Abstract
Anti-β-2 glycoprotein I antibodies (anti-B2GPI) are often cited as the major pathogenically relevant antibody in antiphospholipid syndrome (APS), but it is unclear if there is clinical evidence to support this theory. We performed a systematic review to determine if immunoglobulin G anti-B2GPI positivity was independently associated with thrombotic and/or obstetric manifestations of APS. We searched MEDLINE, EMBASE, The Cochrane Library, and clinicaltrials.gov electronic databases through April 2020 for prospective studies that met prespecified design criteria. Of 4758 articles identified through computer-assisted search, 4 studies examining obstetric outcomes and 2 studies examining thrombotic outcomes were included for qualitative assessment. The presence of anti-B2GPI had only a weak independent association with thrombosis and was, at best, inconsistently associated with obstetric complications. A quantitative assessment could not be performed because of study heterogeneity. The overall quality of the evidence was very low. Although anti-B2GPI are commonly thought to mediate APS manifestations, clinical evidence is lacking with very low-quality data to support a weak association with thrombosis.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by thrombotic or obstetric complications in patients with persistent antiphospholipid antibodies (aPL). The major antigenic target of these antibodies is thought to be β-2 glycoprotein I (B2GPI), which was first identified in 1990 as the cofactor for anticardiolipin (aCL) binding.1 Anti-β-2 glycoprotein I antibodies (anti-B2GPI) have been observed to increase thrombus formation in a dose-dependent manner in rodent models2,3 and mediate thrombosis through suppression of tissue factor pathway inhibitor type I, platelet and neutrophil activation, and inhibition of protein C and antithrombin activity.4-10
Although anti-B2GPI are shown to promote thrombosis based on in vitro and animal experiments, their clinical significance as detected by current laboratory methods remains uncertain. Existing literature consists primarily of retrospective studies that attempt to correlate the presence of anti-B2GPI with prior clinical events. Results vary widely, as do the patient populations, isotypes of anti-B2GPI, and type of clinical manifestations examined. In general, anti-B2GPI is reported to correlate with thrombosis, but some studies find an association only with the immunoglobulin G (IgG) isotype,11-16 whereas others find a significant link with IgM isotypes as well.17-20 However, some show that neither isotype is associated with thrombosis,21-23 and in 1 study, IgM anti-B2GPI was actually associated with a reduced risk of stroke.24 In a systematic review and meta-analysis of patients with aPL without SLE, Reynaud and colleagues25 reported that anti-B2GPI was associated with increased risk of arterial events only and not venous thromboembolism (VTE). The reported association between anti-B2GPI and obstetric events is similarly variable, with some studies noting an association,16,20,26-28 whereas others find no association.29,30
Therefore, although anti-B2GPI is often cited as the major pathogenically relevant antibody in APS, understanding of its clinical relevance remains elusive. In contrast, lupus anticoagulants (LAs) (and to a lesser extent, aCL) are generally thought to correlate with clinical APS manifestations.31,32 Determining whether (and the degree to which) anti-B2GPI positivity is independently associated with clinical outcomes is important because isolated anti-B2GPI positivity is occasionally encountered during evaluation for APS. To this end, we performed a systematic review to identify if IgG anti-β-2 glycoprotein I positivity was independently associated with thrombotic and/or obstetric manifestations of APS.
Methods
Literature search
A comprehensive search was performed using the MEDLINE, EMBASE, The Cochrane Library, and clinicaltrials.gov electronic databases. The keywords (MeSH terms) used were as follows: (1) “beta 2-Glycoprotein I,” (2) “Glycoproteins,” (3) “Antiphospholipid Syndrome,” (4) “Phospholipids,” (5) “Antibodies, Antiphospholipid.” The databases were searched from inception to April 15, 2020.
Titles and abstracts of all publications identified by the search were extracted and reviewed for relevance to the study by 1 author (D.J.). Articles were excluded if they dealt with an unrelated topic, were not available in the English language, or examined a pediatric population. We excluded reviews, case reports, editorials, commentaries, and conference abstracts. Studies that prospectively evaluated APS manifestations among patients identified for anti-B2GPI status were included for full manuscript review by 2 authors (D.J. reviewed all articles and M.C., D.G., and W.L. each reviewed one-third of the articles). All included articles were extracted for first author’s name, year of publication, stated objective, patient population, number of subjects, length of follow-up, thrombotic and obstetric outcomes, and aPL testing characteristics, including type of assay used, positivity threshold, units of measurement, and number of controls used to establish reference ranges. When critical information was not reported, authors of the original publications were contacted. All articles wherein there was a discrepancy between the 2 reviewers were discussed among the group for a consensus on inclusion or exclusion.
