Key Points
Patients with BALT lymphoma show an indolent clinical course, yet disease management at diagnosis is not well defined.
Our retrospective study shows that BALT lymphoma can often be managed expectantly and not require therapy for many years.
Abstract
Although patients with bronchus-associated lymphoid tissue (BALT) lymphoma show an indolent clinical course, appropriate disease management at diagnosis is not well defined. This study aimed to compare 3 treatment strategies for patients with BALT lymphoma: active surveillance, systemic chemotherapy or immunotherapy at diagnosis, or complete surgical resection at diagnosis. We conducted a retrospective study of all patients with new diagnoses of marginal zone lymphoma (MZL) involving the lung who were treated at the Memorial Sloan Kettering Cancer Center between 1995 and 2017. Primary BALT lymphoma was defined as disease confined to the lungs and adjacent lymph nodes. Active surveillance was defined as a documented observation plan and ≥3 months of follow-up before initiating treatment. Overall survival (OS) and event-free survival (EFS) were compared between treatment groups. We reviewed 200 consecutive patients with MZL involving the lung; 123 met the inclusion criteria and were managed by active surveillance (47%), complete surgical resection (41%), or systemic chemotherapy or immunotherapy (11%). With a median follow-up of >60 months, surgical resection was associated with a superior EFS compared with active surveillance and systemic treatment (6-year EFS: 74% vs 65% vs 62%, respectively; P = .013). Larger lesions and thrombocytopenia were associated with shorter EFS. All groups had excellent OS at 6 years (93%), albeit with a slight superiority for surgical resection (100%) over active surveillance (91%) and systemic treatment (76%) (P = .024). BALT lymphoma is an indolent disease that can often be managed expectantly and not require therapy for many years.
Introduction
Bronchus-associated lymphoid tissue (BALT) lymphoma is a rare subtype of extranodal marginal zone lymphoma (MZL) that typically does not compromise pulmonary function and is often diagnosed incidentally.1 This entity was described in 1963 as pseudolymphoma because it was not possible to diagnose a malignancy on the basis of morphology alone before the advent of immunohistochemistry and clonality studies. In a minority of patients, pseudolymphoma progressed and a morphologic diagnosis of lymphoma was possible on subsequent biopsy; however, the majority of patients had no significant progression. With immunohistochemistry and clonality studies, such cases are now mostly diagnosed as BALT lymphoma.2
Currently, oncologists may opt to treat BALT lymphoma early, in contrast to other subtypes of MZL which are often managed expectantly, because of concern that disease progression will result in irreparable damage to the lungs, but earlier surgery or chemotherapy might result in more morbidity than the disease itself.
Data about the disease course of BALT lymphoma comes from a few limited retrospective series assessing active interventions3-7 that demonstrated patients have an excellent overall prognosis.3-5 Equal or superior progression-free survival (PFS) but not overall survival (OS) was found when disease was fully resected compared with disease that was partially resected or treated with systemic chemotherapy or immunotherapy. In other indolent lymphomas, large series have assessed the implementation of active surveillance strategies, demonstrating no OS benefit for systemic interventions.8,9 In a small retrospective study of 11 patients with untreated BALT lymphoma who were observed expectantly (median time of observation, 28 months), treatment was required for only 3 patients, 2 of them because of progression outside the lungs.10 This retrospective study aimed to evaluate the presentation and natural course of primary BALT lymphoma in patients who were managed expectantly and in those managed actively after diagnosis.
Methods
Patients
We reviewed all cases of patients with newly diagnosed MZL involving the lung who presented to Memorial Sloan Kettering (MSK) Cancer Center between 1995 and 2017. All pathology specimens were reviewed by the MSK Cancer Center Department of Pathology. The study was approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki guidelines. Primary BALT lymphoma was defined as disease confined to the lungs and hilar and mediastinal lymph nodes.3,4 Patients younger than age 18 years with evidence of other sites of extranodal disease or with lymphadenopathy outside the hilar and mediastinal regions (raising the possibility of nodal or extranodal MZL with secondary lung involvement) were excluded,7 as were patients with concurrent malignancy, including other lymphoid histology.
