Key Points
The first model capable of predicting 30-day mortality from ICH in allo-HSCT patients was developed.
The LAWS score showed good discrimination and calibration power, and was externally validated by geographical validation.
Abstract
Intracranial hemorrhage (ICH) is a rare but fatal central nervous system complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, factors that are predictive of early mortality in patients who develop ICH after undergoing allo-HSCT have not been systemically investigated. From January 2008 to June 2020, a total of 70 allo-HSCT patients with an ICH diagnosis formed the derivation cohort. Forty-one allo-HSCT patients with an ICH diagnosis were collected from 12 other medical centers during the same period, and they comprised the external validation cohort. These 2 cohorts were used to develop and validate a grading scale that enables the prediction of 30-day mortality from ICH in all-HSCT patients. Four predictors (lactate dehydrogenase level, albumin level, white blood cell count, and disease status) were retained in the multivariable logistic regression model, and a simplified grading scale (termed the LAWS score) was developed. The LAWS score was adequately calibrated (Hosmer-Lemeshow test, P > .05) in both cohorts. It had good discrimination power in both the derivation cohort (C-statistic, 0.859; 95% confidence interval, 0.776-0.945) and the external validation cohort (C-statistic, 0.795; 95% confidence interval, 0.645-0.945). The LAWS score is the first scoring system capable of predicting 30-day mortality from ICH in allo-HSCT patients. It showed good performance in identifying allo-HSCT patients at increased risk of early mortality after ICH diagnosis. We anticipate that it would help risk stratify allo-HSCT patients with ICH and facilitate future studies on developing individualized and novel interventions for patients within different LAWS risk groups.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has become an increasingly widespread and potentially curative treatment of various malignant and nonmalignant disorders of the hematopoietic system. Concomitantly, posttransplant complications that give rise to significant morbidity and mortality are becoming more frequently recognized. The central nervous system (CNS) is commonly affected after allo-HSCT,1,2 which can be related to issues such as drug toxicity, metabolic factors, infections, and cerebrovascular factors.3,4 The main CNS complications after allo-HSCT include seizures, CNS infections, posterior reversible encephalopathy syndrome, and ischemic and hemorrhagic strokes.4-7
Among all the various types of CNS complications, intracranial hemorrhage (ICH) is particularly fatal even though it is rarely diagnosed. Unlike ICH in the general population, which usually results from hypertension or the use of anticoagulants,8 posttransplant ICH has more complicated causes. The etiology includes but is not limited to thrombocytopenia, acute graft-versus-host disease (GVHD), previous CNS episodes such as CNS leukemia, and infectious causes.9,10 The incidence of ICH ranges from 0.33% to 3.42% among patients who have undergone allo-HSCT,2,4,9-12 which is higher than that in the general population (0.01%-0.03%).8 Advancements in imaging techniques such as computed tomography (CT) scanning and magnetic resonance imaging (MRI) enable clinicians to detect ICH quickly and initiate the corresponding interventions once a hemorrhagic event is suspected. However, compared with patients without ICH, allo-HSCT patients with ICH still have a dismal prognosis, with a 5-year overall survival (OS) rate of ∼17% to 27%,7,10 and a high proportion of patients die within 48 hours after ICH onset.12 In addition, neuropathologic studies showed that ICH was prevalent in patients who died after transplantation.1,13 It is therefore necessary to better characterize the outcome of posttransplant ICH.
Several studies attempted to identify independent risk factors for ICH development after allo-HSCT, including pretransplant CNS leukemia, grade III to IV acute GVHD, delayed platelet engraftment/low platelet number, systemic infection, and low fibrinogen level.7,10,12 However, predictors of early mortality in patients who develop ICH after allo-HSCT have never been investigated. Although there are several mortality prediction models for ICH in the general population or in patients with hematologic malignancies,14-16 no prediction model has been developed and validated in allo-HSCT recipients.
We used a nationwide data set of 111 allo-HSCT patients who developed ICH after transplantation to develop and externally validate the first simple scoring system capable of identifying patients at higher risk of early death. This system can help hematologists without extensive experience in stroke neurology to risk stratify allo-HSCT patients promptly and accurately at the time of ICH onset and can improve the communication between clinicians and patients’ families. The score also makes it possible to identify patients who might benefit from alternative ICH treatments and facilitates prospective trials focused on developing individualized and novel therapeutic strategies for posttransplant ICH patients at different risks of early mortality.
Methods
Study patients
A total of 6938 patients received allo-HSCT at Peking University People’s Hospital from January 2008 to June 2020. Conditioning regimens and stem cell harvest have been described in previous studies.17,18 We retrospectively reviewed the electronic medical records and identified 121 patients who were clinically diagnosed with ICH. Of these, we first excluded 39 patients without neuroimaging-confirmed ICH. Ten patients were then excluded because the bleeding was diagnosed before allo-HSCT. Two more patients with posttraumatic ICH were excluded. Each patient was evaluated and diagnosed by both a senior hematologist and a neurologist. Eventually, we identified 70 patients with posttransplant ICH confirmed by CT/MRI scanning, who formed the derivation cohort. For external validation, we used an independent data set of 41 posttransplant ICH patients who received allo-HSCT during the same period of time at 12 other medical centers in China. The same inclusion and exclusion criteria were used during patient enrollment.
The study protocol was approved by the institutional review board and ethics committee of each participating hospital in accordance with the Declaration of Helsinki.
Definitions
ICH was defined as any bleeding occurring in an intraparenchymal hemorrhage, subarachnoid hemorrhage (SAH), subdural hemorrhage, or epidural hemorrhage.14,15,19,20 ICH diagnosis was confirmed by results of neuroimaging tests, including CT/MRI scanning. If more than one neuroimaging test result was available after allo-HSCT, the date of the first CT/MRI scan showing ICH was defined as the diagnosis date of ICH. Acute and chronic GVHD were diagnosed and assessed based on published criteria.21,22 Systemic infections included invasive fungal infections, sepsis, and viremia.12 The disease status was evaluated before transplantation according to published criteria.7,12,17,23,24 Patients with leukemia were categorized as standard risk if they were in their first or second complete remission of acute leukemia without [t(9;22)(q34;q11)] or in the chronic phase of chronic myeloid leukemia. Patients with lymphoma were categorized as standard risk if they were in their first or second complete remission, had partial remission, or had stable disease. Those with myelodysplastic syndrome with bone marrow blasts <20% or aplastic anemia were also classified as standard risk. All other patients were considered to be high risk.