Results
Study selection
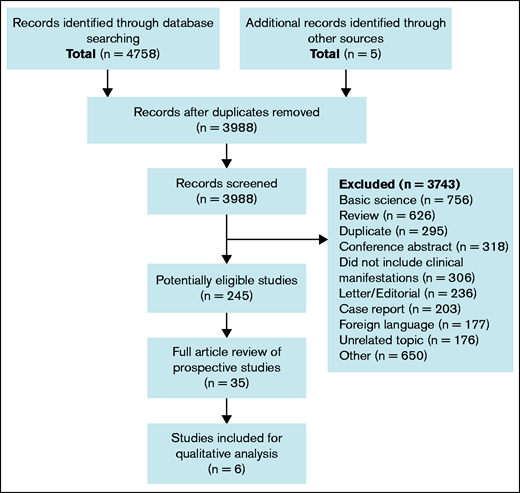
A computer-assisted database search identified 4758 articles (Figure 1). An additional 5 articles were identified through review of references cited. After removal of duplicates, 3988 works were screened for relevancy by abstract and title. Among 245 potentially eligible articles, 35 were identified based on title and abstract screen as prospective studies and reviewed in full.
Literature search results. Flow diagram of study selection process for systematic review.
Literature search results. Flow diagram of study selection process for systematic review.
Description of studies
In total, 6 of the 35 prospective studies are included. The most common reasons for exclusion of prospective studies were that the study design was, upon full-text review, deemed to be retrospective, the cutoff value for anti-B2GPI positivity was below Sydney criteria threshold,33 the relationship between anti-B2GPI and clinical manifestations was not reported, and outcomes were not assessed for their relationship to individual isotypes of anti-B2GPI, but rather as a composite of IgG and IgM.
Among the 6 included studies, 4 looked primarily at obstetric outcomes (Table 1), and 2 looked at thrombotic outcomes (Table 2). The numbers of included anti-B2GPI+patients tended to be small, ranging from 7 to 64. All studies used in-house assays for antibody detection with reference ranges derived from 20 to 200 normal controls. Positivity for anti-B2GPI was defined by percentile in 3 studies, number of standard deviations from the mean in 1, and units per milliliter in 2.
Relationship between IgG anti-B2GPI positivity and APS obstetric clinical manifestations
| . | . | . | . | . | . | Obstetric outcomes . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author (y) . | No. of participants . | No. of anti-B2GPI (+) . | Anti-B2GPI positivity cutoff . | No. of controls for assay calibration . | Length of follow-up . | Composite . | Fetal loss . | Preeclampsia/ eclampsia . | Placental abruption . | Intrauterine growth restriction . |
| Chauleur (2010) | 142 aPL(+) and 142 matched aPL(−) controls with history of embryonic loss | 47 IgG (+) 20 IgM (+) | 99th percentile | 200 | Duration of pregnancy through 3 mo postpartum | — | Not associated | aOR 4.61 | Trend (aOR 2.43, P = .07) | Not associated |
| Lockshin (2012) | 144 pregnant women with aPL positivity | 37 IgG (+) 22 IgM (+) | 25 units/mL (positive) 40 units/mL (high titer) | 60 | Duration of pregnancy and through 3 mo postpartum | Not associated | — | — | — | — |
| Lynch (1999) | 325 low-risk primigravida | NR | 50th percentile. Reported outcomes for 75th, 95th, and 99th percentile | NR | Duration of pregnancy | — | Not associated | Not associated | — | — |
| Yelnik (2016) | 54 aPL(+) pregnant women | 23 IgG (+) | 40 units/mL | 60 | Duration of pregnancy through 12 wk postpartum | Not associated | — | — | — | — |
| . | . | . | . | . | . | Obstetric outcomes . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author (y) . | No. of participants . | No. of anti-B2GPI (+) . | Anti-B2GPI positivity cutoff . | No. of controls for assay calibration . | Length of follow-up . | Composite . | Fetal loss . | Preeclampsia/ eclampsia . | Placental abruption . | Intrauterine growth restriction . |
| Chauleur (2010) | 142 aPL(+) and 142 matched aPL(−) controls with history of embryonic loss | 47 IgG (+) 20 IgM (+) | 99th percentile | 200 | Duration of pregnancy through 3 mo postpartum | — | Not associated | aOR 4.61 | Trend (aOR 2.43, P = .07) | Not associated |
| Lockshin (2012) | 144 pregnant women with aPL positivity | 37 IgG (+) 22 IgM (+) | 25 units/mL (positive) 40 units/mL (high titer) | 60 | Duration of pregnancy and through 3 mo postpartum | Not associated | — | — | — | — |
| Lynch (1999) | 325 low-risk primigravida | NR | 50th percentile. Reported outcomes for 75th, 95th, and 99th percentile | NR | Duration of pregnancy | — | Not associated | Not associated | — | — |
| Yelnik (2016) | 54 aPL(+) pregnant women | 23 IgG (+) | 40 units/mL | 60 | Duration of pregnancy through 12 wk postpartum | Not associated | — | — | — | — |
aOR, adjusted odds ratio; NR, not reported. Dashes denote outcomes that were not examined in a given study.
