Key Points
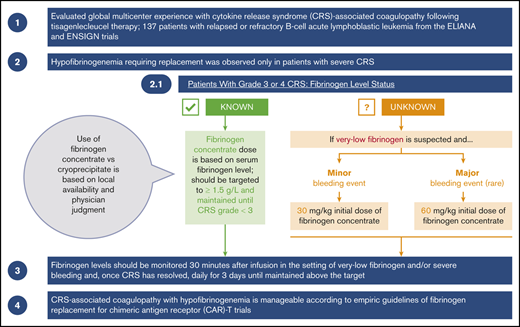
CRS-associated coagulopathy with hypofibrinogenemia is manageable according to empiric guidelines of fibrinogen replacement.
Monitoring of fibrinogen levels in patients with moderate and severe CRS is essential for avoiding potentially fatal bleeding events.
Abstract
Cytokine release syndrome (CRS) is a systemic inflammatory response associated with chimeric antigen receptor T-cell (CAR-T) therapies. In severe cases, CRS can be associated with coagulopathy and hypofibrinogenemia. We present our global multicenter experience with CRS-associated coagulopathy after tisagenlecleucel therapy in 137 patients with relapsed or refractory B-cell acute lymphoblastic leukemia from the ELIANA and ENSIGN trials. These trials included clinical guidelines for fibrinogen replacement during CRS-associated coagulopathy. Hypofibrinogenemia requiring replacement was observed only in patients with severe CRS. A higher percentage of patients who required replacement were <10 years old, compared with those who did not require replacement. Twenty-three patients received replacement for hypofibrinogenemia (<1.5 g/L); 9 of them developed marked hypofibrinogenemia (<1 g/L). Very low fibrinogen levels (<1 g/L) were documented in patients before maximal CRS (n = 1), during maximal CRS (n = 7), and at CRS improvement (n = 1). Although hypofibrinogenemia was the most clinically significant coagulopathy, some patients also developed prolonged prothrombin time and activated partial thromboplastin time and increased international normalized ratio, further increasing the risk of bleeding. Hypofibrinogenemia was effectively managed using fibrinogen concentrate or cryoprecipitate replacement; severe (grade 4) bleeding events were rare (n = 2). CRS-associated coagulopathy with hypofibrinogenemia is manageable according to empiric guidelines of fibrinogen replacement for CAR-T trials. Fibrinogen concentrate should be used when cryoprecipitate is not reliably available. Monitoring fibrinogen levels in patients with moderate or severe CRS is essential for avoiding potentially fatal bleeding events. These trials were registered at www.clinicaltrials.gov as #NCT02435849 and #NCT02228096.
Introduction
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy that has been approved by the US Food and Drug Administration, European Medicines Agency, Swissmedic, and Japanese Pharmaceuticals and Medical Devices Agency. Tisagenlecleucel demonstrated high efficacy and a manageable safety profile in a phase 2 multicenter global registration trial (ELIANA) and a phase 2 multicenter trial in the United States (ENSIGN) in pediatric and young adult patients with relapsed or refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL).1-3 The most common nonhematologic adverse event after tisagenlecleucel therapy is cytokine release syndrome (CRS), a systemic inflammatory response caused by the activation of immune cells and the release of high levels of cytokines. As presented in the current report, any grade CRS was observed in 79% of patients and grades 3 and 4 CRS in 42% in ELIANA and ENSIGN, respectively.
Coagulopathy with hypofibrinogenemia has been reported in association with severe CRS in early reports of tisagenlecleucel therapy in pediatric patients with r/r B-ALL.4,5 Severe hypofibrinogenemia has also been reported in association with chemotherapy and infection/sepsis and is used in the diagnostic criteria of macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH) (fibrinogen <2.5 g/L and <1.5 g/L, respectively).6 In addition to secondary fibrinolysis through disseminated intravascular coagulation (DIC), one hypothesis is that severely reduced fibrinogen in MAS/HLH is caused by primary hyperfibrinogenolysis. For example, fibrinogen degradation may be linked to plasminogen secretion by activated macrophages in response to interleukin-1β and tumor necrosis factor production by activated lymphocytes.7 MAS/HLH substantially overlaps CRS; they share many clinical and laboratory features and pathophysiology, including coagulopathy.8
CRS-associated coagulopathy, including DIC, prolonged prothrombin time (PT)/activated partial thromboplastin time (aPTT), and hypofibrinogenemia, is associated with moderate to severe CRS.9 Although PT and aPTT may be prolonged soon after the onset of CRS, fibrinogen is an acute-phase reactant. Fibrinogen can be elevated during the first few days of CRS and later decreased, often to profoundly low levels, during maximal CRS and at the time of CRS improvement. The risk of bleeding during this phase of profound hypofibrinogenemia can be high, warranting close monitoring and replacement therapy.
In this report, we describe our global multicenter experience with CRS-associated coagulopathy in 137 pediatric and young adult patients with B-ALL treated with tisagenlecleucel in 2 trials. We also present the clinical guidelines we developed for the management of CRS-associated coagulopathy in the trials.
Materials and methods
Study design and patients
Details of the ELIANA and ENSIGN trials have been described previously.2,3 Individual patient data from these 2 single-arm, multicenter, phase 2 trials of tisagenlecleucel in pediatric and young adult patients with r/r B-ALL were examined to further characterize CRS-associated coagulopathy. Pooling of data was possible because of the nearly identical study designs and guidelines for grading and management of CRS. In brief, patients enrolled in ELIANA and ENSIGN received lymphodepleting chemotherapy (fludarabine and cyclophosphamide) before a single infusion of tisagenlecleucel. The patients were then monitored for efficacy and safety outcomes.2 ELIANA enrolled and treated patients at 25 global sites in 11 countries, and patients in ENSIGN were treated at 13 sites in the United States. The study protocols and all amendments were reviewed by the independent ethics committee or institutional review board of each center.
Cytokine release syndrome grading
CRS was graded according to the University of Pennsylvania (Penn) grading scale, as previously described.10 Grade 1 was considered a mild reaction treated with supportive care only. Grade 2 constituted a moderate reaction with some signs of organ dysfunction related to CRS (grade 2 creatinine or grade 3 liver function test results) and hospitalization for management of CRS-related symptoms, including neutropenic fever and a need for IV therapies (not including fluid resuscitation for hypotension) or parenteral nutrition. Grade 3 was a more severe reaction requiring hospitalization for the management of symptoms related to organ dysfunction (grade 3 creatinine or grade 4 liver function test results) related to CRS; hypotension treated with multiple IV fluid boluses or low-dose vasopressors; coagulopathy requiring fresh frozen plasma (FFP), cryoprecipitate, or fibrinogen concentrate; and hypoxemia requiring supplemental oxygen via nasal cannula, high-flow oxygen, bilevel positive airway pressure, or continuous positive airway pressure. Grade 4 CRS involved life-threatening complications, including hypotension requiring high-dose vasopressors or hypoxia requiring mechanical ventilation.