Procedures
The electronic medical records of the derivation cohort were used to identify prognostic factors associated with 30-day mortality from posttransplant ICH. We first compared the characteristics of the patients who died within 30 days after the ICH diagnosis vs those of the patients who survived this period. Candidate prognostic factors were identified by univariate analysis of 37 clinical and laboratory variables. The laboratory data around the time of ICH diagnosis were recorded.
Multivariate modeling was then performed by using logistic regression. Selected categorical and dichotomized continuous variables identified by the univariate analysis (P < .15) were included in the multivariate analysis. Continuous variables were dichotomized by maximizing Youden’s index (sensitivity + specificity – 1). When selecting the variables retained in the final prediction score, factors were chosen based on the statistical significance (P < .05 or P < .1), clinical relevance, and easy accessibility in different hospitals. Rounded β coefficients derived from the multivariate logistic regression were used to establish a scoring system for predicting 30-day mortality in posttransplant ICH patients.
For internal validation, the bootstrap method was used to generate 1000 bootstrap samples equal to the size of the derivation cohort by sampling with replacement from the derivation cohort.25-27 For external validation, an independent external validation cohort was used as previously described. We characterized the model performance by assessing its discrimination and calibration capability. Discrimination was assessed by generating receiver-operating characteristic curves and calculating C-statistics. Calibration was evaluated by using the calibration plot to visualize the agreement between the observed and predicted probability of early deaths. A perfect calibration plot was indicated by a 45° diagonal line. In addition, the Hosmer-Lemeshow test was used to assess how the predicted probability fit the observed probability of 30-day mortality. Decision-curve analysis (DCA) is widely used to determine the clinical utility of multivariable prediction models by calculating a “net benefit,” which is estimated as the true-positive results minus the false-positive results.27-32 DCA was used to assess the usefulness of the risk score in indicating the risk of 30-day mortality and informing treatment decisions (eg, using more intensive or novel treatments for ICH) compared with the “treating all” strategy (as if all allo-HSCT patients would die within 30 days of ICH diagnosis) or the “treating none” strategy (as if no allo-HSCT patient would die within 30 days of ICH diagnosis).
Statistical analysis
Categorical variables were analyzed by using the χ2 test, and continuous variables were analyzed by using the nonparametric Mann-Whitney test. For the survival analysis, we used Kaplan-Meier analysis to generate survival curves and compared them by using the log-rank test. The starting point was the diagnosis of ICH, and the primary end point was ICH-related death within 30 days of ICH diagnosis. The difference in the discriminating power of receiver-operating characteristic curves was compared by using the DeLong test. Data analysis was performed with SPSS 24.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) and R studio 1.2 (RStudio, PBC, Boston, MA).
Results
Patient characteristics
Between January 2008 and June 2020, a total of 70 of the 6938 patients (1.01%) who had received allo-HSCT at Peking University People’s Hospital were diagnosed with ICH on the basis of neuroimaging, and they comprised the derivation cohort. Twenty-five (35.7%) patients died within 30 days of ICH diagnosis. The median OS of the 70 patients from the time of ICH diagnosis was 84 days (range, 0-2355 days). Forty-one patients who developed ICH after allo-HSCT at 12 other medical centers formed the external validation cohort. Twenty-one (51.2%) of the patients from the external validation cohort died within 30 days of ICH diagnosis. The median OS of these 41 patients was 30 days (range, 0-769 days). In all 111 patients from both cohorts, the OS at 30 days and 2 years were 58.6% and 38.7%, respectively (supplemental Figure 1). The characteristics of patients within the external validation cohort were distinct from those of patients in the derivation cohort, such as more patients with a high-risk disease status (P = .008), fewer patients being infused with stem cells harvested from bone marrow and peripheral blood (P < .001), more patients with ICHs of multiple types (P = .029), and lower platelet counts at ICH diagnosis (P = .009). Detailed demographic features and clinical characteristics at the time of ICH diagnosis of the patients from the 2 cohorts are shown in supplemental Table 1.
Considering the long duration of patient enrollment in the derivation cohort, we compared the baseline characteristics of patients over time (2008-2013 vs 2014-2020) (supplemental Table 2). The incidence of ICH was comparable in patients recruited during the 2 periods (0.95% vs 1.04%; P = .800). In addition, there was no significant difference in 30-day mortality between patients who received allo-HSCT during the period 2008 to 2013 and those who received it during the period 2014 to 2020 (40.9% vs 33.3%; P = .54).
Clinical features and treatments of ICH in allo-HSCT patients
Among all 111 patients included in both cohorts, the median time from stem cell infusion to diagnosis of ICH was 84 days, and 54.05% of ICHs developed within 100 days after allo-HSCT. Regarding the localization of ICH, 64 patients (57.7%) had intraparenchymal hemorrhage, 13 patients (11.7%) had SAH, 7 patients (9.9%) had subdural hemorrhage, 2 patients (1.8%) had epidural hemorrhage, and 21 patients (18.9%) had multifocal hemorrhages. The majority of patients (97.3%) were symptomatic at the time of ICH occurrence. Multiple symptoms often occurred in the same patients simultaneously. Common clinical manifestations that prompted a brain CT/MRI scan included impaired consciousness in 54 patients (48.6%), headache in 50 patients (45.0%), seizure in 46 patients (41.4%), partial paralysis in 23 patients (20.7%), vomiting in 17 patients (15.3%), and elevated blood pressure in 9 patients (8.1%). Most of the patients (92.7%, n = 110) were thrombocytopenic (platelet count <100 × 109/L) at ICH diagnosis, and the median platelet count was 31.7 × 109/L (range, 1.00-249.0 × 109/L).
Only 8 patients (7.2%) were eligible for neurosurgical interventions for various reasons, such as the location of the bleeding, delayed detection of ICH, and low platelet counts. Thus, most patients received conservative treatment instead, which mainly included monitoring and nursing care; lowering of blood pressure; reversal of coagulopathies by administration of fresh frozen plasma, prothrombin complex concentrates, and platelet transfusion; intracranial pressure control; and seizure treatment.33,34 There was no significant change in the number of patients receiving surgery (P = .301) over time. For patients with ICH associated with CNS infections, appropriate anti-infective agents were given. There was no statistically significant difference (P = .610) in the 30-day mortality rate of patients who underwent surgery (50.0%) and those who received conservative treatment only (40.8%).