Clinical data set
Data were extracted from the electronic medical records and from pathology and radiology reports. Patients were grouped by first-line treatment: complete surgical resection, active surveillance, or initial systemic therapy. Active surveillance was defined as an explicit documentation of intent to observe the patient and ≥3 months of clinical and imaging follow-up before initiating therapy after completing the diagnostic workup. Surgical resection was defined as a complete excision of all pulmonary lesions without evidence of residual disease on postsurgery imaging and without additional systemic treatment. Systemic therapy was defined as first-line treatment with anti-CD20 monotherapy or chemoimmunotherapy. Lesion sizes were defined as the longest diameter.
Statistics
Descriptive statistics are presented for categorical data as percentage (and number of patients) and for numeric data as median (interquartile range). We compared baseline characteristics between patients treated expectantly or actively, using Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for numeric variables. Survival was calculated from the date of biopsy confirming the diagnosis in all groups; active surveillance was considered as a first-line treatment in these analyses. Measures of survival and their definitions are OS, time to death as a result of any cause or the end of follow-up; PFS, time to computed tomography imaging that documented disease progression per the Cheson criteria in lymphoma,11 death, or the end of follow-up; and event-free survival (EFS), time to second-line treatment, death, or the end of follow-up. The median follow-up time was calculated for survivors only. We used the log-rank test for univariable comparison of survival between groups and the Cox proportional hazard regression model for comparison between groups and for multivariable modeling. Tests of the proportional hazards assumption were conducted using Schoenfeld residuals. All analyses were performed using R 3.4.1 (R Foundation).
Results
Baseline clinical characteristics
Medical records of 200 consecutive patients with MZL involving the lung were reviewed, and 123 met the criteria for primary BALT lymphoma. Of the remaining 77 patients, 25 had concurrent malignancy and 52 had extrathoracic involvement. Only 68 patients meeting all other criteria for primary BALT lymphoma (n = 127) had a bone marrow biopsy, and 4 of them (6%) had bone marrow involvement. Initial treatment strategies for patients in the analysis were active surveillance (47%; n = 58), surgical resection (41%; n = 51), or systemic treatment (11%; n = 14; rituximab monotherapy [n = 7] or rituximab plus other chemotherapy [n = 5] or chemotherapy alone [n = 2]). In all patients, surgical resection was performed as part of the initial diagnostic workup. Median age was 66 years (IQR, 54-74 years) and women predominated (63%; n = 78). Patients had high rates of current smoking or history of smoking (61%; n = 75) and of autoimmune or connective tissue diseases (21%; n = 26) (Table 1). In addition, patients with a concurrent malignancy (n = 25) were excluded from this analysis, representing 16% (25 of 152) of all adult patients with primary BALT lymphoma; 11 of them (44%) had a primary carcinoma of the lung.