CRS was managed according to a protocol-specific algorithm, which is included in the Kymriah prescribing information.1 CRS was considered to have resolved when patients were afebrile for 24 hours and vasopressors had been discontinued for 24 hours, regardless of the resolution of other associated complications, including coagulopathy.
Evaluation and management of coagulopathy after tisagenlecleucel infusion
Coagulation panels, including PT or international normalized ratio (INR), aPTT, and serum fibrinogen, were measured per protocol in patient blood samples collected on the day of, but before, tisagenlecleucel infusion, and on days 7, 14, and 28 after tisagenlecleucel infusion. D-dimer levels were not consistently measured by investigators; thus, data are not available. In patients who developed CRS, coagulation panels were measured more frequently during the clinical course, upon development of CRS, and after resolution of CRS (per protocol, on days 2, 4, 7, 11, 14, 17, 21, and 28 after tisagenlecleucel infusion). Close monitoring for additional levels of fibrinogen, PT or INR, and aPTT was recommended in patients with severe CRS (grade 3 and 4), patients receiving FFP and/or fibrinogen replacement, and/or patients experiencing bleeding, as deemed clinically appropriate by the physician. Frequent fibrinogen level monitoring was recommended at least once per day in patients who had hypofibrinogenemia or were receiving fibrinogen replacement for severe bleeding and within 30 minutes of the initial fibrinogen replacement infusion. Management of coagulopathy and replacement therapy with cryoprecipitate, fibrinogen concentrate, and/or FFP was based on the investigator’s clinical judgment. Guidelines for the management of hypofibrinogenemia were subsequently added to the trial protocols.2 The use of fibrinogen concentrate vs cryoprecipitate was based on local availability and the physician’s judgment.
Statistical analysis
Individual patient data from the ELIANA and ENSIGN trials were pooled in this analysis by using SAS software, version 9.4 (SAS Institute). Characterization of CRS-associated coagulopathy includes summary statistics. Nominal P-values were calculated by using the χ2 test without adjustment for multiplicity and should be interpreted with caution. The analyses included in this manuscript are exploratory in nature.
Results
Baseline demographics and disease characteristics
In total, 137 pediatric and young adult patients with B-ALL were treated with tisagenlecleucel. Baseline demographics and disease characteristics were examined in patients who developed different grades of CRS and in patients who did not develop CRS. Age at diagnosis, number of previous lines of therapy, and number of prior allogeneic stem cell transplantations were generally balanced among patients with (regardless of severity) and without CRS (Table 1).
Baseline demographics and disease burden by maximum CRS grade
| . | No CRS (n = 29) . | Maximum CRS grade . | |||
|---|---|---|---|---|---|
| Grade 1/2 (n = 51) . | Grade 3 (n = 27) . | Grade 4 (n = 30) . | Grade 3/4 (n = 57) . | ||
| Age at initial diagnosis, y | |||||
| Median (min-max) | 7 (0-17) | 6 (1-19) | 8 (0-17) | 9 (1-21) | 8 (0-21) |
| Number of previous lines of therapy | |||||
| Median (min-max) | 3 (1-8) | 3 (1-9) | 4 (2-7) | 3 (1-6) | 3 (1-7) |
| Number of prior allogeneic stem cell transplantations, n (%) | |||||
| 0 | 11 (38) | 24 (47) | 11 (41) | 17 (57) | 28 (49) |
| 1 | 15 (52) | 26 (51) | 13 (48) | 12 (40) | 25 (44) |
| 2 | 3 (10) | 1 (2) | 3 (11) | 1 (3) | 4 (7) |
| Karnofsky/Lansky performance status | |||||
| Median (min-max) | 90 (50-100) | 90 (50-100) | 90 (70-100) | 90 (50-100) | 90 (50-100) |
| Leukemic blasts in bone marrow by flow cytometry, % | |||||
| n | 27 | 48 | 25 | 28 | 53 |
| Median (min-max) | 50 (0-97) | 40 (0-96) | 56 (1-99) | 64 (2-97) | 60 (1-99) |
| Bone marrow tumor burden at enrollment, n (%)* | |||||
| <50% | 10 (34.5) | 22 (43.1) | 6 (22.2) | 5 (16.7) | 11 (19.3) |
| ≥50% | 19 (65.5) | 29 (56.9) | 21 (77.8) | 25 (83.3) | 46 (80.7) |
| CNS status, n (%)† | |||||
| CNS-1 | 22 (75.9) | 44 (86.3) | 26 (96.3) | 26 (86.7) | 52 (91.2) |
| CNS-2 | 6 (20.7) | 7 (13.7) | 1 (3.7) | 3 (10.0) | 4 (7.0) |
| CNS-3 | 1 (3.4) | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 | 1 (3.3) | 1 (1.8) |
| Extramedullary disease, n (%)‡ | |||||
| Yes | 6 (20.7) | 7 (13.7) | 1 (3.7) | 2 (6.7) | 3 (5.3) |
| No | 23 (79.3) | 44 (86.3) | 26 (96.3) | 28 (93.3) | 54 (94.7) |
| . | No CRS (n = 29) . | Maximum CRS grade . | |||
|---|---|---|---|---|---|
| Grade 1/2 (n = 51) . | Grade 3 (n = 27) . | Grade 4 (n = 30) . | Grade 3/4 (n = 57) . | ||
| Age at initial diagnosis, y | |||||
| Median (min-max) | 7 (0-17) | 6 (1-19) | 8 (0-17) | 9 (1-21) | 8 (0-21) |
| Number of previous lines of therapy | |||||
| Median (min-max) | 3 (1-8) | 3 (1-9) | 4 (2-7) | 3 (1-6) | 3 (1-7) |
| Number of prior allogeneic stem cell transplantations, n (%) | |||||
| 0 | 11 (38) | 24 (47) | 11 (41) | 17 (57) | 28 (49) |
| 1 | 15 (52) | 26 (51) | 13 (48) | 12 (40) | 25 (44) |
| 2 | 3 (10) | 1 (2) | 3 (11) | 1 (3) | 4 (7) |
| Karnofsky/Lansky performance status | |||||
| Median (min-max) | 90 (50-100) | 90 (50-100) | 90 (70-100) | 90 (50-100) | 90 (50-100) |
| Leukemic blasts in bone marrow by flow cytometry, % | |||||
| n | 27 | 48 | 25 | 28 | 53 |
| Median (min-max) | 50 (0-97) | 40 (0-96) | 56 (1-99) | 64 (2-97) | 60 (1-99) |
| Bone marrow tumor burden at enrollment, n (%)* | |||||
| <50% | 10 (34.5) | 22 (43.1) | 6 (22.2) | 5 (16.7) | 11 (19.3) |
| ≥50% | 19 (65.5) | 29 (56.9) | 21 (77.8) | 25 (83.3) | 46 (80.7) |
| CNS status, n (%)† | |||||
| CNS-1 | 22 (75.9) | 44 (86.3) | 26 (96.3) | 26 (86.7) | 52 (91.2) |
| CNS-2 | 6 (20.7) | 7 (13.7) | 1 (3.7) | 3 (10.0) | 4 (7.0) |
| CNS-3 | 1 (3.4) | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 | 1 (3.3) | 1 (1.8) |
| Extramedullary disease, n (%)‡ | |||||
| Yes | 6 (20.7) | 7 (13.7) | 1 (3.7) | 2 (6.7) | 3 (5.3) |
| No | 23 (79.3) | 44 (86.3) | 26 (96.3) | 28 (93.3) | 54 (94.7) |
CNS, central nervous system; CSF, cerebrospinal fluid; MRD, minimal residual disease.