LAWS score derivation
Table 1 shows univariate analysis results of 37 variables in the derivation cohort. Seventeen variables were associated with 30-day mortality from posttransplant ICH in the univariate analysis (P < .15). Continuous variables with a P value <.15 were dichotomized. The χ2 test was used to analyze the dichotomized continuous variables and categorical variables; 15 of them were selected for multivariate analysis on the basis of clinical relevance and availability across different medical centers (Table 2).
Univariate analysis comparing ICH patients in the derivation cohort who survived or died within 30 days of ICH diagnosis
| . | Alive (n = 45) . | Dead (n = 25) . | P* . |
|---|---|---|---|
| Demographic features | |||
| Age, range, y | 29 (4-57) | 37 (12-54) | .038 |
| Male sex | 27 (60%) | 21 (84%) | .102 |
| Clinical features related to allo-HSCT | |||
| Primary disease† | .655 | ||
| AML | 13 (28.9%) | 7 (28.0%) | |
| ALL | 15 (33.3%) | 8 (32.0%) | |
| CML | 2 (4.4%) | 0 (0.0%) | |
| MDS | 6 (13.3%) | 6 (24.0%) | |
| AA | 5 (11.1%) | 1 (4.0%) | |
| Others | 4 (8.9%) | 3 (12.0%) | |
| Disease status | .022 | ||
| Standard risk | 38 (84.4%) | 15 (60.0%) | |
| High risk | 7 (15.6%) | 10 (40.0%) | |
| Time from diagnosis to HSCT, range, d | 227 (29-5992) | 208 (75-1750) | .637 |
| No. of transplantation | .091 | ||
| 1 | 44 (97.8%) | 22 (88.0%) | |
| 2 | 1 (2.2%) | 3 (12.0%) | |
| Type of transplantation | .031 | ||
| Haplo-identical | 40 (88.9%) | 17 (68.0%) | |
| HLA match | 5 (11.1%) | 8 (32.0%) | |
| Donor–patient sex | .611 | ||
| Match | 26 (57.8%) | 16 (64.0%) | |
| Mismatch | 19 (42.2%) | 9 (36.0%) | |
| ABO type | .372 | ||
| Match | 23 (51.1%) | 10 (40.0%) | |
| Mismatch | 22 (48.9%) | 15 (60.0%) | |
| Stem cell source | .463 | ||
| PB + BM | 39 (86.7%) | 20 (80.0%) | |
| PB | 6 (13.3%) | 5 (20.0%) | |
| WBC engraftment, range, d | 14 (10-22) | 14 (9-27) [n = 24] | .909 |
| Delayed PLT engraftment (> 30 d) | 22 (48.9%) | 12 (48.0%) | .943 |
| History of acute GVHD | .638 | ||
| None | 16 (35.6%) | 7 (28.0%) | |
| I-II | 22 (48.9%) | 12 (48.0%) | |
| III-IV | 7 (15.6%) | 6 (24.0%) | |
| History of chronic GVHD | .592 | ||
| None | 38 (84.4%) | 20 (80.0%) | |
| Limited | 1 (2.2%) | 0 (0.0%) | |
| Extensive | 6 (13.3%) | 5 (20.0%) | |
| History of TMA | 4 (8.9%) | 5 (20.0%) | .183 |
| History of DIC | 3 (6.7%) | 4 (16.0%) | .212 |
| Systemic infection | 35 (77.8%) | 20 (80.0%) | .828 |
| Clinical features related to ICH | |||
| Time from HSCT to ICH, range, d | 131 (14-1472) | 119 (21-944) | .686 |
| Bleeding site | .505 | ||
| IP | 27 (60.0%) | 18 (72.0%) | |
| SAH | 5 (11.1%) | 4 (16.0%) | |
| SD | 5 (11.1%) | 2 (8.0%) | |
| ED | 2 (4.4%) | 0 (0.0%) | |
| Multiple | 6 (13.3%) | 1 (4.0%) | |
| IVH | 4 (8.9%) | 6 (24.0%) | .083 |
| Preexisting CNS events‡ | 20 (44.4%) | 7 (28.0%) | .176 |
| Hypertension | 6 (13.3%) | 4 (16.0%) | .760 |
| Diabetes | 1 (2.2%) | 2 (8.0%) | .253 |
| Laboratory features | |||
| WBC count (×109/L) | 3.44 (1.31-14.11) | 2.11 (0.20-18.15) | .025 |
| RBC count (×1012/L) | 2.77 (1.57-4.53) | 2.28 (1.73-3.36) | .007 |
| Hemoglobin, g/dL | 90.20 (2.29-152.00) | 73.00 (63.00-113.00) | .008 |
| Platelet count (×109/L) | 55.0 (9.0-249.0) | 24.0 (2.0-74.0) | <.001 |
| PT, s | 11.80 (9.30-17.50) | 13.60 (10.00-20.20) | .045 |
| INR | 1.07 (0.87-1.54) | 1.22 (0.96-1.84) | .033 |
| Fibrinogen, mg/dL | 304.0 (101.0-550.0) | 319.0 (106.0-598.0) | .408 |
| LDH, U/L | 341.0 (155.0-1416.0) | 507.0 (118.0-1500.0) | .038 |
| Urea, mmol/L | 5.22 (1.12-21.12) | 7.78 (3.14-90.18) | .006 |
| Creatinine, µmol/L | 51.0 (21.0-137.0) | 70.0 (22.0-143.0) | .013 |
| Albumin, g/L | 36.5 (28.6-45.0) | 31.9 (21.3-47.1) | .001 |
| Bilirubin, µmol/L | 16.2 (2.0-341.1) | 40.6 (6.9-652.3) | .001 |
| Cholesterol, mmol/L | 4.49 (1.88-8.97) [n = 40] | 3.78 (1.14-16.44) [n = 24] | .560 |
| Triglyceride, mmol/L | 2.75 (1.29-9.89) [n = 40] | 2.50 (0.83-11.32) [n = 24] | .501 |
| . | Alive (n = 45) . | Dead (n = 25) . | P* . |
|---|---|---|---|
| Demographic features | |||
| Age, range, y | 29 (4-57) | 37 (12-54) | .038 |
