 |
| . | Treatment cycles, n . | ||
|---|---|---|---|
| 1-5 (n = 19) . | 6-12 (n = 17) . | 13-16 (n = 28) . | |
| Median PFS, months | 3.8 | 15.4 | 21.6 |
| PFS for extended follow-up, %* | |||
| 12 mo | 27.3 | 58.8 | 96.0 |
| 18 mo | 18.2 | 32.7 | 57.3 |
| 24 mo | 18.2 | 26.1 | 46.9 |
Kaplan-Meier estimates.
PFS per IRF in the ITT population. (A) PFS for patients with MF. (B) PFS for patients with C-ALCL. PFS is defined in Figure 1. Patients were censored at last disease assessment if they withdrew consent, were lost to follow-up, or discontinued treatment because of undocumented disease progression after the last adequate disease assessment.
PFS per IRF in the ITT population. (A) PFS for patients with MF. (B) PFS for patients with C-ALCL. PFS is defined in Figure 1. Patients were censored at last disease assessment if they withdrew consent, were lost to follow-up, or discontinued treatment because of undocumented disease progression after the last adequate disease assessment.
OS.
Although survival was not a prespecified end point in the study, 3-year estimates of OS, after a median follow-up of 45.9 months, were 64.4% with brentuximab vedotin and 61.9% with physician’s choice (HR, 0.75; 95% CI, 0.42-1.32; P = .310; supplemental Figure 3; Table 1). A subanalysis of patients with advanced-stage MF highlighted an improvement in OS with brentuximab vedotin vs physician’s choice (P = .021); details of OS for patients with early and advanced-stage MF are shown in supplemental Figure 4. Overall, there were 23 deaths in the brentuximab vedotin arm and 25 in the physician’s choice arm. Five patients with C-ALCL died in each treatment arm. The high use (69%) of brentuximab vedotin as the subsequent therapy in the physician’s choice arm limits interpretation of survival. In addition, 24% of patients were retreated with brentuximab vedotin. Further details can be found in supplemental Table 2.
TTNT
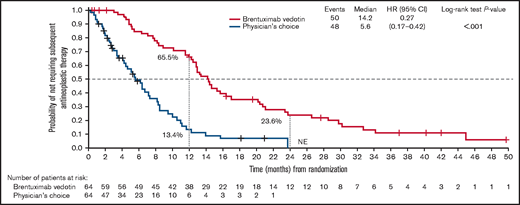
TTNT (median follow-up, 37.3 months) was significantly longer with brentuximab vedotin compared with physician’s choice (14.2 vs 5.6 months; HR, 0.27; 95% CI, 0.17-0.42; P < .001; Figure 3). The probability of patients in the brentuximab vedotin arm and physician’s choice arm, respectively, not requiring subsequent antineoplastic treatment was 65.5% and 13.4% at 1 year and 23.6% and 0% at 2 years.
TTNT in the ITT population. Time to next antineoplastic therapy was defined as the time from randomization to the date of the first documentation of antineoplastic therapy or the last contact date for subjects who never received antineoplastic therapy. NE, not evaluable.
TTNT in the ITT population. Time to next antineoplastic therapy was defined as the time from randomization to the date of the first documentation of antineoplastic therapy or the last contact date for subjects who never received antineoplastic therapy. NE, not evaluable.
Among patients with MF, the median TTNT was 13.4 months (95% CI, 11.4-15.3) in the brentuximab vedotin arm and 5.6 months (95% CI, 3.4-7.2) in the physician’s choice arm. In the C-ALCL group, the median TTNT was 20.6 months (95% CI, 7.0-32.8) in the brentuximab vedotin arm and 7.3 months (95% CI, 2.4-14.0) in the physician’s choice arm. In the brentuximab vedotin arm and physician’s choice arm, the respective probabilities of not requiring subsequent antineoplastic treatment of patients with MF was 63.6% and 8.6% at 1 year, and 16.9% and not estimable (n = 0) at 2 years. For patients with C-ALCL the corresponding values were 71.4% and 31.2% at 1 year, and 42.9% and not estimable (n = 0) at 2 years.