Includes MRD determined by flow cytometry and morphologic blast counts in bone marrow. If there are both MRD and morphologic results, the maximum is shown.
The classification of CNS status includes the following: CNS-1, no lymphoblasts in the CSF regardless of WBC count; CNS-2, WBC <5 cells/µL in the CSF, with the presence of lymphoblasts; CNS-3, WBC of ≥5 cells/µL, with the presence of lymphoblasts.
Excludes CNS disease.
However, in the group of patients who developed severe CRS (grades 3 and 4; 57 patients), a higher percentage of patients (46 of 57; 80.7%) had high blast counts (≥50% blasts in bone marrow) at enrollment, compared with patients experiencing no or low-grade CRS (grades 0-2, 80 patients; 48 [60%] with ≥50% blasts). Despite this association, blast measurement at enrollment may not have been an accurate indicator of blast count at infusion, which was not measured.
CRS and fibrinogen levels
Among patients who experienced CRS, the lowest mean and median fibrinogen levels were observed in those with grade 4 CRS (Table 2). Nine patients (8 with grade 4 CRS, 1 with grade 3) reported severe hypofibrinogenemia (<1 g/L) during CRS. In 1 patient with grade 4 CRS, fibrinogen levels were below the level of detection (<0.4 g/L). Fibrinogen levels <1 g/dL were not reported in any patient with grade 1 or 2 CRS. Thus, patients with grade 4 CRS developed severe hypofibrinogenemia more frequently than patients with lower grades (nominal P < .001).
Lowest fibrinogen level by CRS grade
| . | Grade 1-2 (n = 51) . | Grade 3 (n = 27) . | Grade 4 (n = 30) . | Patients with CRS (n = 108) . | No CRS (n = 29) . |
|---|---|---|---|---|---|
| Lowest fibrinogen, g/L | |||||
| Patients, n | 44 | 26 | 30 | 100 | 29 |
| Mean (SD) | 3.7 (1.2) | 3.2 (1.6) | 1.8 (1.2) | 3.0 (1.6) | 2.7 (0.8) |
| Median | 3.5 | 3.1 | 1.3 | 3.1 | 2.7 |
| Min-max | 1.4-6.3 | 0.9-6.8 | 0.4-5.5 | 0.4-6.8 | 0.8-3.8 |
| Lowest fibrinogen category, n (%), g/L | |||||
| <1 | 0 | 1 (3.7) | 8 (26.7) | 9 (8.3) | 2 (6.9) |
| ≥1 to <1.5 | 1 (2.0) | 4 (14.8) | 8 (26.7) | 13 (12.0) | 0 |
| ≥1.5 | 43 (84.3) | 21 (77.8) | 14 (46.7) | 78 (72.2) | 27 (93.1) |
| . | Grade 1-2 (n = 51) . | Grade 3 (n = 27) . | Grade 4 (n = 30) . | Patients with CRS (n = 108) . | No CRS (n = 29) . |
|---|---|---|---|---|---|
| Lowest fibrinogen, g/L | |||||
| Patients, n | 44 | 26 | 30 | 100 | 29 |
| Mean (SD) | 3.7 (1.2) | 3.2 (1.6) | 1.8 (1.2) | 3.0 (1.6) | 2.7 (0.8) |
| Median | 3.5 | 3.1 | 1.3 | 3.1 | 2.7 |
| Min-max | 1.4-6.3 | 0.9-6.8 | 0.4-5.5 | 0.4-6.8 | 0.8-3.8 |
| Lowest fibrinogen category, n (%), g/L | |||||
| <1 | 0 | 1 (3.7) | 8 (26.7) | 9 (8.3) | 2 (6.9) |
| ≥1 to <1.5 | 1 (2.0) | 4 (14.8) | 8 (26.7) | 13 (12.0) | 0 |
| ≥1.5 | 43 (84.3) | 21 (77.8) | 14 (46.7) | 78 (72.2) | 27 (93.1) |
Median reference range for fibrinogen across 2 age groups (6-10 y and 11-17 y) and 2 reagents = 2.48-2.78 g/L.17 For patients with CRS, nadir fibrinogen is measured during CRS; for patients without CRS, nadir fibrinogen is measured within 1 mo postinfusion.
SD, standard deviation.
We also evaluated coagulation parameters and platelet and white blood cell (WBC) counts within 48 hours of the lowest fibrinogen level by CRS grade. Within this time frame, patients with grades 3 and 4 CRS had lower platelet and WBC counts and prolonged aPTT and PT, compared with patients with grades 1 and 2 CRS (Table 3).