Cohort characteristics by first-line management (N = 123)
| Variable . | Overall . | Active surveillance (n = 58) . | Surgical resection (n = 51) . | Systemic treatment (n = 14) . | P . |
|---|---|---|---|---|---|
| Median age (IQR), y | 66 (54-74) | 67 (56-76) | 64 (54-71) | 60 (54-68) | .2 |
| Female sex | 78 (63) | 39 (67) | 31 (61) | 8 (57) | .7 |
| Smoking status | .027 | ||||
| Never | 48 (39) | 25 (43) | 14 (27) | 9 (64) | |
| Current | 12 (9.8) | 3 (5.2) | 9 (18) | 0 (0) | |
| Past (≤10 y) | 11 (8.9) | 3 (5.2) | 6 (12) | 2 (14) | |
| Distant past (>10 y) | 52 (42) | 27 (47) | 22 (43) | 3 (21) | |
| COPD | 20 (17) | 9 (16) | 7 (14) | 4 (29) | .4 |
| Autoimmune/connective tissue disease | 26 (21) | 14 (24) | 7 (14) | 5 (36) | .2 |
| Sjogren’s syndrome | 7 (5.7) | 3 (5.2) | 2 (3.9) | 2 (14) | .3 |
| Systemic lupus erythematosus | 6 (4.9) | 4 (6.9) | 2 (3.9) | 0 (0) | .7 |
| Rheumatoid arthritis | 1 (0.8) | 0 (0) | 1 (2.0) | 0 (0) | .5 |
| Psoriasis | 4 (3.3) | 1 (1.7) | 1 (2.0) | 2 (14) | .09 |
| Other | 11 (8.9) | 7 (12) | 3 (5.9) | 1 (7.1) | .6 |
| History of cancer | 30 (24) | 17 (29) | 12 (24) | 1 (7.1) | .2 |
| Cancer during follow-up | 4 (3.3) | 2 (3.4) | 1 (2.0) | 1 (7.1) | .5 |
| Dyspnea | 32 (26) | 17 (29) | 8 (16) | 7 (50) | .03 |
| B symptoms | 3 (2.4) | 1 (1.7) | 2 (3.9) | 0 (0) | .7 |
| KPS ≤70 | 7 (5.7) | 3 (5.2) | 2 (3.9) | 2 (14) | .07 |
| Variable . | Overall . | Active surveillance (n = 58) . | Surgical resection (n = 51) . | Systemic treatment (n = 14) . | P . |
|---|---|---|---|---|---|
| Median age (IQR), y | 66 (54-74) | 67 (56-76) | 64 (54-71) | 60 (54-68) | .2 |
| Female sex | 78 (63) | 39 (67) | 31 (61) | 8 (57) | .7 |
| Smoking status | .027 | ||||
| Never | 48 (39) | 25 (43) | 14 (27) | 9 (64) | |
| Current | 12 (9.8) | 3 (5.2) | 9 (18) | 0 (0) | |
| Past (≤10 y) | 11 (8.9) | 3 (5.2) | 6 (12) | 2 (14) | |
| Distant past (>10 y) | 52 (42) | 27 (47) | 22 (43) | 3 (21) | |
| COPD | 20 (17) | 9 (16) | 7 (14) | 4 (29) | .4 |
| Autoimmune/connective tissue disease | 26 (21) | 14 (24) | 7 (14) | 5 (36) | .2 |
| Sjogren’s syndrome | 7 (5.7) | 3 (5.2) | 2 (3.9) | 2 (14) | .3 |
| Systemic lupus erythematosus | 6 (4.9) | 4 (6.9) | 2 (3.9) | 0 (0) | .7 |
| Rheumatoid arthritis | 1 (0.8) | 0 (0) | 1 (2.0) | 0 (0) | .5 |
| Psoriasis | 4 (3.3) | 1 (1.7) | 1 (2.0) | 2 (14) | .09 |
| Other | 11 (8.9) | 7 (12) | 3 (5.9) | 1 (7.1) | .6 |
| History of cancer | 30 (24) | 17 (29) | 12 (24) | 1 (7.1) | .2 |
| Cancer during follow-up | 4 (3.3) | 2 (3.4) | 1 (2.0) | 1 (7.1) | .5 |
| Dyspnea | 32 (26) | 17 (29) | 8 (16) | 7 (50) | .03 |
| B symptoms | 3 (2.4) | 1 (1.7) | 2 (3.9) | 0 (0) | .7 |
| KPS ≤70 | 7 (5.7) | 3 (5.2) | 2 (3.9) | 2 (14) | .07 |
Data presented as no. (%) or median (IQR). Boldface P values indicate statistical significance (P < .05).
COPD, chronic obstructive pulmonary disease; KPS, Karnofsky performance status.