| Male sex | 27 (60%) | 21 (84%) | .102 |
| Clinical features related to allo-HSCT | |||
| Primary disease† | .655 | ||
| AML | 13 (28.9%) | 7 (28.0%) | |
| ALL | 15 (33.3%) | 8 (32.0%) | |
| CML | 2 (4.4%) | 0 (0.0%) | |
| MDS | 6 (13.3%) | 6 (24.0%) | |
| AA | 5 (11.1%) | 1 (4.0%) | |
| Others | 4 (8.9%) | 3 (12.0%) | |
| Disease status | .022 | ||
| Standard risk | 38 (84.4%) | 15 (60.0%) | |
| High risk | 7 (15.6%) | 10 (40.0%) | |
| Time from diagnosis to HSCT, range, d | 227 (29-5992) | 208 (75-1750) | .637 |
| No. of transplantation | .091 | ||
| 1 | 44 (97.8%) | 22 (88.0%) | |
| 2 | 1 (2.2%) | 3 (12.0%) | |
| Type of transplantation | .031 | ||
| Haplo-identical | 40 (88.9%) | 17 (68.0%) | |
| HLA match | 5 (11.1%) | 8 (32.0%) | |
| Donor–patient sex | .611 | ||
| Match | 26 (57.8%) | 16 (64.0%) | |
| Mismatch | 19 (42.2%) | 9 (36.0%) | |
| ABO type | .372 | ||
| Match | 23 (51.1%) | 10 (40.0%) | |
| Mismatch | 22 (48.9%) | 15 (60.0%) | |
| Stem cell source | .463 | ||
| PB + BM | 39 (86.7%) | 20 (80.0%) | |
| PB | 6 (13.3%) | 5 (20.0%) | |
| WBC engraftment, range, d | 14 (10-22) | 14 (9-27) [n = 24] | .909 |
| Delayed PLT engraftment (> 30 d) | 22 (48.9%) | 12 (48.0%) | .943 |
| History of acute GVHD | .638 | ||
| None | 16 (35.6%) | 7 (28.0%) | |
| I-II | 22 (48.9%) | 12 (48.0%) | |
| III-IV | 7 (15.6%) | 6 (24.0%) | |
| History of chronic GVHD | .592 | ||
| None | 38 (84.4%) | 20 (80.0%) | |
| Limited | 1 (2.2%) | 0 (0.0%) | |
| Extensive | 6 (13.3%) | 5 (20.0%) | |
| History of TMA | 4 (8.9%) | 5 (20.0%) | .183 |
| History of DIC | 3 (6.7%) | 4 (16.0%) | .212 |
| Systemic infection | 35 (77.8%) | 20 (80.0%) | .828 |
| Clinical features related to ICH | |||
| Time from HSCT to ICH, range, d | 131 (14-1472) | 119 (21-944) | .686 |
| Bleeding site | .505 | ||
| IP | 27 (60.0%) | 18 (72.0%) | |
| SAH | 5 (11.1%) | 4 (16.0%) | |
| SD | 5 (11.1%) | 2 (8.0%) | |
| ED | 2 (4.4%) | 0 (0.0%) | |
| Multiple | 6 (13.3%) | 1 (4.0%) | |
| IVH | 4 (8.9%) | 6 (24.0%) | .083 |
| Preexisting CNS events‡ | 20 (44.4%) | 7 (28.0%) | .176 |
| Hypertension | 6 (13.3%) | 4 (16.0%) | .760 |
| Diabetes | 1 (2.2%) | 2 (8.0%) | .253 |
| Laboratory features | |||
| WBC count (×109/L) | 3.44 (1.31-14.11) | 2.11 (0.20-18.15) | .025 |
| RBC count (×1012/L) | 2.77 (1.57-4.53) | 2.28 (1.73-3.36) | .007 |
| Hemoglobin, g/dL | 90.20 (2.29-152.00) | 73.00 (63.00-113.00) | .008 |
| Platelet count (×109/L) | 55.0 (9.0-249.0) | 24.0 (2.0-74.0) | <.001 |
| PT, s | 11.80 (9.30-17.50) | 13.60 (10.00-20.20) | .045 |
| INR | 1.07 (0.87-1.54) | 1.22 (0.96-1.84) | .033 |
| Fibrinogen, mg/dL | 304.0 (101.0-550.0) | 319.0 (106.0-598.0) | .408 |
| LDH, U/L | 341.0 (155.0-1416.0) | 507.0 (118.0-1500.0) | .038 |
| Urea, mmol/L | 5.22 (1.12-21.12) | 7.78 (3.14-90.18) | .006 |
| Creatinine, µmol/L | 51.0 (21.0-137.0) | 70.0 (22.0-143.0) | .013 |
| Albumin, g/L | 36.5 (28.6-45.0) | 31.9 (21.3-47.1) | .001 |
| Bilirubin, µmol/L | 16.2 (2.0-341.1) | 40.6 (6.9-652.3) | .001 |
| Cholesterol, mmol/L | 4.49 (1.88-8.97) [n = 40] | 3.78 (1.14-16.44) [n = 24] | .560 |
| Triglyceride, mmol/L | 2.75 (1.29-9.89) [n = 40] | 2.50 (0.83-11.32) [n = 24] | .501 |
Laboratory data proximate to the time of ICH diagnosis are reported. AA, aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BM, bone marrow; CML , chronic myelogenous leukemia; DIC, disseminated intravascular coagulation; HLA, human leukocyte antigen; MDS, myelodysplastic syndrome; PB, peripheral blood; PLT, platelet; PT, prothrombin time; RBC, red blood cell; TMA, thrombotic microangiopathy.
For continuous variables, median and range are displayed, and the nonparametric Mann-Whitney test was used for calculating the P value. For categorical variables, number and percentage of total patients are displayed, and the χ2 test was used for significance calculation.
Others include lymphoma, acute mixed-lineage leukemia, lymphoma, chronic lymphocytic leukemia, and Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis.
CNS diseases diagnosed before ICH diagnosis, including CNS leukemia, CNS infections, reversible posterior leukoencephalopathy syndrome, and ischemic stroke.