Maximum abnormalities of coagulation parameters and platelets within 48 hours of lowest fibrinogen level by CRS grade
| . | Patients, n . | Mean (SD) . | Median . | Min-max . |
|---|---|---|---|---|
| Grade 1-2 CRS | ||||
| Platelet, ×109/L | 44 | 83.2 (69.4) | 67.5 | 5.0-276.0 |
| WBC, ×109/L | 44 | 1.2 (1.2) | 0.7 | 0-4.2 |
| aPTT, s | 42 | 37.3 (12.8) | 34.3 | 25.0-100.4 |
| PT, s | 32 | 14.1 (1.7) | 14.1 | 10.1-20.0 |
| INR | 27 | 1.1 (0.2) | 1.1 | 0.9-1.7 |
| Grade 3 CRS | ||||
| Platelet, ×109/L | 24 | 34.9 (29.5) | 25.5 | 9.0-146.0 |
| WBC, ×109/L | 22 | 0.6 (0.8) | 0.2 | 0-2.7 |
| aPTT, s | 23 | 42.4 (9.9) | 40.8 | 27.1-66.4 |
| PT, s | 17 | 14.8 (3.5) | 14.3 | 10.8-26.5 |
| INR | 16 | 1.3 (0.4) | 1.2 | 1.0-2.5 |
| Grade 4 CRS | ||||
| Platelet, ×109/L | 30 | 36.5 (26.5) | 27.5 | 5.0-114.0 |
| WBC, ×109/L | 30 | 0.5 (1.1) | 0.2 | 0-4.7 |
| aPTT, s | 26 | 63.0 (36.6) | 49.4 | 31.3-200.0 |
| PT, s | 13 | 19.2 (9.3) | 18.5 | 10.8-47.1 |
| INR | 19 | 1.6 (0.5) | 1.5 | 1.0-3.0 |
| . | Patients, n . | Mean (SD) . | Median . | Min-max . |
|---|---|---|---|---|
| Grade 1-2 CRS | ||||
| Platelet, ×109/L | 44 | 83.2 (69.4) | 67.5 | 5.0-276.0 |
| WBC, ×109/L | 44 | 1.2 (1.2) | 0.7 | 0-4.2 |
| aPTT, s | 42 | 37.3 (12.8) | 34.3 | 25.0-100.4 |
| PT, s | 32 | 14.1 (1.7) | 14.1 | 10.1-20.0 |
| INR | 27 | 1.1 (0.2) | 1.1 | 0.9-1.7 |
| Grade 3 CRS | ||||
| Platelet, ×109/L | 24 | 34.9 (29.5) | 25.5 | 9.0-146.0 |
| WBC, ×109/L | 22 | 0.6 (0.8) | 0.2 | 0-2.7 |
| aPTT, s | 23 | 42.4 (9.9) | 40.8 | 27.1-66.4 |
| PT, s | 17 | 14.8 (3.5) | 14.3 | 10.8-26.5 |
| INR | 16 | 1.3 (0.4) | 1.2 | 1.0-2.5 |
| Grade 4 CRS | ||||
| Platelet, ×109/L | 30 | 36.5 (26.5) | 27.5 | 5.0-114.0 |
| WBC, ×109/L | 30 | 0.5 (1.1) | 0.2 | 0-4.7 |
| aPTT, s | 26 | 63.0 (36.6) | 49.4 | 31.3-200.0 |
| PT, s | 13 | 19.2 (9.3) | 18.5 | 10.8-47.1 |
| INR | 19 | 1.6 (0.5) | 1.5 | 1.0-3.0 |
Median reference ranges across 2 age groups (6-10 y and 11-17 y) and 2 reagents: aPTT = 31.0-32.8 s; PT = 11.7-11.8 s; INR = 1.04.17
Treatment and timing of CRS-associated coagulopathy
Among patients with grades 3 and 4 CRS, baseline demographics and disease characteristics were generally similar for those who received fibrinogen concentrate or cryoprecipitate or did not receive replacement therapy (supplemental Table 1). Replacement therapy was given more frequently to patients <10 years of age (n = 10; 43.5%) and all 5 Asian patients received replacement therapy. Supplemental Table 2 presents baseline demographics and disease characteristics for patients enrolled in the ELIANA and ENSIGN trials. Use of fibrinogen concentrate or cryoprecipitate to manage hypofibrinogenemia was only seen among the group of patients with grades 3 and 4 CRS (Table 4). Four of 27 (14.8%) patients with grade 3 CRS and 19 of 30 (63.3%) patients with grade 4 CRS were treated with fibrinogen replacement therapy. No patients had hypofibrinogenemia as the sole criterion for grades 3 and 4 CRS. From a regional perspective, cryoprecipitate was most commonly used in the United States and was available at 18 sites in the United States, Australia, Canada, and Germany. In contrast, fibrinogen concentrate was the preferred option at the remaining 7 sites in Austria, Belgium, France, Italy, Japan, Norway, and Spain. Overall, fibrinogen concentrate was used for replacement therapy in fewer patients than cryoprecipitate (cryoprecipitate in 17 of 23 [73.9%] patients and fibrinogen concentrate in 6 of 23 patients [26.1%]). FFP was used early during CRS coagulopathy at the investigator’s discretion but was not recommended for management of hypofibrinogenemia because of concerns of volume overload.
Fibrinogen concentrate or cryoprecipitate administration by CRS grade
| Fibrinogen concentrate or cryoprecipitate administration . | Grade 1-2 (n = 51) . | Grade 3 (n = 27) . | Grade 4 (n = 30) . | All patients (N = 108) . |
|---|---|---|---|---|
| Patients, n (%) | 0 | 4 (14.8) | 19 (63.3) | 23 (21.3) |
| Fibrinogen concentrate, n (%) | 0 | 1 (3.7) | 5 (16.7) | 6 (5.6) |
| Cryoprecipitate, n (%) | 0 | 3 (11.1) | 14 (46.7) | 17 (15.7) |
| Fibrinogen concentrate or cryoprecipitate administration . | Grade 1-2 (n = 51) . | Grade 3 (n = 27) . | Grade 4 (n = 30) . | All patients (N = 108) . |
|---|---|---|---|---|
| Patients, n (%) | 0 | 4 (14.8) | 19 (63.3) | 23 (21.3) |
| Fibrinogen concentrate, n (%) | 0 | 1 (3.7) | 5 (16.7) | 6 (5.6) |
| Cryoprecipitate, n (%) | 0 | 3 (11.1) | 14 (46.7) | 17 (15.7) |
For patients with grades 3 and 4 CRS, the median time to onset was 6 days (range, 2-33). The median duration of a grade 3 or 4 CRS episode was 10 days (range, 4-36). The median onset of severe hypofibrinogenemia (<1 g/L) and peak ferritin was 8 days (range, 4-13) and 9 days (range, 4-34), respectively. Fibrinogen concentrate or cryoprecipitate was administered during the same time frame at a median of 9 days, as well (range, 5-14; Table 5). Very low fibrinogen levels (<1 g/L) were documented in patients before maximal CRS (n = 1), during maximal CRS (n = 7), and at CRS improvement (n = 1).