Relationship between IgG anti-B2GPI positivity and APS thrombotic clinical manifestations
| First author (y) . | No. of participants . | No. of anti-B2GPI (+) . | Anti-B2GPI positivity cutoff . | No. controls for assay calibration . | Length of follow-up . | Thrombosis . |
|---|---|---|---|---|---|---|
| Forastiero (2005) | 194 patients with persistently positive LA or aCL | 64 IgG (+) 59 IgM (+) | 99th percentile | 95 | Median of 45 mo | Increased risk |
| Wahl (1998) | 71 inpatients admitted for acute VTE | 7 IgG (+) | 3 SD from mean | 20 | Mean 4.9 y | Increased risk |
| First author (y) . | No. of participants . | No. of anti-B2GPI (+) . | Anti-B2GPI positivity cutoff . | No. controls for assay calibration . | Length of follow-up . | Thrombosis . |
|---|---|---|---|---|---|---|
| Forastiero (2005) | 194 patients with persistently positive LA or aCL | 64 IgG (+) 59 IgM (+) | 99th percentile | 95 | Median of 45 mo | Increased risk |
| Wahl (1998) | 71 inpatients admitted for acute VTE | 7 IgG (+) | 3 SD from mean | 20 | Mean 4.9 y | Increased risk |
SD, standard deviation.
Obstetric events
Among the 4 studies that evaluated for obstetric complications, 1 studied healthy pregnant women who were tested for aPL and then followed for obstetric events34 ; 2 followed a cohort of known aPL-positive pregnant women,35,36 and 1 examined outcomes of a second pregnancy among women who had a history of prior embryonic loss and aPL positivity.37 Three of the 4 found no association between anti-B2GPI positivity and adverse pregnancy outcome.34-36 One reported that anti-B2GPI was independently associated with preeclampsia, but not with fetal loss, placental abruption, or intrauterine fetal growth restriction.37
Thrombotic events
Both studies reporting on thrombotic outcomes noted an association with IgG anti-B2GPI. Wahl and colleagues38 evaluated 71 patients admitted for acute VTE for aPL expression and reported that among 7 patients persistently positive for IgG anti-B2GPI, 6 experienced recurrent VTE upon mean follow-up of 4.9 years, correlating with a hazard ratio of 16.3 compared with those who were negative for anti-B2GPI on a multivariate analysis adjusting for age and site of first thrombotic event. Forastiero and colleagues39 studied 194 patients who were persistently positive for LA or aCL and reported that the presence of IgG anti-B2GPI was independently associated with increased risk of arterial or venous thrombosis, with a multivariate hazard ratio of 2.97.
Discussion
Based on our systematic review, IgG anti-B2GPI has a weak independent association with thrombosis. There does not appear to be a significant impact on the risk of fetal loss, placental abruption, intrauterine growth restriction, or composite obstetric complications, and studies examining an association between IgG anti-B2GPI and preeclampsia/eclampsia have yielded inconsistent results. There is no evidence that isolated IgG anti-B2GPI positivity predicts clinical events. Despite the prospective methodology, the overall quality of evidence is very low, and a quantitative assessment could not be performed given the heterogeneity in the patient populations and outcomes examined in the studies.