Clinical findings
Most patients presented with a single lesion (76%; n = 94) of median size 2.5 cm (IQR, 1.5-3.5 cm) and without lobar predominance. A minority (5%; n = 6) presented with thoracic nodal involvement, and 24% (n = 30) presented with pleural involvement or effusion. Overall, few patients had B symptoms, cytopenia, or elevated lactate dehydrogenase (Tables 1 and 2). Data about forced expiratory volume in 1 second (FEV1) was available for 60 patients, and 15% had lower-than-expected FEV1 (<80% expected value; n = 9). Data regarding diffusing capacity of the lungs for carbon monoxide (DLCO) was available for 59 patients, and 36% had lower-than-expected DLCO (<75% expected value; n = 21), including 13 with mild dysfunction (65% to 74% expected value of DLCO).12
Imaging, pathology, and laboratory characteristics by first-line management (N = 123)
| Characteristic . | Overall . | Active surveillance (n = 58) . | Surgical resection (n = 51) . | Systemic treatment (n = 14) . | P . |
|---|---|---|---|---|---|
| Both lungs | 10 (8) | 8 (14) | 0 (0) | 2 (14) | .007 |
| No. of lesions | .005 | ||||
| 1 | 94 (76) | 39 (67) | 46 (90) | 9 (64) | |
| ≥2 | 29 (24) | 19 (33) | 5 (9.8) | 5 (36) | |
| Median size (IQR), cm | 2.50 (1.50-3.50) | 2.35 (1.50-2.80) | 2.60 (1.60-3.45) | 5.50 (3.48-7.45) | <.001 |
| Size category, cm* | .3 | ||||
| ≤1 | 9 (7.3) | 5 (8.6) | 3 (5.9) | 1 (7.1) | |
| >1 to ≤2 | 35 (28) | 20 (34) | 14 (27) | 1 (7.1) | |
| >2 | 79 (64) | 33 (57) | 34 (67) | 12 (86) | |
| Pleural involvement | 23 (19) | 13 (22) | 9 (18) | 1 (7.1) | .5 |
| Pleural effusion | 14 (11) | 5 (8.6) | 6 (12) | 3 (21) | .4 |
| Nodal disease in chest | 6 (4.9) | 3 (5.2) | 3 (5.9) | 0 (0) | >.9 |
| Ki67 (percent positive)† | 5 (5-10) | 5 (5-10) | 5 (5-10) | 8 (5-12) | .7 |
| Anemia (Hb <11 g/dL) | 11 (8.9) | 8 (14) | 3 (5.9) | 0 (0) | .2 |
| Neutropenia (<1.5 × 103/µL) | 2 (1.6) | 1 (1.7) | 0 (0) | 1 (7.1) | .2 |
| Thrombocytopenia (<150 × 103/µL) | 7 (5.7) | 4 (6.9) | 1 (1.9) | 2 (14) | .15 |
| LDH >ULN | 9 (7.3) | 6 (10) | 2 (3.9) | 1 (7.1) | .8 |
| Characteristic . | Overall . | Active surveillance (n = 58) . | Surgical resection (n = 51) . | Systemic treatment (n = 14) . | P . |
|---|---|---|---|---|---|
| Both lungs | 10 (8) | 8 (14) | 0 (0) | 2 (14) | .007 |
| No. of lesions | .005 | ||||
| 1 | 94 (76) | 39 (67) | 46 (90) | 9 (64) | |
| ≥2 | 29 (24) | 19 (33) | 5 (9.8) | 5 (36) | |
| Median size (IQR), cm | 2.50 (1.50-3.50) | 2.35 (1.50-2.80) | 2.60 (1.60-3.45) | 5.50 (3.48-7.45) | <.001 |
| Size category, cm* | .3 | ||||
| ≤1 | 9 (7.3) | 5 (8.6) | 3 (5.9) | 1 (7.1) | |
| >1 to ≤2 | 35 (28) | 20 (34) | 14 (27) | 1 (7.1) | |
| >2 | 79 (64) | 33 (57) | 34 (67) | 12 (86) | |
| Pleural involvement | 23 (19) | 13 (22) | 9 (18) | 1 (7.1) | .5 |
| Pleural effusion | 14 (11) | 5 (8.6) | 6 (12) | 3 (21) | .4 |
| Nodal disease in chest | 6 (4.9) | 3 (5.2) | 3 (5.9) | 0 (0) | >.9 |
| Ki67 (percent positive)† | 5 (5-10) | 5 (5-10) | 5 (5-10) | 8 (5-12) | .7 |
| Anemia (Hb <11 g/dL) | 11 (8.9) | 8 (14) | 3 (5.9) | 0 (0) | .2 |
| Neutropenia (<1.5 × 103/µL) | 2 (1.6) | 1 (1.7) | 0 (0) | 1 (7.1) | .2 |
| Thrombocytopenia (<150 × 103/µL) | 7 (5.7) | 4 (6.9) | 1 (1.9) | 2 (14) | .15 |
| LDH >ULN | 9 (7.3) | 6 (10) | 2 (3.9) | 1 (7.1) | .8 |
Data presented as no. (%) or median (IQR). Boldface P values indicate statistical significance (P < .05).