Timing of onset, peak ferritin, and fibrinogen concentrate or cryoprecipitate administration in patients with grade 3 or 4 CRS
| . | . | Timing of onset, d . | |
|---|---|---|---|
| Patients, n . | Median . | Min-max . | |
| Onset of CRS episode | 57 | 2 | 1-22 |
| Onset of grade 3 or 4 CRS | 57 | 6 | 2-33 |
| Duration of CRS episode | 57 | 10 | 4-36 |
| Onset of fibrinogen <1 g/L | 9 | 8 | 4-13 |
| Peak ferritin | 57 | 9 | 4-34 |
| Fibrinogen concentrate/cryoprecipitate administration | 23 | 9 | 5-14 |
| . | . | Timing of onset, d . | |
|---|---|---|---|
| Patients, n . | Median . | Min-max . | |
| Onset of CRS episode | 57 | 2 | 1-22 |
| Onset of grade 3 or 4 CRS | 57 | 6 | 2-33 |
| Duration of CRS episode | 57 | 10 | 4-36 |
| Onset of fibrinogen <1 g/L | 9 | 8 | 4-13 |
| Peak ferritin | 57 | 9 | 4-34 |
| Fibrinogen concentrate/cryoprecipitate administration | 23 | 9 | 5-14 |
Median duration and range for patients with maximum grade 3 or 4 CRS.
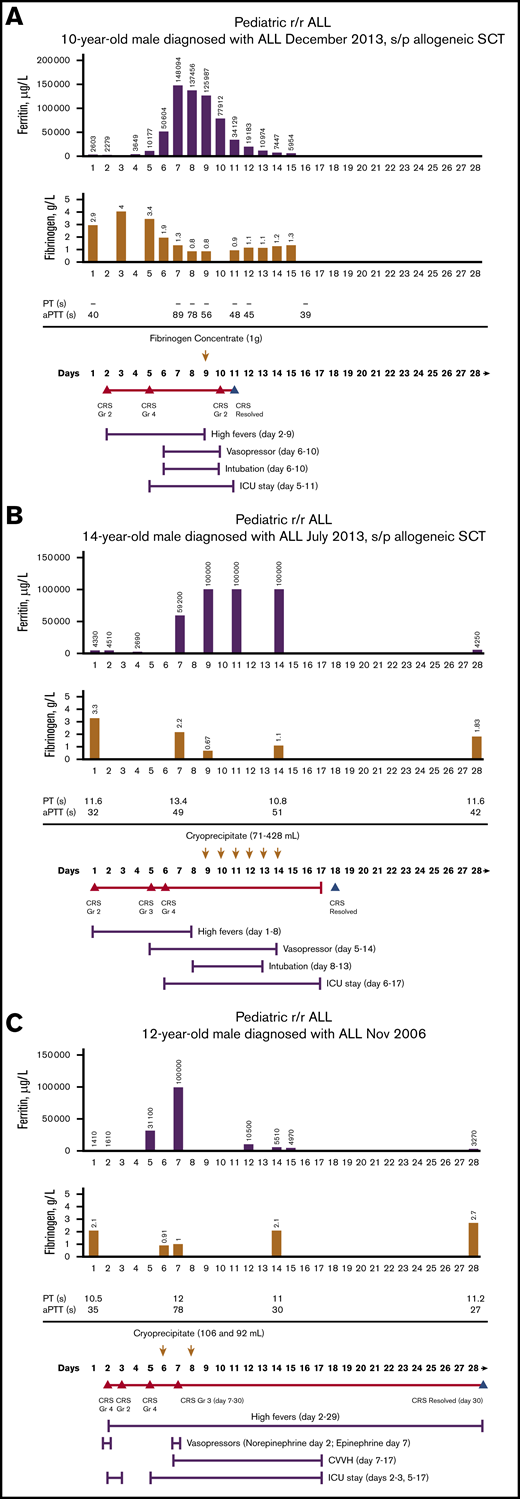
Figure 1A-C shows the time course of fibrinogen, ferritin, PT, aPTT, and CRS and the corresponding management in 3 representative patients who needed treatment of CRS-associated coagulopathy with fibrinogen concentrate or cryoprecipitate. Among all 3 representative patients, there was hyperferritinemia, prolonged PT and aPTT, and hypofibrinogenemia during grades 3 and 4 CRS.
Time course of treatment of CRS-associated coagulopathy on patients treated with tisagenlecleucel. Patient 1 (A), patient 2 (B), patient 3 (C). Days after tisagenlecleucel infusion; day 1, infusion day. Arrows, the day of blood product replacement with fibrinogen concentrate; orange triangles, CRS onset by grade; blue triangles, CRS resolution. ALL, acute lymphoblastic leukemia; Gr, grade; ICU, intensive care unit; s/p, status post; SCT, stem cell transplantation.
Time course of treatment of CRS-associated coagulopathy on patients treated with tisagenlecleucel. Patient 1 (A), patient 2 (B), patient 3 (C). Days after tisagenlecleucel infusion; day 1, infusion day. Arrows, the day of blood product replacement with fibrinogen concentrate; orange triangles, CRS onset by grade; blue triangles, CRS resolution. ALL, acute lymphoblastic leukemia; Gr, grade; ICU, intensive care unit; s/p, status post; SCT, stem cell transplantation.
Bleeding events and CRS
At the beginning of the trial, the use of fibrinogen replacement was based on the investigator’s judgement. Once the importance of CRS-associated coagulopathy became apparent, specific guidance for fibrinogen monitoring and replacement was developed.
Overall, of the 57 patients with grade 3 and 4 CRS, 23 received fibrinogen replacement and 34 did not. Thirteen of the 23 patients (57%) who received fibrinogen replacement at any time experienced bleeding events within 8 weeks after infusion (highest grade 1 [n = 1], grade 2 [n = 6], grade 3 [n = 4], and grade 4 [n = 2]). Among the 34 patients with grade 3 and 4 CRS who did not receive fibrinogen replacement, 13 (38%) experienced bleeding events (highest grade 1 [n = 6], grade 2 [n = 3], and grade 3 [n = 4]; Table 6). Of note, median fibrinogen level was 1.15 g/L (range, 0.40-3.71) in patients with fibrinogen replacement compared with 3.12 g/L (range, 0.83-6.75) in patients without fibrinogen replacement. The lowest fibrinogen level by bleeding grade is shown in Table 7. Six patients (2 each with grade 2, grade 3, and grade 4 bleeding) had severe hypofibrinogenemia (<1 g/L) during bleeding. Five patients with severe hypofibrinogenemia (<1 g/L) experienced no bleeding (Table 7).