Odds ratios for categorical and dichotomized continuous variables with a P ≤ .15 in univariate analysis
| . | Dichotomized value . | Odds ratio (95% CI) . | P* . |
|---|---|---|---|
| Age† | >37 y | 2.67 (0.97-7.36) | .055 |
| Sex† | Male | 3.50 (1.03-11.91) | .038 |
| Disease status† | High risk | 3.62 (1.162-11.269) | .022 |
| No. of transplantation† | 2 | 6.00 (0.59-61.07) | .091 |
| Type of transplantation† | Haplo-identical | 0.266 (0.076-0.930) | .031 |
| IVH† | — | 3.237 (0.817-12.829) | .083 |
| WBC count† (×109/L) | <2.4 | 5.495 (1.899-15.902) | .001 |
| RBC count (×1012/L) | <2.74 | 6.00 (1.77-20.31) | .002 |
| Hemoglobin† (g/dL) | <84.5 | 5.47 (1.74-17.20) | .002 |
| PLT count† (×109/L) | <43 | 6.59 (2.09-20.82) | .001 |
| PT (s) | >14.1 | 5.01 (1.63-15.43) | .003 |
| INR† | >1.31 | 5.33 (1.56-18.19) | .005 |
| LDH† (U/L) | >426 | 4.89 (1.71-13.98) | .002 |
| Urea† (mmol/L) | >5.53 | 4.57 (1.46-14.32) | .007 |
| Creatinine† (µmol/L) | >70 | 5.89 (1.96-17.66) | .001 |
| Albumin† (g/L) | <32.7 | 6.91 (2.24-21.36) | <.001 |
| Bilirubin† (µmol/L) | >15.3 | 11.0 (2.32-52.28) | .001 |
| . | Dichotomized value . | Odds ratio (95% CI) . | P* . |
|---|---|---|---|
| Age† | >37 y | 2.67 (0.97-7.36) | .055 |
| Sex† | Male | 3.50 (1.03-11.91) | .038 |
| Disease status† | High risk | 3.62 (1.162-11.269) | .022 |
| No. of transplantation† | 2 | 6.00 (0.59-61.07) | .091 |
| Type of transplantation† | Haplo-identical | 0.266 (0.076-0.930) | .031 |
| IVH† | — | 3.237 (0.817-12.829) | .083 |
| WBC count† (×109/L) | <2.4 | 5.495 (1.899-15.902) | .001 |
| RBC count (×1012/L) | <2.74 | 6.00 (1.77-20.31) | .002 |
| Hemoglobin† (g/dL) | <84.5 | 5.47 (1.74-17.20) | .002 |
| PLT count† (×109/L) | <43 | 6.59 (2.09-20.82) | .001 |
| PT (s) | >14.1 | 5.01 (1.63-15.43) | .003 |
| INR† | >1.31 | 5.33 (1.56-18.19) | .005 |
| LDH† (U/L) | >426 | 4.89 (1.71-13.98) | .002 |
| Urea† (mmol/L) | >5.53 | 4.57 (1.46-14.32) | .007 |
| Creatinine† (µmol/L) | >70 | 5.89 (1.96-17.66) | .001 |
| Albumin† (g/L) | <32.7 | 6.91 (2.24-21.36) | <.001 |
| Bilirubin† (µmol/L) | >15.3 | 11.0 (2.32-52.28) | .001 |
The χ2 test was used for statistical comparisons.
Variables included in multivariate analysis.
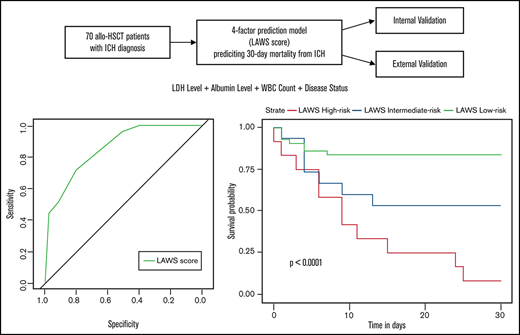
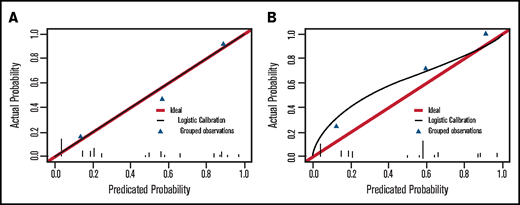
Two independent predictors of 30-day mortality after ICH (P < .05), lactate dehydrogenase (LDH) level and albumin level, were identified by the full logistic regression model. We also included 2 other variables, white blood cell (WBC) count and disease status, in our final 4-predictor prognostic model. There were several reasons for including WBC count and disease status in the final model. First, both of these variables performed well in the univariate analysis, and their levels of significance were just above 0.05 in the full multivariate regression analysis (Table 1; supplemental Table 3). Second, they are easily accessed at ICH onset, and there are studies supporting their relevance to ICH prognosis.14,35 In the final 4-predictor model, all 4 factors selected remained significant (P < .05) (Table 3). Above all, compared with the 2-factor prediction model including only LDH level and albumin level (C-statistic, 0.801; 95% confidence interval [CI], 0.697-0.905), the addition of WBC count and disease status (C-statistic, 0.862; 95% CI, 0.777-0.946) significantly increased the discrimination ability of the final model (P = .0338) (Figure 1A). The final 4-predictor model indicated good agreement between the observed and predicted probability of 30-day mortality from ICH in the derivation cohort according to the Hosmer-Lemeshow test (P = .702) and the calibration plot (Figure 2A).
Results of the multivariable logistic regression model for the derivation cohort (n = 70)
| Characteristic . | β coefficient . | SE . | Odds ratio (95% CI) . | P . | Assigned score . | Acronym . |
|---|---|---|---|---|---|---|
| LDH >426 U/L | 1.74 | 0.67 | 5.72 (1.54-21.18) | .009 | 3 | L |
| Albumin <32.7 g/L | 2.00 | 0.72 | 7.41 (1.80-30.53) | .006 | 3 | A |
| WBC count <2.4 × 109/L | 1.33 | 0.66 | 3.80 (1.04-13.88) | .044 | 2 | W |
| High-risk status | 1.67 | 0.78 | 5.33 (1.16-24.53) | .032 | 3 | S |
| Characteristic . | β coefficient . | SE . | Odds ratio (95% CI) . | P . | Assigned score . | Acronym . |
|---|---|---|---|---|---|---|
| LDH >426 U/L | 1.74 | 0.67 | 5.72 (1.54-21.18) | .009 | 3 | L |
| Albumin <32.7 g/L | 2.00 | 0.72 | 7.41 (1.80-30.53) | .006 | 3 | A |
| WBC count <2.4 × 109/L | 1.33 | 0.66 | 3.80 (1.04-13.88) | .044 | 2 | W |
| High-risk status | 1.67 | 0.78 | 5.33 (1.16-24.53) | .032 | 3 | S |
SE, standard error.