Hb, hemoglobin; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Largest tumor.
There were 74 patients with available data for evaluation.
Patients who underwent surgical resection were less likely to have ever smoked (P = .03) or to present with dyspnea (P = .03) or multiple lung lesions (P = .005). No patients with bilateral lung lesions underwent a surgical resection, but 14% of patients who received active surveillance and 14% receiving systemic treatment had bilateral lung lesions (P = .007). Patients receiving systemic treatment were likely to have larger lesions than those in the other 2 groups (median, 5.5 cm for systemic treatment vs 2.35 cm for active surveillance and 2.6 cm for surgical resection; P < .001). Apart from tumor size, there were no significant differences in clinical characteristics between the active surveillance and systemic treatment groups at baseline (Tables 1 and 2).
Treatment outcomes
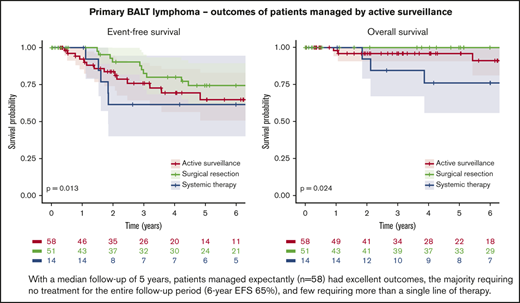
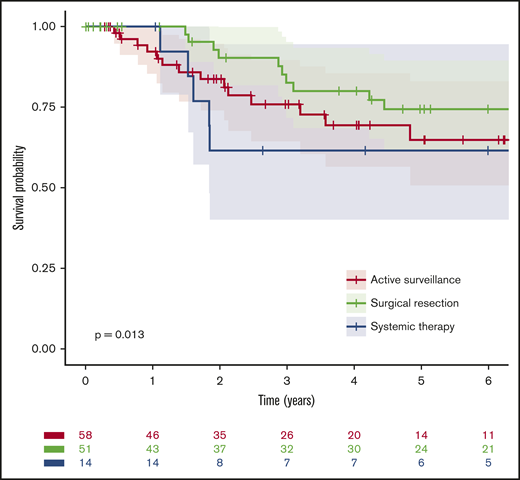
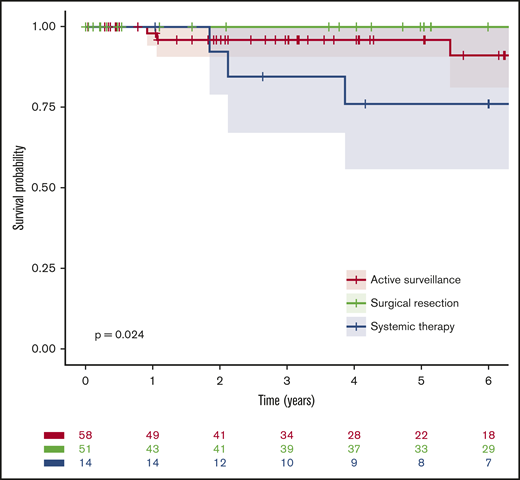
Median follow-up was 5 years (60 months; IQR, 25-109 months), and 10 deaths occurred; median OS for the entire cohort was not reached, and the 6-year OS was 93% (95% confidence interval [CI], 88%-99%). Notably, there were high survival rates in all 3 groups: the estimated 6-year OS rates were 91% (95% CI, 81-100), 100%, and 76% (95% CI, 56-100) for patients managed by active surveillance, initial complete surgical resection, and initial systemic treatment, respectively (P = .02; Figure 1; Table 3). Likewise, EFS rates were similar for active surveillance and systemic treatment groups but superior after surgical resection: the estimated 6-year EFS rates were 65% (95% CI, 51-83), 62% (95% CI, 40-95), and 74% (95% CI, 62-90), respectively (P = .01; Figure 2; Table 3). Only 14% (95% CI, 7-23) of the patients initially receiving active surveillance were estimated to have required a second systemic treatment or died within 6 years.