Bleeding events in patients with grade 3 or 4 CRS, with and without fibrinogen replacement therapy
| . | Fibrinogen replacement therapy . | |
|---|---|---|
| Yes, n = 23 . | No, n = 34 . | |
| Lowest fibrinogen, n | 23 | 33* |
| Lowest fibrinogen, median (range), g/L | 1.15 (0.40-3.71) | 3.12 (0.83-6.75) |
| Bleeding event, n (%)† | n = 23 | n = 34 |
| No bleeding | 10 (43) | 21 (62) |
| Any grade | 13 (57) | 13 (38) |
| Grade 1 | 1 (4) | 6 (18) |
| Grade 2 | 6 (26) | 3 (9) |
| Grade 3 | 4 (17) | 4 (18) |
| Grade 4 | 2 (9) | 0 |
| . | Fibrinogen replacement therapy . | |
|---|---|---|
| Yes, n = 23 . | No, n = 34 . | |
| Lowest fibrinogen, n | 23 | 33* |
| Lowest fibrinogen, median (range), g/L | 1.15 (0.40-3.71) | 3.12 (0.83-6.75) |
| Bleeding event, n (%)† | n = 23 | n = 34 |
| No bleeding | 10 (43) | 21 (62) |
| Any grade | 13 (57) | 13 (38) |
| Grade 1 | 1 (4) | 6 (18) |
| Grade 2 | 6 (26) | 3 (9) |
| Grade 3 | 4 (17) | 4 (18) |
| Grade 4 | 2 (9) | 0 |
Bleeding event grade corresponds to maximum grade.
One patient did not have a documented fibrinogen level.
Number of patients with an event.
Lowest fibrinogen level by bleeding event grade
| . | Lowest fibrinogen category, g/L, n (%) . | ||
|---|---|---|---|
| Bleeding event, grade . | <1 . | ≥1 to <1.5 . | ≥1.5 . |
| No bleeding | 5 (16.7) | 9 (30) | 16 (53.3) |
| 1 | 0 | 1 (14.3) | 6 (85.7) |
| 2 | 2 (22.2) | 2 (22.2) | 5 (55.6) |
| 3 | 2 (25) | 0 | 6 (75) |
| 4 | 0 | 0 | 2 (100) |
| . | Lowest fibrinogen category, g/L, n (%) . | ||
|---|---|---|---|
| Bleeding event, grade . | <1 . | ≥1 to <1.5 . | ≥1.5 . |
| No bleeding | 5 (16.7) | 9 (30) | 16 (53.3) |
| 1 | 0 | 1 (14.3) | 6 (85.7) |
| 2 | 2 (22.2) | 2 (22.2) | 5 (55.6) |
| 3 | 2 (25) | 0 | 6 (75) |
| 4 | 0 | 0 | 2 (100) |
Grade ≥3 bleeding was reported in 10 of the 57 patients experiencing grade 3 and 4 CRS (17.5%; Table 6; supplemental Table 3). Among those patients, 2 had severe hypofibrinogenemia (<1 g/L), 1 of whom died of a fatal cerebral hemorrhage (grade 4 bleeding event) on day 14 after tisagenlecleucel infusion. When coagulopathy with hypofibrinogenemia was detected 8 days after infusion, the patient who died had resolving CRS, abdominal compartment syndrome, and renal failure. The fibrinogen level was 0.6 g/L, PT was 13.7 seconds, aPTT was 35.0 seconds, and platelets were 76 × 109/L. The patient’s other medical conditions included Down syndrome. The patient received 6 consecutive daily doses of cryoprecipitate. On day 14 after infusion, the patient’s aPTT had normalized, but fibrinogen remained low at 0.83 g/L. Computed tomography of the head revealed acute parenchymal hemorrhage, and the patient died of the bleeding event. The hemorrhage was attributed to tisagenlecleucel and/or lymphodepleting chemotherapy. In addition, the patient’s leukemia, prior chemotherapy, thrombocytopenia, coagulopathy, abdominal compartment syndrome, continuous venovenous hemodiafiltration (CVVH), and hypertension were considered to be possible contributory factors.
There was only 1 other patient with grade 4 bleeding (epistaxis, hematuria, and hemoptysis, all grade 4). Fibrinogen replacement was initiated based on clinical judgement (active bleeding); fibrinogen levels were not reported.
Discussion
Consistent with the experience from an earlier single-center study,11 CRS-associated coagulopathy with hypofibrinogenemia was observed in pediatric and young adult patients with B-ALL who were treated with tisagenlecleucel in the multicenter ELIANA and ENSIGN trials. Coagulopathy with hypofibrinogenemia was more frequent in patients who experienced severe CRS.5,9 Very low fibrinogen levels (<1 g/L) were documented in patients before maximal CRS (n = 1), during maximal CRS (n = 7), and at CRS improvement (n = 1). The need for frequent monitoring of serum fibrinogen levels, even after the resolution of acute CRS symptoms, was emphasized in the ELIANA and ENSIGN treatment protocols, as fibrinogen levels may initially be elevated as an acute-phase reactant during the acute CRS phase and then later decreased to profoundly low levels, with increased risk of life-threatening bleeding. Because the timing of severe hypofibrinogenemia can lag behind the onset of severe CRS, close monitoring of fibrinogen levels in patients with grade 3 or 4 CRS should be continued.