Patient responses by disease subtype
Analysis of response by disease subtype showed that ORR4, ORR, and CR rates per IRF were higher with brentuximab vedotin than with physician’s choice in patients with MF and those with C-ALCL (Table 3). ORR4 per IRF with brentuximab vedotin was 5 times higher than with physician’s choice in patients with MF (50% vs 10%) and almost 3.5 times higher than physician’s choice in patients with C-ALCL (69% vs 20%). Six of the 11 patients treated with brentuximab vedotin who achieved CR had C-ALCL. Responses by CD30 expression level and large-cell transformation status at the 2-year data cutoff have been reported separately.18
Patient response per IRF by baseline disease subtype and stage per investigator in the ITT population
| . | Patients, n (%) . | |||||||
|---|---|---|---|---|---|---|---|---|
| . | Brentuximab vedotin (n = 64) . | Physician’s choice (n = 64) . | ||||||
| . | Total . | ORR4 . | ORR . | CR . | Total . | ORR4 . | ORR . | CR . |
| MF | 48 (75) | 24 (50) | 31 (65) | 5 (10) | 49 (77) | 5 (10) | 8 (16) | 0 |
| Stage | ||||||||
| IA-IIA | 15 (31) | 6 (40) | 8 (53) | 1 (7) | 18 (37) | 4 (22) | 5 (28) | 0 |
| IIB | 19 (40) | 12 (63) | 13 (68) | 3 (16) | 19 (39) | 1 (5) | 3 (16) | 0 |
| IIIA-IIIB | 4 (8) | 2 (50) | 3 (75) | 0 | 2 (4) | 0 | 0 | 0 |
| IVA | 2 (4) | 2 (100) | 2 (100) | 1 (50) | 9 (18) | 0 | 0 | 0 |
| IVB | 7 (15) | 2 (29) | 4 (57) | 0 | 0 | — | — | — |
| Unknown | 1 (2) | 0 | 1 (100) | 0 | 1 (2) | 0 | 0 | 0 |
| C-ALCL | 16 (25) | 11 (69) | 11 (69) | 6 (38) | 15 (23) | 3 (20) | 5 (33) | 1 (7) |
| Involvement | ||||||||
| Skin only | 9 (56) | 8 (89) | 8 (89) | 4 (44) | 11 (73) | 3 (27) | 5 (45) | 1 (9) |
| Extracutaneous disease | 7 (44) | 3 (43) | 3 (43) | 2 (29) | 4 (27) | 0 | 0 | 0 |
| . | Patients, n (%) . | |||||||
|---|---|---|---|---|---|---|---|---|
| . | Brentuximab vedotin (n = 64) . | Physician’s choice (n = 64) . | ||||||
| . | Total . | ORR4 . | ORR . | CR . | Total . | ORR4 . | ORR . | CR . |
| MF | 48 (75) | 24 (50) | 31 (65) | 5 (10) | 49 (77) | 5 (10) | 8 (16) | 0 |
| Stage | ||||||||
| IA-IIA | 15 (31) | 6 (40) | 8 (53) | 1 (7) | 18 (37) | 4 (22) | 5 (28) | 0 |
| IIB | 19 (40) | 12 (63) | 13 (68) | 3 (16) | 19 (39) | 1 (5) | 3 (16) | 0 |
| IIIA-IIIB | 4 (8) | 2 (50) | 3 (75) | 0 | 2 (4) | 0 | 0 | 0 |
| IVA | 2 (4) | 2 (100) | 2 (100) | 1 (50) | 9 (18) | 0 | 0 | 0 |
| IVB | 7 (15) | 2 (29) | 4 (57) | 0 | 0 | — | — | — |
| Unknown | 1 (2) | 0 | 1 (100) | 0 | 1 (2) | 0 | 0 | 0 |
| C-ALCL | 16 (25) | 11 (69) | 11 (69) | 6 (38) | 15 (23) | 3 (20) | 5 (33) | 1 (7) |
| Involvement | ||||||||
| Skin only | 9 (56) | 8 (89) | 8 (89) | 4 (44) | 11 (73) | 3 (27) | 5 (45) | 1 (9) |
| Extracutaneous disease | 7 (44) | 3 (43) | 3 (43) | 2 (29) | 4 (27) | 0 | 0 | 0 |
One patient in each arm had incomplete staging data and are not included in the table: 1 patient in the brentuximab vedotin arm had a PR, and 1 patient in the physician’s choice arm had no response.