OS from diagnosis by first-line management. For the purpose of this analysis, active surveillance and surgical resection were considered as the first line of treatment.
OS from diagnosis by first-line management. For the purpose of this analysis, active surveillance and surgical resection were considered as the first line of treatment.
Survival analyses for differences in time-to-event (years) by first-line management
| Survival . | No. (%) . | Events . | Median OS or EFS (95% CI) . | 6-year OS or EFS (95% CI) . | P . |
|---|---|---|---|---|---|
| OS | |||||
| All patients | 123 | 10 | NR | 0.93 (0.88-0.99) | .024 |
| Active surveillance | 58 (47) | 6 | NR | 0.91 (0.81-1.00) | |
| Surgical resection | 51 (41) | 1 | NR | 1.00 (1.00-1.00) | |
| Systemic treatment | 14 (11) | 3 | NR | 0.76 (0.56-1.00) | |
| EFS | |||||
| All patients | 123 | 37 | 9.1 (7.7-NR) | 0.69 (0.59-0.79) | .013 |
| Active surveillance | 58 (47) | 17 | 7.2 (6.8-NR) | 0.65 (0.51-0.83) | |
| Surgical resection | 51 (41) | 12 | NR (12.8-NR) | 0.74 (0.62-0.90) | |
| Systemic treatment | 14 (11) | 8 | 7.3 (1.8-NR) | 0.62 (0.40-0.95) |
| Survival . | No. (%) . | Events . | Median OS or EFS (95% CI) . | 6-year OS or EFS (95% CI) . | P . |
|---|---|---|---|---|---|
| OS | |||||
| All patients | 123 | 10 | NR | 0.93 (0.88-0.99) | .024 |
| Active surveillance | 58 (47) | 6 | NR | 0.91 (0.81-1.00) | |
| Surgical resection | 51 (41) | 1 | NR | 1.00 (1.00-1.00) | |
| Systemic treatment | 14 (11) | 3 | NR | 0.76 (0.56-1.00) | |
| EFS | |||||
| All patients | 123 | 37 | 9.1 (7.7-NR) | 0.69 (0.59-0.79) | .013 |
| Active surveillance | 58 (47) | 17 | 7.2 (6.8-NR) | 0.65 (0.51-0.83) | |
| Surgical resection | 51 (41) | 12 | NR (12.8-NR) | 0.74 (0.62-0.90) | |
| Systemic treatment | 14 (11) | 8 | 7.3 (1.8-NR) | 0.62 (0.40-0.95) |
P values correspond to Kaplan-Meier log-rank test.
NR, not reached.
EFS from diagnosis by first-line management. For the purpose of this analysis, active surveillance and surgical resection were considered as the first line of treatment.
EFS from diagnosis by first-line management. For the purpose of this analysis, active surveillance and surgical resection were considered as the first line of treatment.