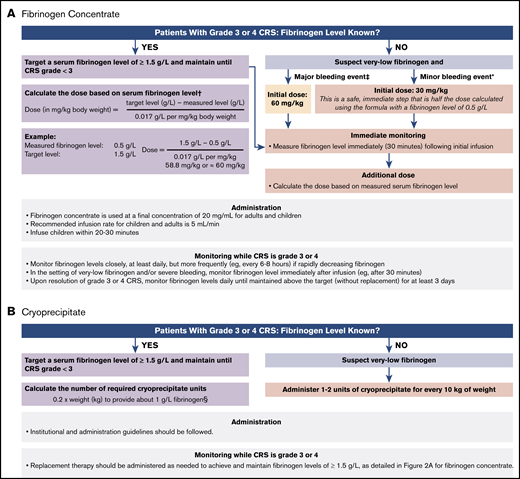
Because production of cryoprecipitate has been phased out throughout much of the European Union12 and is not widely available in Japan, fibrinogen concentrate (eg, RiaSTAP, Fibryga) has become the preferred treatment of CRS-associated hypofibrinogenemia. Guidelines for the use of fibrinogen concentrate in the treatment of CRS-associated hypofibrinogenemia were developed during the ELIANA and ENSIGN trials and have been adapted based on clinical experience as guidelines for the management of CRS-associated coagulopathy with hypofibrinogenemia (Figure 2A).13,14 Among patients who experience grade 3 or 4 CRS, according to the Penn grading scale, fibrinogen levels should be monitored frequently (at least once per day), and replacement with fibrinogen concentrate should be instituted for fibrinogen levels <1.5 g/L. Early on, adequate fibrinogen correction should be assessed by measuring fibrinogen levels 30 to 60 minutes after the initial replacement infusion. If significant bleeding is observed in CRS grades 3 and 4, hypofibrinogenemia should be strongly suspected, even in the absence of known fibrinogen levels, and replacement should be initiated. The initial dose of fibrinogen concentrate is determined by the severity of the bleeding event and the initial fibrinogen concentration. For minor bleeding events, 30 mg/kg can be safely administered, whereas 60 mg/kg is recommended for major bleeding events (Figure 2A). Subsequent doses can be calculated based on serum fibrinogen levels. Serum fibrinogen should be closely monitored, especially in the context of concurrent bleeding tendency or active bleeding, low platelets, or anticoagulation, and replacement therapy should be administered as needed to maintain fibrinogen levels of ≥1.5 g/L until CRS is grade <3. Late-onset hypofibrinogenemia may occur after grade 3 or 4 CRS; therefore, daily serum fibrinogen monitoring should be considered until fibrinogen levels are maintained above the target (without replacement) for at least 3 days.
Guidelines for use of replacement to treat CRS-associated hypofibrinogenemia or coagulopathy. Fibrinogen concentrate (A), cryoprecipitate (B). *Minor bleeding events include epistaxis, intramuscular bleeding, and menorrhagia. †From RiaSTAP Prescribing Information.13 ‡Major bleeding events include head trauma and intracranial hemorrhage. For patients without bleeding, fibrinogen levels should be monitored and maintained at >1.5 g/L. §Each unit of cryoprecipitate contains at least 150 mg fibrinogen per unit. Two units of cryoprecipitate per 10 kg body weight generally increases fibrinogen concentration by 1 g/L, except in cases of DIC or continued bleeding with massive transfusion.
Guidelines for use of replacement to treat CRS-associated hypofibrinogenemia or coagulopathy. Fibrinogen concentrate (A), cryoprecipitate (B). *Minor bleeding events include epistaxis, intramuscular bleeding, and menorrhagia. †From RiaSTAP Prescribing Information.13 ‡Major bleeding events include head trauma and intracranial hemorrhage. For patients without bleeding, fibrinogen levels should be monitored and maintained at >1.5 g/L. §Each unit of cryoprecipitate contains at least 150 mg fibrinogen per unit. Two units of cryoprecipitate per 10 kg body weight generally increases fibrinogen concentration by 1 g/L, except in cases of DIC or continued bleeding with massive transfusion.
In regions where cryoprecipitate is readily available or preferable, institutional and administration guidelines should be followed. Each unit of cryoprecipitate contains at least 150 mg of fibrinogen per unit. Two units of cryoprecipitate per 10 kg of body weight generally increases fibrinogen concentration by 1 g/L, except in cases of DIC or continued bleeding with massive transfusion.14 Therefore, the number of required cryoprecipitate units can be calculated as follows: 0.2 × weight (in kilograms) to provide ∼1 g/L fibrinogen (Figure 2B). Replacement therapy should be administered as needed to achieve and maintain fibrinogen levels of ≥1.5 g/L, as detailed for fibrinogen concentrate.
CRS-associated coagulopathy and hypofibrinogenemia were managed with fibrinogen concentrate or cryoprecipitate replacement during the ELIANA and ENSIGN trials. As a result, we developed clinical guidelines for the ELIANA and ENSIGN trials for the monitoring and management of CRS-associated hypofibrinogenemia and the use of fibrinogen concentrate. In patients who developed grade 3 or 4 CRS, frequent monitoring of fibrinogen, PT, aPTT, platelet levels, and signs of clinical bleeding was recommended. Among the patients with grade 3 or 4 CRS who did or did not receive fibrinogen replacement therapy, approximately half in each group experienced bleeding events. However, patients who received fibrinogen replacement seemed to have lower median fibrinogen levels and were likely more prone to bleeding than those who did not receive fibrinogen replacement. It is important to note that at the beginning of the trial, there was no guidance provided for managing hypofibrinogenemia, and the use of fibrinogen replacement was based on investigator judgement. In some cases, fibrinogen replacement was not initiated before the patients had a clinically significant bleeding event.
CRS-associated hypofibrinogenemia increases the risk of bleeding, and these patients should be appropriately monitored and managed. Fortunately, severe bleeding events (grade 4) in the context of low fibrinogen were uncommon and were reported in only 2 patients, 1 of whom had a fatal cerebral hemorrhage. It is likely that other factors including, but not limited to, procoagulant and anticoagulant factor abnormalities and thrombocytopenia contribute to the incidence and severity of the bleeding. It is also possible that the patients at the highest risk of bleeding received replacement, which decreased their risk of bleeding to a level similar to that of the patients who did not require replacement. In addition, some patients received fibrinogen replacement when the bleeding was already present, not before. As such, replacement cannot prevent a bleeding event that has already occurred. Last, the guidelines for replacement evolved over time, based on clinical experience, and replacement at the beginning of the trial was based on investigator judgment and clinical signs of bleeding rather than strict fibrinogen monitoring.
Patients with CRS-associated coagulopathy, as well as MAS/HLH that occurs in the setting of CRS, should be monitored and managed according to the proposed management guidelines for hypofibrinogenemia. Coagulopathy with hypofibrinogenemia is also a prominent feature and one of the diagnostic criteria of MAS/HLH, a syndrome that substantially overlaps CRS. MAS/HLH encompasses a wide array of related life-threatening conditions, whereby ineffective immune responses are characterized by an uncontrolled hyperinflammatory response.15 The clinical presentation of MAS/HLH includes fever, hepatosplenomegaly, cytopenias, hypertriglyceridemia, liver dysfunction, elevated serum ferritin and transaminases, coagulopathy with hypofibrinogenemia, and neurological abnormalities. Laboratory parameters include highly elevated ferritin (16 000-500 000 mg/dL) and decreased fibrinogen (<1.5 g/L).16
In patients with CRS-associated coagulopathy, we found low fibrinogen levels that was out of proportion with other coagulation parameters, such as PT and aPTT. This finding is consistent with those in other reports of HLH in which low fibrinogen was reported in most patients, some of whom still had normal levels of other coagulation factors.15 The latest onset of hypofibrinogenemia observed in the clinical trial was at day 13. Hypofibrinogenemia often occurs as the patient begins to improve systemically; as a result, hypofibrinogenemia may be overlooked in these patients. Thus, low fibrinogen is a particularly sensitive indicator of CRS-associated coagulopathy and should be frequently monitored during and after grade 3 or 4 CRS. In patients who experience grade 3 or 4 CRS in the absence of bleeding, fibrinogen levels should be monitored daily until the resolution of CRS and until fibrinogen levels are ≥1.5 g/L without replacement for 3 days.