Discrimination capability of the multivariate regression logistic model in the derivation and external validation cohorts. (A) Receiver-operating characteristic curve of the final 4-predictor model (LDH level, albumin level, WBC count, and disease status), the 2-predictor model (LDH level and albumin level), and the simplified LAWS score in the derivation cohort. The C-statistics were 0.862 (95% CI, 0.777-0.946), 0.801 (95% CI, 0.697-0.905), and 0.859 (95% CI, 0.776-0.945), respectively. (B) Receiver-operating characteristic curve of the simplified LAWS score in the external validation group. The C-statistic was 0.795 (95% CI, 0.645-0.945).
Discrimination capability of the multivariate regression logistic model in the derivation and external validation cohorts. (A) Receiver-operating characteristic curve of the final 4-predictor model (LDH level, albumin level, WBC count, and disease status), the 2-predictor model (LDH level and albumin level), and the simplified LAWS score in the derivation cohort. The C-statistics were 0.862 (95% CI, 0.777-0.946), 0.801 (95% CI, 0.697-0.905), and 0.859 (95% CI, 0.776-0.945), respectively. (B) Receiver-operating characteristic curve of the simplified LAWS score in the external validation group. The C-statistic was 0.795 (95% CI, 0.645-0.945).
Calibration plot of the final 4-predictor model for predicting 30-day mortality of ICH in allo-HSCT patients. (A) Calibration plot in the derivation cohort (Hosmer-Lemeshow test, P = .702). (B) Calibration plot in the external validation cohort (Hosmer-Lemeshow test, P = .261).
Calibration plot of the final 4-predictor model for predicting 30-day mortality of ICH in allo-HSCT patients. (A) Calibration plot in the derivation cohort (Hosmer-Lemeshow test, P = .702). (B) Calibration plot in the external validation cohort (Hosmer-Lemeshow test, P = .261).
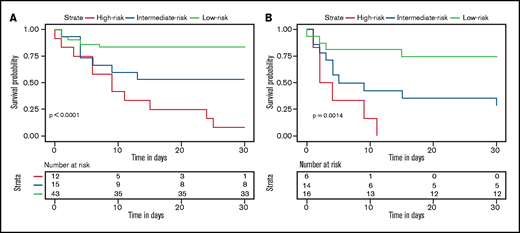
To incorporate these 4 variables into a simplified prediction score, each variable was weighted on the basis of its β coefficient obtained from the multivariable analysis. LDH level >426 U/L scored 3 points, albumin level <32.7 g/L scored 3 points, WBC count <2.4 × 109/L scored 2 points, and high risk scored 3 points (Table 3). This resulted in the LAWS score, named from its 4 parameters. Eleven (91.7%) of 12 patients with a LAWS score of 8 to 11 died within 30 days after ICH diagnosis, in contrast to 7 (46.7%) of 15 patients with a score of 5 to 6, and 7 (16.3%) of 43 patients with a score of 6 or 7 (Table 4). Accordingly, we separated patients with ICH after allo-HSCT into 3 risk groups: high risk (score, 8-11), intermediate risk (score, 5-6), and low risk (score, 0-3). The LAWS score was able to distinguish posttransplant ICH patients with different risks of early death (P < .0001) (Figure 3A). Similar to the original 4-predictor model, the simplified LAWS score had good discrimination power (C-statistic, 0.859; 95% CI, 0.776-0.945) (Figure 1A).
Observed 30-day mortality rates of ICH in allo-HSCT patients by risk group in the derivation and external validation cohorts
| Risk group . | LAWS score . | Derivation cohort (n = 70) . | External validation (n = 36) . | All patients (N = 106) . |
|---|---|---|---|---|
| Low | 0-3 | 7/43 (16.3%) | 4/16 (25.0%) | 11/59 (18.6%) |
| Intermediate | 5-6 | 7/15 (46.7%) | 10/14 (71.4%) | 17/29 (58.6%) |
| High | 8-11 | 11/12 (91.7%) | 6/6 (100.0%) | 17/18 (94.4%) |
| Risk group . | LAWS score . | Derivation cohort (n = 70) . | External validation (n = 36) . | All patients (N = 106) . |
|---|---|---|---|---|
| Low | 0-3 | 7/43 (16.3%) | 4/16 (25.0%) | 11/59 (18.6%) |
| Intermediate | 5-6 | 7/15 (46.7%) | 10/14 (71.4%) | 17/29 (58.6%) |
| High | 8-11 | 11/12 (91.7%) | 6/6 (100.0%) | 17/18 (94.4%) |
Data are number of patients who died/total number of patients within each LAWS risk group (%).
Thirty-day survival of allo-HSCT patients diagnosed with ICH according to the LAWS score in the derivation and external validation groups. High risk denotes patients with LAWS scores of 8 to 11, intermediate risk denotes patients with LAWS scores of 5 to 6, and low risk denotes patients with LAWS scores of 0 to 3. (A) Survival curve of the derivation cohort (log-rank test, P < .0001). (B) Survival curve of the derivation cohort (log-rank test, P = .0014).
Thirty-day survival of allo-HSCT patients diagnosed with ICH according to the LAWS score in the derivation and external validation groups. High risk denotes patients with LAWS scores of 8 to 11, intermediate risk denotes patients with LAWS scores of 5 to 6, and low risk denotes patients with LAWS scores of 0 to 3. (A) Survival curve of the derivation cohort (log-rank test, P < .0001). (B) Survival curve of the derivation cohort (log-rank test, P = .0014).