Survival analyses
In univariable survival analysis, the variables associated with shorter EFS, other than treatment type, were lesion size (hazard ratio [HR], 1.2; 95% CI, 1.03-1.34 for every centimeter; P = .02) and thrombocytopenia (platelet count <150 × 103/μL; HR, 8.49; 95% CI, 3.1-23.1; P < .001). Bilateral lung involvement and number of lesions (≥2 lesions) were not prognostically significant.
The low number of deaths precluded performing a multivariable analysis for OS. In a multivariable model for EFS that included treatment type, lesion size, and presence of thrombocytopenia, the risk of an event was lower with surgical resection than with active surveillance (HR, 0.36; 95% CI, 0.16-0.77; P = .014); the effect of systemic treatment was not significant. Thrombocytopenia was also independently associated with an increased risk of an EFS event (HR, 8.34; 95% CI, 2.9-24.02; P < .001), as was each centimeter increase in the size of the largest lesion (HR, 1.2; 95% CI, 1.02-1.41; P = .028).
Discussion
Primary BALT lymphoma has been recognized as an exceptionally indolent condition in the majority of patients since as early as 1963.2 But this rare neoplasm may be overtreated because of its strategic location in the lung. This study aimed to evaluate an active surveillance approach compared with surgical and systemic interventions. With a median follow-up of 5 years, patients managed expectantly (n = 58) had excellent outcomes, the majority requiring no treatment during the entire follow-up period (6-year EFS, 65%), and few patients requiring more than a single line of therapy. The few patients who received early systemic therapy (n = 14) likely presented with a more aggressive disease and did not fare better than patients in the other groups (6-year EFS, 62%). Meanwhile, as could be expected for the limited number of patients with disease amenable to surgery, those who underwent a complete surgical resection (n = 51) experienced a lower rate of progression (6-year EFS, 74%). OS was excellent in all groups (6-year OS, 93%).
Despite its notably higher number of patients managed expectantly, our cohort resembles previously described series. Most patients presented in their 60s and women slightly predominated; we found high rates of current smoking or history of smoking (61%), chronic obstructive pulmonary disease (17%), and autoimmune or connective tissue diseases (21%).3,6,7 As with previous studies, lung involvement was mostly unilateral and localized to a single lobe.3,13
Also in keeping with previous series, we observed differences in EFS by treatment modality, but OS was excellent.3,4 In the largest series of patients with BALT lymphoma published to date (N = 205), patients who underwent a surgical resection had a 6-year PFS of ∼80% compared with ∼65% in those treated with systemic therapy. However, as with our data, OS was similar between the groups, with an approximate 6-year OS for the entire cohort of 85%.3 Notably, in that study, which described treatment patterns across 17 different international centers, >95% of the patients received either surgical or systemic treatment. Multiple other contemporary series of BALT lymphoma, all describing similarly excellent long-term outcomes, demonstrated the general preference for an active treatment approach.4,5,14-16
This preference for active treatment, despite the similarities in cases and survival outcomes between our cohort and others, may reflect concern among oncologists about the potential for compromised pulmonary function if lymphoma progresses. However, pulmonary symptoms may be caused by other factors, such as chronic obstructive pulmonary disease or concurrent infection, which should be ruled out before concluding that lymphoma is the cause. In other indolent lymphomas, several criteria are used to determine the need for therapy. Broadly, these include the development of symptoms, a predefined burden of disease, the pace of progression, and suspicion of transformation to an aggressive lymphoma.8,9 Inferring similar criteria for BALT lymphoma from a retrospective cohort that was not treated uniformly is challenging, but our data suggest that the presence of thrombocytopenia or larger lesions (we would empirically suggest >3.5 cm) could serve as a guide. Finally, whether patients with a localized BALT lymphoma that has not been fully excised should be referred to resection is questionable. Our data and the literature suggest that active surveillance may be appropriate for a large proportion of these patients.3,4
The main limitation of this study, beyond being retrospective and conducted at a single center, is the inclusion of very few patients treated with systemic chemotherapy or immunotherapy at presentation who were characterized by a higher tumor burden and more symptomatic disease. The mix of patients in our cohort contrasts with previous studies in which most patients who did not undergo a complete surgical resection were treated with systemic therapy. Nonetheless, the fact that so few patients were treated with systemic therapy in our study serves to strengthen our observation that active surveillance is feasible and associated with excellent outcomes.