Patient responses by baseline disease stage or extracutaneous involvement
The superiority of brentuximab vedotin over physician’s choice was consistent across all stages of MF in terms of ORR4 (stages IA-IIA, 40% vs 22%; stage IIB, 63% vs 5%; stages IIIA-IIIB, 50% vs 0%; stage IVA, 100% vs 0%; and stage IVB, 29% vs not available), ORR, and CR rates, and in patients with C-ALCL and skin-only disease (ORR4, 89% vs 27%; and ORR, 89% vs 45%) or extracutaneous sites of involvement (ORR4, 43% vs 0%; and ORR, 43% vs 0%; Table 3).
Patient responses by tumor-node-metastasis-blood stage
The ORR in patients with MF and disease with nodal or blood involvement was higher in those who received brentuximab vedotin than in those who received physician’s choice (supplemental Table 3); the CR rate was also superior with brentuximab vedotin in the case of nodal involvement. Among 48 patients with blood involvement at baseline (patients with B2 disease were ineligible), ORR4 was 50% and ORR was 65% (CR, 10%; partial response [PR], 55%) with brentuximab vedotin, whereas ORR4 was 10% and ORR was 16% (PR, 16%) with physician’s choice. For patients with C-ALCL, a higher ORR4 was observed for brentuximab vedotin vs physician’s choice in those with nodal (69% vs 20%) or visceral (69% vs 20%) involvement (supplemental Table 4). ORR and CR in patients with C-ALCL was higher with brentuximab vedotin vs physician’s choice in disease with nodal or visceral involvement. Patients treated with brentuximab vedotin demonstrated a higher ORR4 vs physician’s choice for all stages of skin involvement in patients with MF (T1-T4) and those with C-ALCL (T1-T3) (supplemental Tables 3 and 4).
Dosage
The median duration of treatment and relative dose intensity were reported in the primary analysis, during which 3 patients were still receiving brentuximab vedotin, and all patients who were receiving physician’s choice had discontinued bexarotene and methotrexate.15
Peripheral neuropathy
In the safety population, 44 of 66 (67%) patients in the brentuximab vedotin arm and 4 of 62 (6%) patients in the physician’s choice arm experienced PN (standardized Medical Dictionary for Regulatory Activities query), most of which was grade 1 or 2; 6 patients experienced grade 3 PN, the highest grade reported. In most patients in the brentuximab vedotin arm, the maximum grade of PN was grade 1 (18 of 44 patients) or 2 (20 of 44 patients); 6 patients had grade 3 events; and there were no grade 4 events. Of the 44 patients with PN, 23 (52%) required at least 1 brentuximab vedotin dose modification (delay, reduction, or dose held), with 9 patients (14%) permanently discontinuing brentuximab vedotin treatment.
By the final data cutoff, 86% (38 of 44) of patients with any grade PN in the brentuximab vedotin arm had complete resolution (26 of 44) or improvement to grade 1 or 2 (12 of 44), as compared with 36 of 44 (82%) patients in the primary analysis (data cutoff, 31 May 2016; Table 4). Eighteen brentuximab vedotin–treated patients had ongoing PN (grade 1, n = 15; grade 2, n = 3), as compared with 22 patients in the primary analysis. No patients had ongoing grade 3 or 4 PN.
Resolution, improvement, and duration of PN (SMQ) in the safety population
| . | Brentuximab vedotin (n = 44) . | Physician’s choice (n = 4) . | ||
|---|---|---|---|---|
| Data cutoff . | 31 May 2016 . | 28 September 2018 . | 31 May 2016 . | 28 September 2018 . |
| Patients with resolution or improvement of PN events, n (%) Patients with resolution of all PN events, n (%) Median time to resolution, wk Patients with improvement in PN events by ≥1 grade, n (%) Median time to improvement, wk | 36 (82) 22 (50) 27.0 14 (32) 8.0 | 38 (86) 26 (59) 33.0 12 (27) 15.0 | 1 (25) 1 (25) 2.0 0 — | 2 (50) 2 (50) 10.5 0 — |
| Patients with ongoing PN events, n (%) Maximum severity grade 1, n (%) Maximum severity grade 2, n (%) | 22 (50) 17 (39) 5 (11) | 18 (41) 15 (34) 3 (7) | 3 (75) 1 (25) 2 (50) | 2 (50) 1 (25) 1 (25) |
| . | Brentuximab vedotin (n = 44) . | Physician’s choice (n = 4) . | ||
|---|---|---|---|---|
| Data cutoff . | 31 May 2016 . | 28 September 2018 . | 31 May 2016 . | 28 September 2018 . |
| Patients with resolution or improvement of PN events, n (%) Patients with resolution of all PN events, n (%) Median time to resolution, wk Patients with improvement in PN events by ≥1 grade, n (%) Median time to improvement, wk | 36 (82) 22 (50) 27.0 14 (32) 8.0 | 38 (86) 26 (59) 33.0 12 (27) 15.0 | 1 (25) 1 (25) 2.0 0 — | 2 (50) 2 (50) 10.5 0 — |
| Patients with ongoing PN events, n (%) Maximum severity grade 1, n (%) Maximum severity grade 2, n (%) | 22 (50) 17 (39) 5 (11) | 18 (41) 15 (34) 3 (7) | 3 (75) 1 (25) 2 (50) | 2 (50) 1 (25) 1 (25) |
Discussion
The primary analysis from the international, randomized, phase 3 ALCANZA trial demonstrated the favorable efficacy and tolerability of brentuximab vedotin in previously treated patients with CD30-expressing MF or C-ALCL.15 For these patients, effective treatment options are limited, and durable responses are rare.2 Demonstration of clinical benefit as measured by improved responses (ORR4 and CR), PFS, and patient-reported symptoms in the ALCANZA trial compared with a physician’s choice of methotrexate or bexarotene led to the regulatory approval of brentuximab vedotin and established it as an effective treatment option in CD30-expressing cases.