LAWS score validation
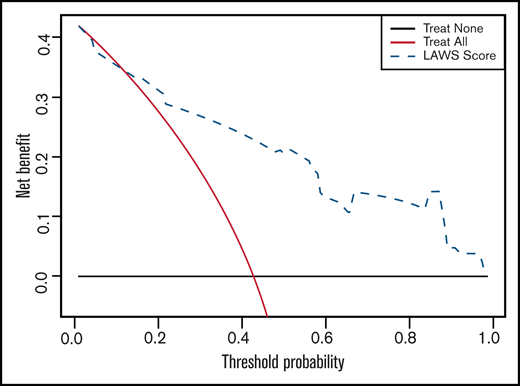
We performed 2 independent validations of the LAWS score. First, it was internally validated by using the bootstrap method. The optimism-corrected C-statistic was 0.829 (95% CI, 0.826-0.832). We then applied the LAWS score to the external validation cohort, in which 36 (87.8%) of 41 patients had complete data for the LAWS score calculation. The LAWS score had good discrimination ability (C-statistic, 0.795; 95% CI, 0.645-0.945) (Figure 1B). In addition, the LAWS score was adequately calibrated to the external validation cohort, as shown in the Hosmer-Lemeshow test (P = .261) and the calibration plot (Figure 2B). Four (25.0%) of 16 patients in the low LAWS risk group, 10 (71.4%) of 14 patients in the intermediate LAWS risk group, and 6 (100.0%) of 6 patients in the high LAWS risk group died within 30 days of ICH diagnosis (Table 4). Consistent with the derivation cohort, Kaplan-Meier analysis showed that in the external validation cohort, patients with a higher LAWS risk had a higher 30-day mortality rate (P = .0014) (Figure 3B). DCA revealed that our model had a positive net benefit for predicting 30-day mortality and indicating the need for further ICH treatments compared with the treating-all or treating-none strategies (Figure 4). Particular benefit of using the LAWS score was seen for physicians who would consider patients at higher risk of mortality and in need of novel ICH treatments when the predicted 30-day mortality risk ranged from 12% to 99%.
Decision-curve analysis using the LAWS score for predicting 30-day mortality of ICH in 106 patients from both the derivation and external validation cohorts. Black line (treat none) shows the net benefit when we assume no allo-HSCT patient dies within 30 days of ICH diagnosis; gray line (treat all) shows the net benefit when we assume all allo-HSCT patients die within 30 days of ICH diagnosis; and dashed line (LAWS score) shows the net benefit when we manage allo-HSCT patients with ICH according to the predicted risk of 30-day mortality estimated by LAWS score. The preferred management strategy is the one with the highest net benefit at each given threshold probability.
Decision-curve analysis using the LAWS score for predicting 30-day mortality of ICH in 106 patients from both the derivation and external validation cohorts. Black line (treat none) shows the net benefit when we assume no allo-HSCT patient dies within 30 days of ICH diagnosis; gray line (treat all) shows the net benefit when we assume all allo-HSCT patients die within 30 days of ICH diagnosis; and dashed line (LAWS score) shows the net benefit when we manage allo-HSCT patients with ICH according to the predicted risk of 30-day mortality estimated by LAWS score. The preferred management strategy is the one with the highest net benefit at each given threshold probability.
Discussion
Although previous studies have reported that the OS of allo-HSCT patients with ICH is significantly inferior to that of patients without ICH,7,10,12 we observed that posttransplant ICH patients actually had greatly varying OS, ranging from 0 to 2355 days. Hence, there is an urgent need to establish a clinical prediction method that can be used to quickly identify ICH patients at higher risk of early mortality whose prognosis is particularly dismal under standard ICH treatments and who may benefit from more novel ICH treatments and interventions.36,37
Several studies have sought to identify independent factors associated with poor outcome in ICH patients and to derive a prediction model to determine survival outcomes. However, none of them was developed or validated in allo-HSCT patients.14-16,19 In the general population, the ICH score, which includes 5 variables (the Glasgow Coma Scale [GCS], bleed volume, and other variables), is widely used to predict 30-day mortality due to intracerebral hemorrhage.16,34,38 However, the ICH score does not take into consideration other types of ICH, such as SAH or bleeding at multiple locations. In addition, applying this ICH score to posttransplant patients requires hematologists to determine the GCS and calculate the ICH volume at the onset of ICH, which makes it not simple to use. Another study developed a simple model including 4 predictors (albumin level, LDH level, age, and relapsed disease status) for predicting all-cause mortality after ICH onset in patients with leukemia.14 However, that study did not disclose the number of patients who had received allo-HSCT.
To address the unmet need for a prognostic model in allo-HSCT patients with ICH, we developed and externally validated the LAWS score using a data set of 111 stringently enrolled allo-HSCT patients who had fresh ICH occurring after transplantation. This is the largest data set of such patients to date. We successfully identified 4 factors (LDH level, albumin level, WBC count, and disease status [high or standard risk]) and retained them in the final prediction model, of which LDH level and albumin level were independent risk factors for early mortality (P < .05). These 4 predictors all have reasonable significance levels and are easily accessed when an ICH diagnosis is made.39-41 Importantly, they are clinically relevant to the prognosis of ICH and allo-HSCT patients according to previous studies.42-44 Previous studies have shown the prognostic role of increased LDH levels and low albumin levels in a broad range of disease conditions, including ICH, hematologic malignancies, and HSCT.45-51 Therefore, it is not surprising that they are successfully identified by our multivariate analysis, although neither of them should be considered a prognostic predictor specific to ICH in allo-HSCT patients. Neither of these factors was associated with disease relapse in our study. Furthermore, the disease status, which took into account both whether the patient had experienced relapse or achieved complete remission after induction therapy and the primary disease, is an independent risk factor for inferior engraftment, increased extensive chronic GVHD incidence, and worse nonrelapse-related mortality after allo-HSCT.17,23,24
There is some controversy regarding the association between WBC count and ICH outcomes. Traditionally, it was believed that a high WBC count and inflammation could aggravate secondary brain injury after ICH and adversely affect ICH development.15,52 However, several studies failed to identify a high WBC count as an independent predictor of hematoma growth, neurologic deterioration, or mortality.53 In contrast, there is increasing evidence supporting the adverse influence of a lower-than-normal WBC count on ICH outcome: sufficient WBC count and acute inflammation can have a protective role in ICH,35 whereas low WBC count is related to an increased incidence of viral and fungal CNS infection.9,54
During our clinical practice, we managed our patients mainly based on the recommendations set forth in the current American Heart Association/American Stroke Association guidelines (supplemental Table 4). Several modifications were made, considering that the guidelines were developed on the basis of ICH occurring in the general population.34 First, very few of our patients received open surgery although their hematoma-mediated mass effect might be alleviated by surgical evacuation. Because our patients were frequently not eligible for invasive procedures due to thrombocytopenia or to their poor general condition.7,9,12,55,56 Second, almost all of our patients developed ICH while they were still being monitored in the transplantation unit because of transplantation-related complications. Therefore, whether the patient should be transferred to the intensive care unit was decided by a multidisciplinary team that included hematologists, neurologists, neurosurgeons, and intensive care unit physicians. Once bleeding was suspected, we closely monitored the vital signs and neurologic status of the patients who remained in the transplantation unit. Third, prothrombin complex concentrates, fresh frozen plasma, and platelet transfusion are commonly used to limit hematoma expansion, whereas antihypertensive agents are not frequently administered. Posttransplant ICH is often associated with thrombocytopenia and coagulation disorders rather than with hypertension.7,9,12