In summary, BALT lymphoma is an indolent disease that can be managed expectantly after initial diagnosis in most patients without requiring therapy for many years. Our finding that patients with fully resected disease had slightly better survival could represent a selection bias toward earlier detection, fitter patients, or a biologically less-active disease. In asymptomatic patients with small nodules, initial observation of the pace of the disease may help identify the minority of patients who will benefit from treatment while avoiding or delaying its potential risks for the vast majority.
Please send requests for data to David J. Straus at strausd@mskcc.org.
Acknowledgments
Editorial support in the preparation of this article was provided by Hannah Rice, Editor in the Life Sciences.
This research was funded, in part, by a Cancer Center Support grant from the National Institutes of Health/National Cancer Institute (P30 CA008748).
Authorship
Contribution: E.J. and D.J.S. designed the study; E.J. and Y.L. extracted the data; E.D. performed the statistical analyses; S.R., A.D.Z., M.L.P., C.H.M., C.P., A.N., S.M.H., J.F.G., A.M., P.H., M.J.M., A.K., C.L.B., and A.Y. contributed patients to the study; and all authors helped write and review the manuscript.
Conflict-of-interest disclosure: E.J. served as an advisor for AstraZeneca and Epizyme. Y.L. received an Abstract Achievement Award for presenting this study as an abstract at the 2019 American Society of Hematology Annual Meeting and Exposition. A.D.Z. has received grants and personal fees from MEI Pharmaceutical, Gilead (including Kite), Bristol Myers Squibb (including Celgene and Juno), Novartis, Roche (including Genentech), BeiGene, AbbVie, Pharmacyclics, AstraZeneca (including Acerta), Amgen, Pfizer, and Janssen outside the submitted work. None of these disclosures impact recommendations or findings in this manuscript. A.N. served as an advisor at Janssen and Morphosys, has received honoraria from Medscape and Pharmacyclics and research funding from Pharmacyclics and Rafael Pharma. These disclosures are not directly relevant to this article. S.M.H. has served as a consultant, received honoraria from, or participated in advisory boards for ADC Therapeutics, C4 Therapeutics, Celgene, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, Seattle Genetics, Takeda, Verastem, and Vividion Therapeutics, has received research support for clinical trials from ADC Therapeutics, Aileron, Celgene, Daiichi Sankyo, Forty Seven, Kyowa Hakko Kirin, Millennium/Takeda, Seattle Genetics, Verastem, Portola Pharmaceuticals, and Trillium Therapeutics. J.F.G. is an employee of Janssen Pharmaceuticals, starting after participation in this project. A.M. has received research support from Miragen, Seattle Genetics, Merck, Bristol Myers Squibb, and Incyte and honoraria from Imbrium Therapeutics, Merck, and Seattle Genetics. C.L.B. has received research support from Janssen, Novartis, Epizyme, Xynomic, and Bayer, honoraria from Dava Oncology, and has served as a consultant for Kite Pharma, Juno Therapeutics, Life Sci, GLG Pharma, and Seattle Genetics. A.Y. is an employee of AstraZeneca, starting after participation in this project. D.J.S. has received research support from the Lymphoma Foundation, Rob and Karen Schneider and The Louis Schneider and Harry Davis Memorial Trust, The Adam R. Spector Foundation, The David R. and Patricia D. Atkinson Foundation, and Mr. and Mrs. Ernest Dicker. The remaining authors declare no competing financial interests.
The current affiliation for C.H.M. is Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL.
The current affiliation for J.F.G. is Janssen Pharmaceuticals, Raritan, NJ.
The current affiliation for A.Y. is AstraZeneca, New York, NY.
Correspondence: David J. Straus, Lymphoma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, SR‐441B, New York, NY 10065; e-mail: strausd@mskcc.org.