This final analysis from ALCANZA shows that, with longer follow-up and increased treatment exposure, brentuximab vedotin provides a durable, robust clinical benefit in previously treated patients with CD30-expressing MF or C-ALCL through improved global response rates (ORR4 per IRF, 54.7% vs 12.5%; P < .001; including long-lasting CRs) and prolonged PFS (median, 16.7 vs 3.5 months; P < .001), when compared with physician's choice. These findings therefore support the primary analysis of ALCANZA and are also consistent with the results of other smaller studies of brentuximab vedotin in relapsed/refractory CTCL.6,15,19,20 These single-arm phase 2 studies included more CTCL subtypes not studied in ALCANZA such as SS and lymphomatoid papulosis (LyP), and lower CD30 levels, down to 0%. Brentuximab vedotin at the standard dose demonstrated significant clinical activity in treatment-refractory or advanced MF, SS, or LyP, with a wide range of CD30 expression levels.
In the final analysis, treatment with brentuximab vedotin provided clinically meaningful extensions in median TTNT compared with physician’s choice (median, 14.2 vs 5.6 months; median difference, 8.6 months; P < .001), sparing patients from the burden of additional rounds of subsequent antineoplastic treatment in the months immediately after treatment of relapsed disease. Consistently, TTNT in the physician’s choice arm was similar to that reported in a retrospective analysis of 198 patients with MF/SS requiring systemic therapy, who had a median TTNT of 5.4 months.21
TTNT was shorter than PFS in the brentuximab vedotin arm; median TTNT and PFS were 13.4 and 16.1 months in patients with MF and 20.6 and 27.5 months in patients with C-ALCL, respectively. A possible explanation for this result is that patients with CTCL often require treatment of symptomatic deterioration without meeting the criteria for PD.22
There was no difference in OS between treatment arms in the ITT population, although a subanalysis highlighted an improvement in OS in patients with advanced-stage MF (P = .021). OS was not a prespecified end point in the study because of the challenges of assessing OS in patients who received multiple subsequent therapies. Limiting the assessment of OS according to assigned therapy is the fact that 62% of patients in the physician’s choice arm received brentuximab vedotin as a subsequent treatment, so OS data should be interpreted with caution.
The subgroup analyses of response showed that treatment with brentuximab vedotin led to improved, clinically meaningful, durable responses and consistently higher ORR4, CR rate, and ORR than with physician’s choice across disease subtypes, stages, and compartments. Of note, in the C-ALCL subgroup, PFS was 22.2 months longer with brentuximab vedotin vs physician’s choice (27.5 vs 5.3 months; HR, 0.35; 95% CI, 0.13-0.95). The longer follow-up reported in these analyses allowed for continued monitoring of PN, a common side effect of brentuximab vedotin.13,14,23 With additional follow-up, 86% of cases of PN in patients treated with brentuximab vedotin had resolved completely or had improved by at least 1 grade. Of the patients with ongoing PN, 18 in the brentuximab vedotin arm had grade 1 (n = 15) or 2 (n = 3) PN and 2 in the physician’s choice arm had grade 1 (n = 1) or 2 (n = 1) PN; there were no ongoing grade 3 or 4 PN events.