Using these therapeutic strategies, the 30-day mortality rates due to ICH in the allo-HSCT patients in the low LAWS risk group (18.6%) and the intermediate LAWS risk group (58.6%) were no higher than that of the general population (13.1%-61%).57 However, >90% of the patients in the high LAWS risk group died within 30 days of ICH. These data and the development of the LAWS score can contribute to improving the management of posttransplant ICH patients in several ways. First, our findings indicate that it is reasonable to manage the low- and intermediate-risk patients according to the current American Heart Association/American Stroke Association guidelines, and a nihilistic clinical response should never be taken, although ICH on top of allo-HSCT has traditionally been considered fatal.3 Second, the dismal prognosis of high-risk patients shows that the treatments currently in use are not sufficient for them, and novel treatments for ICH may be considered, which include minimal invasive surgery (MIS), novel hemostatic agents, and neuroprotective agents36,58-69 (supplemental Table 4). The DCA results also showed that it would be beneficial to use the LAWS score to aid clinicians in selecting patients at higher risk of early mortality and in more urgent need of novel ICH treatments other than the standard ones.28-30 However, more clinical trials are warranted to further determine the beneficial role of these novel treatments. Finally, our prognostic scale can facilitate prospective trials on the development of novel and individualized ICH treatments by better defining the target patient groups included in a clinical trial, balancing randomizations, and providing a consistent criterion for patient evaluation and assessment of treatment efficacy among different trials.16,70
In our opinion, among the novel ICH treatments undergoing clinical trials, MIS in particular sheds new light on ICH treatment, especially for patients whose condition is deteriorating posttransplant who may benefit from alleviation of the hematoma-mediated mass effect but are too vulnerable to endure traditional open craniotomy for iatrogenic brain injury.34,71-74 Moreover, Babadjouni et al37 highlighted the potential synergistic effects of combining MIS and the use of neuroprotective agents, a scenario in which primary and secondary injuries due to ICH can be simultaneously targeted.
The current study has certain limitations. First, it is a retrospective study. Although we attempted to mitigate this inherent flaw by performing internal and external validations, further validations in large, prospective cohorts are still needed to better assess the performance of the LAWS score and to help us adjust it where necessary. Given that ICH in allo-HSCT patients is rare, and a prospective study is not feasible in the short term,2,4,9-12 we believe the retrospective nature of our study is necessary.75,76 Second, we could have underestimated the incidence of posttransplant ICH, and this might have adversely affected the accuracy of our prediction model. To illustrate, patients with minor bleeding but without typical ICH symptoms that prompted a CT/MRI scan were neglected. In addition, patients who were too sick to receive a CT/MRI scan despite presenting with highly suspected ICH symptoms were also excluded due to a lack of solid neuroimaging evidence. In addition, even though we do not consider GCS scores and the volume of ICH measured by CT scans simple and straightforward parameters for a clinical scoring system, they could have been important for determining the outcome of ICH in our allo-HSCT patients. Unfortunately, relevant information was not available for most of the patients included in our study. Finally, although our findings showed that patients with high LAWS risk scores had a dismal prognosis under the current treatments and interventions, at present, our study is not able to answer whether any of the promising novel ICH treatments we suggested would definitely improve the outcome in ICH patients from the high LAWS risk group. In reality, until now, no prediction model has been routinely used to guide medical decisions in ICH. The ICH score is only recommended for the initial evaluation of ICH patients.16,34
In summary, we generated the largest data set of posttransplant ICH patients, and developed and externally validated the first simple scoring system that can be used to effectively risk stratify 3 distinct groups of patients based on the 30-day mortality. It is named LAWS after the 4 variables (LDH level, albumin level, WBC count, and disease status) that comprise the score. Although we acknowledge that this system needs to be further validated in large, prospective cohorts, its simplicity, accuracy, and clinical usefulness still support its potential use as a preliminary tool to promptly evaluate allo-HSCT patients and select high-risk patients once a bleeding is suspected. Its capability of stratification of post-transplant ICH patients also facilitates the prospective trials that focus on developing novel and individualized therapeutic strategies for ICH in the allo-HSCT patients.
Acknowledgments
The authors thank all the patients who participated in this study and their families. The authors are also grateful for the support from the Department of Medical Records Library, Peking University People’s Hospital in terms of searching for medical records.
This work was supported by The Beijing Natural Science Foundation (No. H2018206423), The National Natural Science Foundation of China (No. 81970113), The Key Program of National Natural Science Foundation of China (No. 81730004), and The National Key Research and Development Program of China (No. 2017YFA0105503).
The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Authorship
Contribution: X.R. and Xiaohui Zhang contributed to the concept and design; X.R., Q.Q., X.C., H.F., X.M., Yu Wang, Yawei Zheng, E.J., Y.Y., Y.L., S.C., T.Y., Yuanyuan Zhang, W.H., F.T., W.M., S.W., F.L., D.L., Xiaoying Zhang, Yicheng Zhang, S.F., F.G., H.Y., Dao Wang, Dingming Wan, H.C., Yao Chen, J.W., Yuhong Chen, Ying Wang, K.X., T.L., X.W., H.M., L.L., Z.W., and Y.F. collected the data; X.R., Q.H., Q.Q., X.C., Yawei Zheng, Y.Y., S.C., W.M., F.L., Xiaoying Zhang, S.F., H.Y., Dao Wang, Ying Wang, T.L., H.M., and Z.W. contributed to data analysis and data interpretation; X.R., Q.H., and Q.Q. performed statistical analysis; X.R., Q.H., and Xiaohui Zhang wrote the manuscript; Yingjun Chang, L.X., and X.H. revised the manuscript critically; and Xiaohui Zhang designed, conceived, and supervised the study, analyzed the data, and drafted and revised the manuscript and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaohui Zhang, Peking University People’s Hospital, No. 11 Xizhimen South St, Xicheng District, Beijing, China; e-mail: zhangxh@bjmu.edu.cn.
References
Author notes
X.R., Q.H., and Q.Q. contributed equally to this work.
The data that support the findings of this study are available on request from the corresponding author (zhangxh@bjmu.edu.cn). The data are not publicly available due to privacy or ethical restrictions.
The full-text version of this article contains a data supplement.