Several challenges exist in interpreting this literature. First, there is a lack of standardization among anti-B2GPI testing, which may be done via enzyme-linked immunosorbent assay, chemiluminescence, immunoassays using fluorescence, or multiplex flow. Assays may be performed using a commercial kit or, more commonly, developed in-house. Furthermore, although aCL is typically reported in IgG phospholipid and IgM phospholipid units, no convention exists for anti-B2GPI, which may be expressed as units per milliliter, micrograms per milliliter, optical density value, IgG phospholipid, or IgM phospholipid.40 Several studies included in this analysis reported anti-B2GPI level by percentile or standard deviation from the mean, although this is not recommended by the International Congress on Antiphospholipid Antibodies.41 Interassay correlation is variable,42,43 and some studies have reported that as many as 40% of patients referred for aPL were discovered to have negative results upon retesting at a central laboratory.35 Furthermore, sensitivity and specificity of these assays have improved over time, making older reports examining aPL less reliable.
Second, differences in the patient populations may obscure any true effect specific to anti-B2GPI. The 3 studies that reported a significant relationship between IgG anti-B2GPI positivity and clinical outcomes involved patients who were tested for aPL because of a clinical history suggestive of APS.37-39 In contrast, the 1 report studying healthy pregnant women reported low rates of IgG anti-B2GPI positivity and no association with clinical outcomes.34 It has been well documented that aPL positivity may be transiently seen in various conditions, including HIV, syphilis, malaria, leprosy, end-stage renal disease on hemodialysis, and celiac disease.44 There is also increasing evidence that the anti-B2GPI detected in APS is directed toward domain 1 (D1).45-48 This raises the question of whether studies including patients with a history suggestive of APS and aPL positivity are more likely to capture clinically relevant anti-B2GPI, whereas unselected patient populations may express a heterogeneous group of antibodies directed at other subunits of the B2GPI molecule. Indeed, 2 prospective studies identified in this review looked specifically at the clinical relevance of anti-B2GPI D1 positivity and reported a significant association with rates of thrombosis.49,50 Further progress to standardize anti-B2GPI D1 testing and, ultimately, to clarify its clinical relevance is needed.
Finally, as with any systematic review or meta-analysis, the search strategy and inclusion/exclusion criteria may have impacted our findings. In this review, we sought to include the best available evidence by examining only prospective studies where anti-B2GPI status was defined by Sydney criteria thresholds. It is possible that less restrictive criteria for article inclusion may have yielded a different result.
Prior prospective studies have identified LA as an independent risk factor for thrombosis among aPL carriers51 and that the risk of first thromboembolic event among high-risk subjects who were triple positive for LA, aCL, and anti-B2GPI was particularly elevated at 5.3% per year.52 By contrast, although anti-B2GPI is often cited as the most pathogenically relevant of the aPLs, we identified only 1 study in which the association between anti-B2GPI and thrombotic outcomes was independent of LA and aCL positivity.39 There is no convincing evidence to support a clear association with obstetric complications.
In summary, we could not find evidence that isolated IgG anti-B2GPI positivity predicts clinical events. The available evidence is of very low quality. A prospective study examining the natural history of patients with persistent anti-B2GPI positivity regardless of prior aPL testing or clinical history is needed to elucidate the clinical relevance of B2GPI and/or anti-B2GPI antibodies. However, such a study would be difficult to execute given the lack of standardized testing and the low rates of isolated anti-B2GPI positivity. We conclude that there is little or no currently available high-quality evidence to support an independent association between isolated IgG anti-B2GPI positivity and the risk of clinical events, such as thrombosis or obstetric complications.
Acknowledgments
The authors are grateful to Karin Dearness, Director of St. Joseph’s Healthcare Hamilton Library Service, for her assistance with performing the computer-assisted database search.
Authorship
Contribution: D.J. was responsible for search strategy and drafting the manuscript; M.C., D.G., and W.L. contributed to critical revision; and all authors contributed to the study design, study selection, data extraction, and interpretation of results.
Conflict-of-interest disclosure: M.C. reports conflicts of interest with various pharmaceutical companies and laboratory services companies but none are relevant to the contents of this paper. The authors declare no competing financial interests.
Correspondence: Debbie Jiang, Division of Hematology, University of Washington, 1100 Fairview Ave N, D5-100, Seattle, WA 98109; e-mail: dcjiang@uw.edu.
References
Author notes
The authors agree to share publication-related data through e-mails to the corresponding author: dcjiang@uw.edu.