Limitations of the ALCANZA study have been discussed.15 However, this final analysis is subject to some additional limitations, mainly relating to OS. The study was not powered to detect the difference between the study arms in OS (nor was OS specified as an end point). The large proportion of patients who received subsequent antineoplastic treatment must also be considered, particularly patients in the physician’s choice arm who were permitted to cross over and receive brentuximab vedotin, and the relatively small number of deaths in each arm.
In summary, the final data from the ALCANZA trial are consistent with the primary analysis.15 These final analyses confirm that patients with CD30-expressing MF or C-ALCL, who are treated with brentuximab vedotin vs physician’s choice, have improved, clinically meaningful, durable responses; prolonged TTNT; a reduction in patient reported-symptoms; resolution of PN over time; and longer PFS. These data also provide reassurance about the long-term efficacy and safety of brentuximab vedotin in this patient population. Further subgroup analyses and QoL investigations have been presented previously. Kim et al18 presented a post hoc analysis of patients with MF enrolled in ALCANZA that explored whether baseline CD30 expression level or large-cell transformation at the time of enrollment affects the efficacy and safety of brentuximab vedotin. To reflect the importance of QoL in CTCL, ALCANZA also evaluated patient-reported outcomes by using the Skindex-29 and EuroQoL 5-Dimension questionnaires, as reported by Dummer et al.24
Acknowledgments
The authors thank the patients who participated in the study and their families; the other investigators and staff at all ALCANZA clinical sites; and the members of the Independent Data Monitoring Committee and Independent Review Committee.
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Hedley Coppock, PhD, and Rebecca Vickers, BSc, of Ashfield MedComms, an Ashfield Health company, funded by Takeda Development Center Americas, Inc. (TDCA), and complied with the Good Publication Practice-3 (GPP3) guidelines (Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015,163:461-464). Thanks also go to Caroline Ojaimi, PhD, of Takeda Development Center Americas, Inc. (TDCA), for editorial support.
Authorship
Contribution: All authors collected and analyzed the data, drafted the manuscript, revised the manuscript critically, and gave final approval to submit the manuscript for publication.
Conflict-of-interest disclosure: S.M.H. reports consultancy or advisory roles for ADCT Therapeutics, Aileron, Forty-Seven, Infinity/Verastem, Kyowa Hakko Kirin, Takeda Development Center Americas, Inc., Lexington, MA, Seagen Inc, Affimed, Angimmune, Beigene, Corvus, Innate Pharma, Kura, MSD, MiRagen, Mundipharma, Portola, and Syros Pharmaceutical; and research funding from ADCT Therapeutics, Aileron, Forty-Seven, Infinity/Verastem, Kyowa Hakko Kirin, Takeda Development Center Americas, Inc., Seagen Inc, Celgene, and Trillium. J.J.S. reports consultancy or advisory roles for Helsinn, Kyowa Hakko Kirin, Takeda Development Center Americas, Inc., Innate Pharma, 4SC, and Mallinckrodt. R.D. reports intermittent, project-focused consultancy, and advisory roles for Novartis, MSD, Bristol-Myers Squibb, Roche, Amgen, Takeda Development Center Americas, Inc., Pierre Fabre, and Sun Pharma. M.D. reports employment with UT MD Anderson Cancer Center; membership on board of directors or consultancy or advisory roles for Huya Bioscience, Covance Laboratory Services (formerly MiRagen Therapeutics), Array Biopharma, Cell Medica, Celgene, Kyowa Hakko Kirin, Seagen Inc, Mallinckrodt Pharmaceuticals (formerly Therakos), Takeda Development Center Americas, Inc., Aclaris Therapeutics, and USCLC; honoraria from Aclaris Therapeutics, Array Biopharma, Celgene, Cell Medica, Kyowa Hakko Kirin, Mallinckrodt Pharmaceuticals (formerly Therakos), Takeda Development Center Americas, Inc., Seagen Inc, Therakos, and Jonathan Wood & Associates; and research funding from Eisai, Shape, Allos, Rhizen Pharma, Spatz Foundation, Oncoceuticals, Tetralogics, Takeda Development Center Americas, Inc., Mallinckrodt Pharmaceuticals (formerly Therakos), Kyowa Hakko Kirin, Seagen Inc, and Soligenix. Y.H.K. reports honoraria from