The current management guidelines were developed based on CRS grading from the Penn grading scale. The most recent American Society for Transplantation and Cellular Therapy (ASTCT) consensus scale grades CRS severity mainly by hypotension and hypoxia. Significant organ toxicities and coagulopathy are not part of the grading system.8 Differences between the Penn and ASTCT grading scales include the use of intervention for hypoxia as a grade 3 event (Penn) vs a grade 2 event (ASTCT). In addition, hypoxia requiring positive pressure is considered a grade 3 event (Penn) vs a grade 4 event (ASTCT). Centers using the ASTCT grading scale should consider implementing the guidelines for management of potential CRS-associated coagulopathy in cases wherein mild intervention is used for treatment of hypoxia (grade 2). However, the management guidelines presented in this report are applicable to all patients with severe CRS (grades 3 and 4), regardless of the applied grading scale.
As part of the clinical guidelines for managing CRS-associated coagulopathy and hypofibrinogenemia, replacement of fibrinogen with cryoprecipitate or fibrinogen concentrate, rather than FFP, is preferred to avoid fluid overload in these patients. In addition, severe CRS is commonly associated with capillary leak syndrome, in which hyperpermeability of capillaries allows fluid to leak into the lungs and interstitial tissues, which can be worsened by fluid overload,16 another reason that fibrinogen supplementation should be provided through cryoprecipitate or fibrinogen concentrates. For fibrinogen replacement with fibrinogen concentrate, weight-adjusted dosing is recommended for maintenance of adequate fibrinogen levels.
In addition to monitoring fibrinogen levels and PT/aPTT, platelet counts should also be monitored closely. Platelet transfusion should be administered as appropriate to prevent added risk of bleeding in the context of severe thrombocytopenia and coagulopathy.
Concurrent clinical events and laboratory abnormalities can further complicate the course of treatment, increase the risk of bleeding, and present challenges for the management of coagulopathy in the context of CRS. Ongoing thrombocytopenia, uncontrolled hypertension after withdrawal of vasopressors, and the use of CVVH with anticoagulation for renal replacement therapy often contribute to observed bleeding. For example, the patient who experienced a fatal cerebral hemorrhage had undergone CVVH with continued treatment with furosemide. It is important to note that the risk of bleeding may outweigh the risk of thrombosis in this setting, and caution should be exercised when considering anticoagulants such as low-dose heparin.
In summary, monitoring of patients with CRS-associated coagulopathy and hypofibrinogenemia is imperative to avoid the potential for bleeding events and even life-threatening hemorrhage. Fibrinogen monitoring and replacement with fibrinogen concentrate or cryoprecipitate according to the guidelines developed for the ELIANA and ENSIGN trials is an effective strategy and the recommended treatment option in these patients.
Presented in poster form at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017 (Poster 1276) and American Society of Pediatric Hematology/Oncology Annual Meeting, Pittsburgh, PA, 2-5 May 2018 (Poster 751).
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who participated in the trial, in line with applicable laws and regulations. The data of these trials are made available according to the criteria and process described on www.clinicalstudydatarequest.com.
Acknowledgments
The authors thank Sarah Jablonski Schandle (Healthcare Consultancy Group) for providing medical writing support.
The writing of the manuscript was funded by Novartis Pharmaceuticals Corporation.
The authors dedicate this study to Patricia Wood, who participated actively in the research and drafting of the manuscript.
Authorship
Contribution: T.W.L, S.R., B.D.M., J.B., G.A.Y., S.A.G., D.T.T., and H.H. were involved with patient accrual; T.W.L., S.R., B.D.M., J.B., G.A.Y., S.A.G., D.T.T., and H.H. were involved with clinical care; T.W.L., S.R., B.D.M., J.B., G.A.Y., S.A.G., D.T.T., and H.H. were involved with data collection; P.W., R.A., L.Y., A.C.-A., and L.K.E. were involved with data analysis; and all authors contributed to the manuscript and approved the final manuscript.
Conflict-of-interest disclosure: J.B. has received personal fees, grants, and nonfinancial support from and has served as a trial investigator, a consultant, and an advisory board member for Novartis Pharmaceuticals Corporation. S.A.G. has received grant support and personal fees from Novartis Pharmaceuticals Corporation; has received study support and consulting fees from and has served on study steering committees and science advisory boards for Vertex, Adaptimmune, CBMG, Cure Genetics, Humanigen, Jazz, Kite, Roche, and Servier; and holds a patent (WO 2014011984 A1) related to toxicity management for antitumor activity of CARs managed according to University of Pennsylvania policies. D.T.T. has received nonfinancial support from Novartis Pharmaceuticals Corporation and has served on advisory boards for Janssen, La Roche, Amgen, and Humanigen. S.R. has received personal fees and nonfinancial support from and has served on study steering committees for Novartis Pharmaceuticals Corporation; has served as a trial investigator, a consultant, and an advisory board member for Jazz Pharmaceutical; has received personal fees and nonfinancial support from and has been an advisory board member for Shire; and has received personal fees and nonfinancial support from and served as a trial investigator and an advisory board member for Celgene. T.W.L. has received personal fees from Novartis Pharmaceuticals Corporation, Bayer, Loxo Oncology, and Eli Lilly and grant support from Novartis, Bayer, and Pfizer. P.W. was employed by Novartis during the conduct of the trial. R.A., L.Y., A.C.-A., and L.K.E. are employed by Novartis. B.D.M. has served as a trial investigator and an advisory board member for Novartis Pharmaceuticals Corporation and has received nonfinancial support from Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Patricia Wood died on 25 October 2019.
Correspondence: Jochen Buechner, Oslo University Hospital, PO Box 4950, Nydalen, 0424 Oslo, Norway; e-mail: jocbuc@ous-hf.no.
References
Author notes
The full-text version of this article contains a data supplement.