Eisai, Kyowa Hakko Kirin, Takeda Development Center Americas, Inc., Seagen Inc, Medivir, Innate Pharma, Portola, and Corvus; and research funding from Eisai, Kyowa Hakko Kirin, MSD, Horizon, Takeda Development Center Americas, Inc., Seagen Inc, Soligenix, MiRagen, Forty-Seven, Neumedicine, Innate Pharma, Portola, Trillium, Galderma, and Elorac. P.Q. reports consultancy or advisory roles from Takeda Development Center Americas, Inc., Kyowa Hakko Kirin, Therakos, and 4SC. P.L.Z. reports consultancy, advisory, and/or speakers bureau roles for Verastem, Celltrion, Gilead, Janssen-Cilag, Bristol-Myers Squibb, Servier, Sandoz, MSD, Immune Design, Celgene, Portola, Roche, EUSA Pharma, Kyowa Hakko Kirin, and Sanofi. H.E. reports consultancy or advisory roles for Genentech, Roche, AbbVie, and Pharmacyclics; honoraria from Genentech, Roche, AbbVie, Pharmacyclics, and Takeda Development Center Americas, Inc.; and research funding from Genentech, Roche, AbbVie, Pharmacyclics, ATARA, and Celgene. L.P-B. reports consultancy or advisory roles for and honoraria from Takeda Development Center Americas, Inc.. O.E.A. reports consultancy or advisory roles for Trillium Therapeutics, Actelion, Mallinckrodt, Medivir, Seagen Inc, and Kyowa Hakko Kirin; honoraria from Soligenix; and research funding from Trillium Therapeutics, Pfizer, and Actelion. L.G. reports consultancy or advisory roles for, and research funding from Kyowa Hakko Kirin, Therakos, Helsinn, and Bristol-Myers Squibb. J.A.S. reports consultancy or advisory roles for Takeda Development Center Americas, Inc.. P.L.O-R. reports consultancy or advisory roles for Kyowa Hakko Kirin, Takeda Development Center Americas, Inc., Actelion, 4SC, Innate Pharma, MiRagen, and Bristol-Myers Squibb; and research funding from Meda. M.W. reports research funding and/or travel expenses from and/or is a consultant for Takeda Development Center Americas, Inc., and TEVA Pharmaceuticals. D.C.F. is a member of a Board of Directors or advisory committee for Seagen Inc. J.W. reports an advisory role for and lecture honoraria from Roche, Celgene, Takeda, Janssen-Cilag, Servier, Amgen, BMS, Abbvie, Novartis, and Gilead; research grants from Roche, GSK/Novartis, Takeda, and Janssen-Cilag; and conference travel support from Roche. J.T. is a member of a Board of Directors, advisory committee, and/or speaker’s bureau for (all positions nonremunerated), and/or reports funding facilitating research (paid to third parties) from Janssen Cilag, Celgene, Roche, and Takeda Development Center Americas, Inc.. S.D. reports research funding from Kyowa Hakko Kirin Pharmaceutical. R.S. is a member of a Board of Directors or advisory committee and/or is a consultant for Takeda Development Center Americas, Inc., Seagen Inc, 4SC, ICN, Novartis, Roche, and Johnson & Johnson. J.L. reports employment with Seagen Inc V.B. reports employment with Takeda Development Center Americas, Inc.. M.L. reports employment with Takeda Development Center Americas, Inc.; and equity ownership in Takeda Pharmaceutical Co Ltd. H.M.P. reports research funding and honoraria from, is a member of a Board of Directors or advisory committee and/or is a consultant for Takeda Development Center Americas, Inc., Celgene Corporation, and Eisai. S.W., O.B., and K.T. declare no competing financial interests.
Correspondence: Steven M. Horwitz, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: horwitzs@MSKCC.org.
References
Author notes
Presented in abstract form at the 15th International Conference on Malignant Lymphoma (ICML), Lugano, Switzerland, 18-22 June, 2019 (abstract 232); the European Organisation for Research and Treatment of Cancer (EORTC), Cutaneous Lymphoma Task Force Meeting 2019, Athens, Greece, 26-28 September 2019 (abstract 085); and the 4th World Congress of Cutaneous Lymphomas (WCCL), Barcelona, Spain, 12-14 February, 2020.
The data sets Including the redacted study protocol, redacted statistical analysis plan, and individual participant’s data supporting the results reported in this article, will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its deidentification, in compliance with applicable privacy laws, data protection guidelines, and requirements for consent and anonymization.
The full-text version of this article contains a data supplement.